Abstract
Regulation of immune response was found to play an important role in the course of many diseases such as autoimmune diseases, allergy, malignancy, organ transplantation. The studies on immune regulation focus on the role of regulatory cells (Tregs, Bregs, regulatory myeloid cells) in these disorders. The number and function of Tregs may serve as a marker of disease activity. As in allergy, the depletion of Tregs is observed and the results of allergen-specific immunotherapy could be measured by an increase in the population of IL-10+ regulatory cells. On the basis of the knowledge of anti-cancer immune response regulation, new directions in therapy of tumors are introduced. As the proportion of regulatory cells is increased in the course of neoplasm, the therapeutic action is directed at their inhibition. The depletion of Tregs may be also achieved by an anti-check-point blockade, anti-CD25 agents, and inhibition of regulatory cell recruitment to the tumor site by affecting chemokine pathways. However, the possible favorable role of Tregs in cancer development is considered and the plasticity of immune regulation should be taken into account. The new promising direction of the treatment based on regulatory cells is the prevention of transplant rejection. A different way of production and implementation of classic Tregs as well as other cell types such as double-negative cells, Bregs, CD4+ Tr1 cells are tested in ongoing trials. On the basis of the results of current studies, we could show in this review the significance of therapies based on regulatory cells in different disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Regulation of Immune Response in Allergy
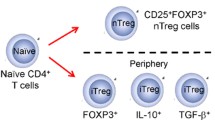
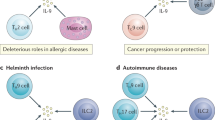
Various abnormalities in regulatory network have long been considered as the main step in development and maintenance of allergic diseases. Regulatory T cells (Tregs) are extremely important part of this process. Among others they inhibit the production of interleukin (IL)-4, IL-5, IL-9, and IL-13 by direct suppression of Th2 cells activation, induce IgG4 instead of immunoglobulin E (IgE) production in B cell, and block the migration of effector T cells into inflamed tissue (Baecher-Allan et al. 2004; Kleer et al. 2004; Palomares et al. 2010). Frequency and functional deficiency of Tregs can be caused by any factor that disturbs immunological balance, such as environmental factors or genetic predisposition (Lambrecht and Hammad 2013). However, Tregs are not the only regulatory cells, which have a role in maintaining allergen tolerance, and if impaired lead to developing allergy. Recently, also B regulatory cells (Bregs) have been described. Like Tregs, Bregs have also a variety of mechanisms of suppression. Their most common mechanism of action is based on IL-10 production and secretion. Other regulatory function can be carried out through transforming growth factor (TGF)-β, Granzyme-B, Fas ligand production, or mediating anti-inflammatory mechanism due to their capacity of inhibitory IgG4 and sialylated IgG production (Braza et al. 2014). Certainly, they are important for the establishment of allergen tolerance (van de Veen et al. 2013).
Many authors agree that during allergy the number of Tregs is often decreased and their function is impaired (Akdis et al. 2004; Lee et al. 2007; Stelmaszczyk-Emmel et al. 2013; Xu et al. 2007). Nevertheless, the role of Tregs in the pathogenesis of pediatric allergic disorders is still unclear and the results obtained from many studies are inconsistent. A large group of authors demonstrated the reduction of the Treg population often accompanied by the impairment of their function (Lee et al. 2007; Stelmaszczyk-Emmel et al. 2013; Xu et al. 2007; Zhang et al. 2008). However, other scientists showed that the frequency of Tregs does not differ between allergic and non-allergic populations (Geraldes et al. 2010; Grindebacke et al. 2004; Ling et al. 2004). The observed discrepancies may have several reasons, including differences in clinical form of the disease, age of the patients, exposure to allergens, drugs used during the therapy (e.g., higher frequency of Tregs during steroid therapy was observed) (Karagiannidis et al. 2004; Lee et al. 2007; Shi et al. 2004). Other important factors, which influence the results include laboratory techniques used for Treg identification, the way of their characterization by different surface and intracellular markers, and the type of the collected clinical materials (peripheral blood, bronchoalveolar lavage fluid, sublingual epithelium or nasal mucosa) (Hartl et al. 2007; Nguyen et al. 2008, 2009; Radulovic et al. 2008; Scadding et al. 2010; Thunberg et al. 2010). There are still many inconsistencies in Breg phenotyping: they are characterized differently by authors, but greater agreement with regard to the direction of changes during allergy can be observed. So far, only reduction of Breg number in peripheral blood of allergic patients was observed (Kamekura et al. 2015; Kim et al. 2016; Noh et al. 2012; Stanic et al. 2015; van de Veen et al. 2013; van der Vlugt et al. 2014).
In addition to a lower number of Tregs, some authors also demonstrated the relationship between the severity of the disease and proportion of Tregs (Lee et al. 2007; Meszaros et al. 2009; Shi et al. 2004). Our studies have shown a strong correlation between relative number of Tregs and clinical manifestation of the disease. Children with characteristic symptoms of respiratory tract allergy (controlled asthma, allergic rhinitis, and allergic conjunctivitis) and additionally coexisting atopic dermatitis and/or food allergy had significantly lower percentage of Tregs in their peripheral blood than children without additional symptoms (Stelmaszczyk-Emmel et al. 2013).
Allergen-specific immunotherapy (ASIT) is nowadays the best and non-symptomatic allergy treatment. Regardless of the type of treatment (subcutaneous, sublingual, oral), ASIT influences the function of many cells: monocytes, B cell, basophils, eosinophils, mast cells, and last but not least T cells. Speaking of T cells, except reducing allergen-specific T cell proliferation, reducing tissue Th2 cytokine production, and increasing tissue Th1 cytokine release, ASIT induces functional Tregs (Akdis and Akdis 2011; Moingeon 2013; Novak et al. 2011; Ring and Gutermuth 2011). Nonetheless, the precise mechanism of ASIT is unknown and it is difficult to assess the treatment benefit in context of Tregs.
Clinical scales to assess the treatment efficiency, such as visual analog scale, medication score, and quality of life questionnaire, are widely used. However, these scales are not always objective and may not give trustworthy results. They require huge patient’s involvement and compliance, and are not easy to perform especially in children. Moreover, the results of ASIT from year to year can be affected by duration and intensity of exposure to allergens, which is never alike between allergic seasons. It would therefore be very helpful if physicians could rely on laboratory tests that would indicate whether a patient is responsive to immunotherapy or not. Nowadays, laboratory monitoring of ASIT can be performed using several test (e.g., specific IgE serum levels, specific serum IgG4 levels, allergen-induced basophils CD63 expression, allergen-specific T cells identify by MCH class II/peptide tetramers), but none of them is sufficient and each has some limitations. Some years ago, Tregs appeared to be a good marker for such assessment. In many studies, clinically successful ASIT went together with increased proportion of Tregs, increased population of IL-10+ cells, and/or hypomethylation of forkhead box P3 (Foxp3) (Fujimura et al. 2011; Lou et al. 2012; Scadding et al. 2010; Sørensen et al. 2013; Suárez-Fueyo et al. 2014; Swamy et al. 2012; Syed et al. 2014). However, other authors did not demonstrate any alterations in the number of Tregs after ASIT (Kim et al. 2011; Lou et al. 2012; Moed et al. 2013; Schubert et al. 2009; Stelmaszczyk-Emmel et al. 2015; Thunberg et al. 2010). And again, typical inconsistencies between different studies (mainly caused by differences in definition of Tregs and method used to identify them, different clinical conditions, etc.) make them difficult to compare. To sum up, the majority of authors agree that efficacy of ASIT does not depend on Foxp3 Tregs, but on IL-10-producing cells, and finally, only IL-10-producing cells were considered as biomarker for ASIT monitoring (Fujimura et al. 2011). Our findings are in accordance with studies in which similar numbers of Tregs before and after treatment were observed. Similarly to the variations in the onset of allergy, the differences between patients with different manifestations of allergy were observed. Small group of patients with additional clinical symptoms (atopic dermatitis, food allergy) showed significant increase of Foxp3 Tregs after ASIT, while in the entire group of patients such phenomenon was not shown (Stelmaszczyk-Emmel et al. 2015).
In short, in the view of a large number of studies Tregs and Bregs influence many immune cells, which participate in the development of allergic reactions. During ASIT, Tregs help inactivate these cells and, as a consequence, reduce severity of patients’ symptoms and improve their quality of life.
Regulation in Malignancy and Therapeutic Options
The recognition of the mechanisms of anti-cancer immune response opened the era of successful cancer immunotherapy. The goal of this immunomodulatory treatment in malignancy is to renew own host defense mechanisms (Aerts and Hegmans 2013). The inhibition of immune regulatory processes is a very important and promising pathway in the immunomodulatory treatment of solid tumors. Here we focus on the possibility of the silencing of the function of Tregs, macrophages polarized to the regulatory population, myeloid-derived suppressor cells (MDSCs), and some suppressive cytokines: IL-17, IL-10, and TGF-β.
Regulatory T Cells
An increased number of Tregs within peripheral blood, lymph nodes, and tumor-infiltrating lymphocytes (TIL) observed in cancer patients were found to be an important negative prognostic factor. There is a growing body of evidence that Tregs are an ideal target for therapy improving an anti-cancer response. The mechanisms contributing to an elevated number of Tregs are as follows: the migration to the tumor site of the cells de novo arising in lymph nodes and the differentiation under the influence of mediators in the tumor environment (TME) (Gallimore and Simon 2008). The main function of Tregs is to inhibit T effector cells: CD4+ and CD8+ lymphocytes, dendritic cells (DCs), and natural killer (NK) cells in the site of immune response (Chaput et al. 2007; Orentas et al. 2006; Woo et al. 2002). In contrast to solid tumors, the role of Tregs in lymphoproliferative disorders is opposite—the simplified explanation is that these cells suppress proliferating B cells (Grygorowicz et al. 2016).
The function of Tregs in malignancy is associated with overexpression of some molecules (Baecher-Allan et al. 2004; Orentas et al. 2006). These molecules are also the markers of Tregs. These are Foxp3, cytotoxic T lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced TNF receptor (GITR; CD357), and lymphocyte-activation gene 3 (LAG-3). Foxp3 is known per se as a negative prognostic factor in solid tumors. Evaluation of immune cell infiltrates (so-called “immunoscoring”) has shown that the increased expression of Foxp3 in lymphocytes or in tumor cells and an increased Foxp3/CD8+ ratio are related to tumor progression (Petersen et al. 2006). On the other hand, the presence of Foxp3-positive lymphocytes in lymphoproliferative disorders is associated with a better prognosis (Tzankov et al. 2008). It was found that malignant B cells die after contact with CD4+/Foxp3+ cells.
A very strong inductor of Tregs is CTLA-4 molecule also known as a strong suppressor of the T effector cell (Teff) function (Avogadri et al. 2011). This antigen is presented on Tregs mainly as an intracellular domain. CTLA-4 is required for Treg-mediated suppression of immune response (Krummey and Ford 2014) and the inhibitory function of CTLA-4 seems to be stronger than that of Foxp3. Tregs lose their function when the expression of CTLA-4 is reduced (Krummey and Ford 2014; Walker and Sansom 2015). CTLA-4 blockade on Teff cells is capable of activating an antitumor response and has been used recently in some solid tumor therapy (Avogadri et al. 2011; Mocellin and Nitti 2013). Thus, by blocking CTLA-4 on Tregs an additional therapeutic effect of this kind of immunotherapy could be achieved. There are two domains of CTLA-4: extracellular and intracellular. The extracellular domain is required for cell function (Tai et al. 2012). CTLA-4 traffic and the expression of this molecule are modified by the tumor environment. We observed the difference in CTLA-4 cellular distribution in lung cancer: the ratio of surface to the intracellular expression of CTLA-4 was higher in TME when compared to peripheral blood (Kwiecien et al. 2017). GITR is constitutively expressed on Tregs similarly to CTLA-4 and the persistent expression of this molecule in the tumor environment was demonstrated (Avogadri et al. 2011). The agonistic anti-GITR monoclonal antibody (mAb) suppresses Tregs and is a promising direction of therapy (Nishikawa and Sakaguchi 2010).
The suppressive molecules, CTLA-4, programmed cell death protein-1 (PD-1), mucin domain containing molecule-3 (TIM-3), and the so-called “check-points,” are expressed on Teff cells and play a role of strong regulators of anti-cancer cytotoxicity. The check-point blockers anti-CTLA-4—ipilimumab and anti PD-1 nivolumab are approved in the treatment of melanoma and non-small cell lung cancer (Postow et al. 2015). PD-1 being expressed on Tregs is known to induce their suppressive and regulatory function. LAG-3 and TIM-3 play a similar role and are also the possible targets for blockade. Thus, the anti-check-point agents which are capable of restoring the anti-cancer function of cytotoxic T lymphocytes (CTLs) are simultaneously the inhibitors of Tregs (Fig. 1).
Tregs are defined by expression of CD25 (α chain IL-2 receptor), which is a possible target for Treg inhibition (Wolf et al. 2015). A classic way of CD25 blockade is to use anti-CD25 mAb. CD25 antibody—daclizumab, approved in humans in transplanthology was investigated in many cancers, but without spectacular promising results. Another method of anti-CD25 action is the use of IL-2 conjugated with diphtheria toxin (denileukin diftitox, ONTAK). The possible reason for the low efficacy of these agents is their opposite effect on Teffs and Tregs which depends on the current immune status of the tumor milieu and the stage of the maturation of targeted cells (Nishikawa and Sakaguchi 2010).
Another possible method for depleting Tregs is to modify their homing mediated by chemokines in the tumor environment. Tregs are recruited to the tumor site by the interaction of CCR4 expressed on highly immunosuppressive cells with CCL17 and CCL22 secreted by DCs and expressed by M2 macrophages (Komohara et al. 2016; Nishikawa and Sakaguchi 2010). The chemokine blocking agents causing Treg depletion in the tumor site are investigated (Kurose et al. 2015). For example, the cancers expressing NY-ESO-I testis antigen are a good model of immunogenicity and immune reaction (Nishikawa and Sakaguchi 2010). The efficacy of anti-CCR4 mAb in this model was observed (Wolf et al. 2015). It is suspected that Tregs are removed by macrophages via antibody-dependent cellular cytotoxicity (ADCC). Ipilimumab causes ADCC by mediating macrophage Fcγ receptors (FcγR) activation (Romano et al. 2015).
Some role of the pathway of arachidonic acid-cyclooxygenases (COXs)-prostaglandins in regulation of immune response in malignancy was described (Liu et al. 2015; O’Callaghan and Houston 2015). The most important is COX-2 alteration and a massive production of PGE-2 was found in the milieu of many cancers. PGE-2 is capable of inhibiting NK cells, reduce maturation of DCs, and enhance production of IL-10. The contribution of PGE-2 in the differentiation of T cells to Tregs was presented (Mougiakakos et al. 2010). Thus, the use of selective COX-2 inhibitors may have some protective anti-cancer properties and the reduction of the risk of some solid tumors was noted (Göbel et al. 2014). The targets for PGE-2 signals are four plasma membrane EP receptors: EP1-4. Each of them is connected with special cellular pathway (O’Callaghan and Houston 2015) and recently EP antagonists seem to be attractive suppressors of tumor growth in preclinical studies.
A targeted therapy using tyrosine kinase inhibitors (IKTs) proved to be very effective in patients with lung adenocarcinoma and activated epidermal growth factor receptor (EGFR) mutations. It was shown that some intracellular pathways in Tregs are also susceptible to IKTs (Wolf et al. 2015). For instance, IKTs are the inhibitors of signal transducers and activators of transcription (STAT) signaling necessary for Foxp3 expression. Other intracellular signaling pathways in Tregs are PI3 K/Akt and mTOR and may be inhibited by IKTs.
There are other studies pointing at some new anti-Treg activities that were revealed, but they need a more detailed investigation. These are the new directions:
-
influencing the metabolic pathways: the suppression of Treg proliferation may be achieved by binding leptin, an adipocyte-derived cytokine (De Rosa et al. 2007),
-
the evaluation of KRAS mutation which contributes to the induction of Tregs (Zdanov et al. 2016), and
-
the measurement of hypoxia which promotes the suppression of immune response (Labiano et al. 2015).
Interestingly, the possible favorable role of Tregs in cancer development is considered. Persistent inflammation is a risk of malignant transformation of epithelial cells of many organs such as lung, bowel, stomach. The role of colon microbiome in chronic inflammation in relation to carcinogenesis was described in the studies on colorectal carcinoma (Feng et al. 2015). Tregs may have a protective anti-cancer role as the cells which contribute to control chronic inflammation. We previously presented the depletion of Tregs in chronic obstructive pulmonary diseases (COPD) (Domagala-Kulawik et al. 2011). COPD is a chronic inflammatory pulmonary disorder and it is recognized as a risk factor for lung cancer. The depletion of Tregs may cause the development of uncontrolled inflammation and promote malignant changes of bronchial epithelium.
It should be noted that the anti-cancer immune reaction is highly individual, dynamic, and plastic. These features are exhibited mainly by cytokines. TGF-β and interleukins, such as IL-10 and IL-6, have a well-documented inhibitory and regulatory function (Burkholder et al. 2014). However, these cytokines interact with the current direction of immune reaction and their function is strictly dependent on “of the moment” character of immune response. TGF-β is a strong suppressor, but it was demonstrated that in the initial stage of carcinogenesis TGF-β is required for the stimulation of CTLs (Quatromoni et al. 2013). The dual role of TGF-β is well visible during the differentiation of naïve Th cells to regulatory populations: the low concentration of this cytokine is capable of inducing Th17 cells, while the high concentration is capable of inducing Tregs (Romagnani 2008). The conversion of Tregs to Th-17 cells seems to be a promising way for therapeutic modification (Burkholder et al. 2014). These two populations are competitive in terms of their number, differentiation, and function. The adverse correlation between them was found in the tumor milieu; however, in our study the opposite relation was observed: the concentration of IL-17A correlated with Tregs in the lung affected by cancer (Kwiecien et al. 2017). Th17 role in malignancy remains controversial (Guery and Hugues 2015) and the high plasticity of Th17 function, dependent on IL-1β, IL-6, IL-23 concentration in the site of cancer, is known (Muranski and Restifo 2013). Recently, Punt et al. (2015) presented the impact of IL-17 and Th17 cells on the prognosis of different cancers and concluded that IL-17 was associated rather with a poor prognosis and that patients exhibiting a high IL-17 level may benefit from anti-IL-17 treatment or from the transfer of Th17 cells. Interestingly, the authors indicate the necessity to distinguish Th17 cells from other populations of IL-17-producing cells (Punt et al. 2015).
Regulatory B Cells
Recently, a new population of cells influencing Tregs was recognized, i.e., Breg set. A deep characteristic of Bregs was presented in part I of this review. The role of Bregs in malignancy is much less defined and recognized than the role of Tregs. A very important direction and the function of Bregs confirmed in many studies is the induction of Treg generation (He et al. 2014). As the main feature of Bregs is IL-10 production, this cytokine seems to be the most important as a Treg inducer.
In the study on gastric cancer, Wang et al. (2015) found that the proportion of CD19+CD24highCD38high Bregs is augmented with the capacity to produce large amounts of IL-10 and TGF-β1. These Bregs positively correlated with the levels of CD4+Foxp3+ Tregs in gastric cancer. Bregs were shown to play an immunosuppressive role in gastric cancer by inhibiting Th cytokine production, but also by converting CD4+CD25– effector T cells to CD4+Foxp3+ Tregs in a TGF-β1-dependent way (Wang et al. 2015). Biragyn et al. (2014) described the phenotype and function of tBregs—tumor-evoked Bregs. These tBregs are CD19+ B cells that express CD25+CD81highB7H-1high and CD20low4-1BBLlow and a constitutively active transcription factor STAT3. Zhou et al. (2014) in the study on a large group of lung cancer patients found an elevated content of Bregs defined as CD19+CD24highCD27+ in the blood of the patients when compared to healthy subjects. It was enhanced by direct contact with lipopolysaccharide-stimulated cancer cells (Zhou et al. 2014). To date, there are no defined anti-Breg therapeutic options in malignancy. It seems that similarly to Tregs by targeting IL-10 and TGF-β, some important Breg markers such as CD19 and CD27 will contribute to inhibiting the cancer-promoting Breg function.
Myeloid Cell Contribution
Regulatory lymphocytes work in a strict connection with other non-lymphoid cell populations. Tumor-associated macrophages form a large group of non-epithelial non-lymphoid cells in TEM. As well as “good” and “bad” lymphocytes among TIL, the distinction of macrophages to anti- and pro-cancer phenotype has been described. M1 type anti-cancer macrophages are defined upon the ability to produce pro-inflammatory cytokines. Thanks to the full repertoire of FcγR, macrophages reveal antigen-dependent cellular phagocytosis (ADCP) and are capable of eliminating tumor cells. The M2 macrophage differentiation is done under the influence of IL-4, IL-13, IL-10, and M-CSF (colony-stimulating factor). Among M2 products, there are also IL-10 and TGF-β (Martinez and Gordon 2014). The following term for M2 characteristics is used: “product of Th2 stimulation, pro-Th2 activation, immunoregulation.” Of M2 population, the M2b and M2c are suspected to be stronger immunoregulators. However, the analysis of macrophages by immunohistochemical staining reveals a transition of one form to another and a double expression of M1/M2 markers (Osinska et al. 2014). The correlation of M2 and Tregs is connected with a poor prognosis in malignancy. An important pathway is represented by high mobility group box 1 protein secreted by tumor cells and is able to enhance the regulatory function of myeloid cells. M2 contributes to the recruitment of Tregs by chemokine pathway CCR4/CCL17/CCL22 (Wolf et al. 2015). The other suppressive effect results from expression PD-L2, a ligand to PD-1 on macrophages. PD-1/PD-L2 ligation results in the inhibition of Teff and stimulation of Tregs. We showed the usefulness of CD163 marker for M2 cell identification (Osinska et al. 2014). CD163 is known to have complex function in inflammation with the prevalence of anti-inflammatory function and is down-regulated by many pro-inflammatory cytokines (Kowal et al. 2011). Not meaningless is the influence of hypoxia on tumor progression and macrophages belong to cell populations hypoxia-dependent (Labiano et al. 2015). Hypoxia changes metabolic pathways by the activity of hypoxia inducible factor, which mediates the suppressive cellular response (Doedens et al. 2010).
Macrophages, in contrast to lymphocytes, are much less explored as a target to immunotherapy. One of the therapeutic options targeting regulatory macrophages is the stimulation of phagocytosis after the targeted therapy. The binding of antibody to tumor cell causes the stimulation of FcγR and enhances phagocytosis. This process was confirmed in leukemia (rituximab) and in solid tumors (anti-HER-2, EGFR treatment) (Weiskopf and Weissman 2015). In addition, in patients with malignancy the FcγR polymorphism causes an increased response to antibody affected tumor cells as it was shown in leukemia, breast cancer (trastuzumab), and colon carcinoma (cetuximab) (Tamura et al. 2011; Weng and Levy 2003). CD47 is a marker on tumor cells, which protects from phagocytosis and is upregulated in many cancers. Thus, anti-CD47 antibodies are able to stimulate phagocytosis being effective after confirmation of CD47 expression on tumor cells. The clinical trials with anti-CD47 antibodies are ongoing (Kong et al. 2016). The conventional anti-cancer treatment was found to improve the effectiveness of immunotherapy. The killed cancer cells are capable of activating ADCP by releasing pro-inflammatory cytokines. A new direction is engineering antibodies to facilitate binding to the receptors, such as the newly formulated anti-CD20, anti-CD19 antibodies, IgG1 variant, cytokine-conjugated antibodies (Lazar et al. 2006). The other promising achievement is blocking of M2 receptors: CD204, folate receptor β, TIM-3, and CSF-1 (Nywening et al. 2016). Some plant-derived triterpenoids exhibit strong immunoactivatory function by anti-M2/MDSCs.
MDSCs are hematopoietic cells which originate from bone marrow in different pathological disorders, among others, and in malignancies. Different phenotypes of MDSCs were described; therefore for identifying these cells, the following markers are used: CD11β, CD14, CD15, CD33, HLA-DR (Katoh and Watanabe 2015; Komohara et al. 2016; Solito et al. 2014). The persistence of MDSC population in TEM is guaranteed by mediators secreted by cancer cells. The function of MDSCs is to inhibit T cell activation, DC differentiation, and to promote Tregs by cell-to-cell contact. The expansion of Tregs occurs thanks to the expression of CD40 on MDSCs and interaction with CD40L on Tregs in the presence of TGF-β and IL-10 (Burkholder et al. 2014). A proper T cell activation and memory-type differentiation depends on arginine, cysteine, and nitric oxide usage and MDSCs inhibit immune response by competitive use of these substrates (Srivastava et al. 2012). MDSCs produce a number of radical species and suppressive cytokines, and in this way favor angiogenesis, vasculogenesis, and metastases. The effective function of these cells is provided by epithelial–mesenchymal transition (Maenhout et al. 2014; Toh et al. 2011). The anti-MDSC strategies consist of blocking of the proliferation and differentiation and of inhibition of the migration and accumulation of these cells by different unspecific agents (Draghiciu et al. 2015; Katoh and Watanabe 2015).
Future Directions in Immunomodulatory Treatment
Malignant disease is a complex disorder, and the immune response against neoplasm is complex. There is a growing body of evidence that only combination therapy is capable of activating the host immune system most effectively. Moreover, many results of in vivo observation indicate that one agent influences many pathways. A good example is a check-point blockade which activates T effector cells while inhibiting Tregs and regulatory macrophages (Burkholder et al. 2014). The same observation concerns the combination of conventional treatment with immunotherapy. A very important role of chemo- radiotherapy is to enhance the antigenicity of tumor cells and thus the presentation of a “new victim” to antigen-presenting cells (APC) inducing cytotoxic attack. Similarly, targeted therapy is able to change the nature of malignant cells to make them well “visible” by the immune system (Galluzzi et al. 2012; Tartour and Zitvogel 2013). The rationale for combining immunotherapy with conventional chemo- and radiotherapy and targeted therapy as well is
-
to achieve the induction of immunogenic cell stress and cell death,
-
to achieve the induction of antigen expression and modulation antigenic repertoire, promoting antigen cross-presentation,
-
to favor releasing pro-inflammatory cytokines which recruit immune cells, support DCs migration to lymph nodes, and induce death cell receptors on tumor cells, and
-
to kill MDSCs and inhibit Foxp3 expression.
Moreover, regulatory/suppressor cells form an actively multiplied population when compared to effector cells and seem to be more susceptible to chemotherapy than the less numerous CTLs. It should be noted that tumor cells also play a specific role of regulators in the tumor site. They overexpress important ligands to death receptors, such as Fas ligand and PD-L1, which induce apoptosis of Fas-positive Teffs (Hoser et al. 2004). Conventional therapy is capable of destroying these pathways.
The Role of Cell Therapies Based on Regulatory T and B Cells in Transplantology
The innate immunity response caused by tissue injury as well as adaptive immunity response resulting from immunologic incompatibility for histocompatibility antigens between donor and recipient need to be controlled in patients undergoing cell and organ transplantation.
Although macrophages, DCs, and T and B cells may participate in the destruction of transplanted cells, they may as well develop tolerance resulting in longer graft survival. The population of regulatory cells participating in the prevention of transplant rejection and graft versus host (GvH) reaction includes CD4+ T cells, CD8+ T cells, Bregs, CD4−CD8− T lymphocytes, NKT cells, γδ T cells, regulatory macrophages, tolerogenic DC (tolDC), MDSCs, and mesenchymal stem cells (Wood et al. 2012). Of the cells listed above, a role of the Tregs has been best understood and are most commonly used in cell therapies.
In the early stages of immune response, the strength as well as the number of recipient regulatory cells (before the transplantation or generated during the response) is not sufficient to get the large number of leukocytes capable of damaging the transplant under control. The combination of immunosuppressive agents received by the patient in order to inhibit the immune response against transplantation antigens depends on the kind of transplantation and protocols used in customized programs. The most commonly prescribed combinations of immunosuppressive drugs include calcineurin inhibitors (tacrolimus or cyclosporin A) and proliferation inhibiting factor (mycophenolate mofetil, rapamycin). Both medication types inhibit the activation of effector T cells but, they may also influence the generation and function of regulatory cells (Battaglia et al. 2005; Calvo-Turrubiartes et al. 2009; Chen et al. 2000; Coenen et al. 2006; Gao et al. 2007; Ligocki and Niederkorn 2015; Safa et al. 2015; Tebbe et al. 2016). Such phenomenon was also confirmed in our observations (Bocian et al. 2010; Korczak-Kowalska et al. 2007; Korecka-Polak et al. 2016).
The use of immunosuppressive agents contributes significantly to ensure graft survival; however, it is associated with a range of side effects (Marcen 2009; van der Net et al. 2016). The cases of long-term transplant survival in humans have been reported in spite of discontinuation of immunosuppression. This applies to patients, who had their immunosuppressive therapy discontinued for clinical reasons (chronic viral infections and tumors), patients who discontinued their immunosuppressive therapy by themselves, some patients who had liver transplanted while participating in programs for the discontinuation of immunosuppressive medicaments, and finally some patients after kidney transplantation treated according to tolerance development protocols.
The limitation of therapies based on immunosuppressive agents is required to increase the length and quality of life of the recipients of cell and organ transplants. The therapies utilizing regulatory cells seem to be promising, as they may allow to reduce the demand for immunosuppression (van der Net et al. 2016). The cell therapy includes the adoptive transfer of regulatory cells, which were prepared in vitro and in vivo generation of alloreactive regulatory cells (Kitchens and Adams 2016; Pierini et al. 2016; Scalea et al. 2016).
Regulatory T Cells
Natural thymus-derived Treg cells (tTregs, formerly nTregs) (Abbas et al. 2013) already present in the recipient’s body during transplantation procedure are transferred into the graft, where they can inhibit the ischemia-induced injuries. Moreover, the lymphatic tissue draining the transplant contains Tregs, which inhibit the proliferation of T lymphocytes. Bregs and tolDC may promote the development of peripherally derived (formerly induced) Tregs (Abbas et al. 2013) from naive T cells (van der Net et al. 2016).
Among other Tregs, the important role in such processes is attributed to Tr1 cells, CD8+ Tregs, and double-negative (DN) T cells (CD4−CD8−) (Wood et al. 2012).
The suppressive activity of Tregs (CD4 + CD25 + Foxp3 + ) results from the influence on maturation and function of APC, synthesis of suppressive cytokines, possible induction of effector cell apoptosis, and possible disruption of metabolic pathways (Juvet et al. 2014; Safinia et al. 2015).
The greatest amount of data concerning the efficacy of therapies based on Tregs in the experimental studies was obtained using mice, rat, canine, porcine, and primate monkey models. Many data indicate the beneficial clinical role of Tregs in controlling the transplant rejection and GvH disease (GvHD) (Kitchens and Adams 2016; Trzonkowski et al. 2009; van der Net et al. 2016).
Despite the fact that different regulatory cells were used in therapeutic trials, most data concern Tregs. The most important molecules allowing for the identification of Tregs are CD4, CD25high, Foxp3, CD127low, and CD45RA.
The important stage in the development of cell therapies included the identification of the markers, which would allow the isolation of Tregs for therapeutic purposes. It has been proved that CD4+CD25+CD127low cells show higher suppressive activity than CD4+CD25high cells (Nadig et al. 2010).
There are two methods of preparation of Tregs for therapeutic purposes:
-
1.
tTregs may be isolated from the peripheral blood, proliferated ex vivo, and administered to the patients (Dieckmann et al. 2001). tTregs are isolated from the transplant recipient, proliferated in adequate conditions, according to the valid protocols. Once CD8+ T cells are removed, T cells are stimulated by beads coated with anti-CD3/anti-CD28 antibodies in the presence of recombinant IL-2. The medium is enriched with rapamycin to inhibit the proliferation of cells other than Tregs. This method leads to obtaining the polyclonal Treg cells (Juvet et al. 2014; Nadig et al. 2010; Peters et al. 2008; Trzonkowski et al. 2009).
-
2.
Alloantigen peripherally derived Tregs (pTregs) may also be prepared. Allogeneic APC cells are therefore used and the culture medium is enriched with TGF-β. pTregs were shown to display higher antigen specificity and it seems that their suppressive activity is stronger or comparable to that of tTregs; however, it requires further studies (Juvet et al. 2014; Landwehr-Kenzel et al. 2014; Sagoo et al. 2011).
Regulatory cells may also develop in situ in transplant recipients, once they received medication, which affect the development of Tregs or conversion of allospecific naive T cells to pTregs. Such therapies may also support the development of CD8+ Tregs and other regulatory cells (Juvet et al. 2014).
Tregs are mainly utilized in the GvHD treatment and prevention, as well as in patient, who received vascularized organ grafts (Scalea et al. 2016; van der Net et al. 2016). In the currently running (since 2014), phase I/II, multicentre clinical trials (ONE Study), Tregs, as well as Tr1, regulatory macrophages, and tolDC are administered to the kidney transplant recipients. Both the effectiveness and safety of the therapy are evaluated (Ferrer et al. 2014; Geissler 2012; Gregori et al. 2012; van der Net et al. 2016).
Type-1 Tregs (CD4+ Tr1 cells) are the second important regulatory cell population within CD4+ cells. Their characteristic properties include the synthesis of large amounts of IL-10 and no expression of Foxp3. The co-expression of CD49b and LAG-3 is also suggested (Gagliani et al. 2013). It has been shown that these cells participate in the development of transplantation tolerance both in animal models (tolerance for pancreatic islets transplants) and in human patients (tolerance for kidney, pancreatic islets, liver, and stem cell transplants) (Ligocki and Niederkorn 2015; Zeng et al. 2015). The clinical trial of administering Tr1 cells to the patients who underwent stem cell transplantation (phase I/II) revealed that these cells induce faster immunological restoration than Tregs, which in turn inhibited mainly GvH reaction (Gregori et al. 2012).
Among the described populations of regulatory CD8+ T cells (CD8 + Treg), there are CD8+CD28− T cells that are worth mentioning, as they inhibit the activation of T cells by promoting the development of tolerogenic DC. These cells were found in patients who had received mAb (alemtuzumab) in induction therapy following renal transplantation. CD8+ Tr cells, which produce IL-10, are to a large extent functionally similar to the CD4+ Tr1 cells (Ligocki and Niederkorn 2015; Wood et al. 2012). The significance of CD8+Foxp3+ T cells for inhibiting immunological response after allogeneic bone marrow transplantation has also been shown (Robb et al. 2012). It has been proven that the presence of CD8+ Tregs may increase survival of allogeneic skin, kidney, pancreatic islets, and heart grafts (Ligocki and Niederkorn 2015). However, regulatory CD8+ T cells have not been used in cell therapies yet.
Peripheral αβ-TCR + CD3 + CD4 − CD8 − NK1.1 − T cells (DN) inhibit the response of CD4+, CD8+ cells, B cells, NK cells, and DC. Due to their antigen-specific mechanism of action, they also prevent the graft rejection and GvHD (Ligocki and Niederkorn 2015; Wood et al. 2012). Most of the data stem from the animal models and suggest the importance of apoptosis as the mechanism of killing target cells by DN cells. The suppressive function of DN cells in human is reversible and does not require the induction of apoptosis of the target cell. The role of these cells in the survival of heart, skin, and pancreatic islets grafts has already been described (Ligocki and Niederkorn 2015). As it was with CD8+ Tregs, there are no reports of trial therapeutic administration of these cells.
Regulatory B Cells
The synthesis of alloantibodies and ability to stimulate CD4+ T cells make B cells important in the process of transplant rejection, especially in its chronic form. The response to antigen results in the development of Bregs, which limit the excessive response and may participate in acceptance of the transplant by the recipient. Many phenotypes of Bregs have been described, as well as a wide range of their mechanism of action (synthesis of suppressive cytokines, cytotoxicity, secretion of anti-inflammatory antibodies, expression of receptors inhibiting the immune response, induction of other regulatory cell populations) (Durand and Chiffoleau 2015; Kim et al. 2015).
The role of IL-10 in Breg function and the role of CD19+CD24highCD38high B cells in the development of tolerance after kidney transplantation have also been noticed (Durand and Chiffoleau 2015; Newell et al. 2010; Kim et al. 2015). The regulatory populations of CD19+CD24highCD27+ and CD19lowFoxp3+ cells also participate in the development of transplantation tolerance (Segundo et al. 2013).
The role of Breg cells in the development of tolerance has been described in various experimental models (Chesneau et al. 2013; Ding et al. 2011; Durand and Chiffoleau 2015; Yan et al. 2002), as well as in patients after cell and organ transplantations (Chesneau et al. 2013, 2014; Clatworthy et al. 2009; Durand and Chiffoleau 2015; Newell et al. 2010; Pallier et al. 2010).
Clinical trials aim the deletion of B cells population followed by promotion of the development of Breg cell population at the later stage of response (Durand and Chiffoleau 2015). It is suggested that the deletion of B cells achieved with mAbs (alemtuzumab, rituximab, basiliximab, ATG) in the presence of alloantigen promotes the development of donor-specific tolerance (Ferrer et al. 2014; Segundo et al. 2013).
Studies concerning the use of Breg cells are complicated by the large number of phenotypes and mechanisms of action of the mentioned Bregs. It is suggested that Breg cells may increase graft survival time by the induction of Tregs within the body (Durand and Chiffoleau 2015; Kim et al. 2015; Lee et al. 2014). As it is in the case of Tregs, the possibility of using medication, which would in vivo promote the development of Breg cells, is being considered, as well as in vitro generation of Breg cells followed by their administration to the patients, but the data are still scarce (Ferrer et al. 2014; Nouel et al. 2014; Scalea et al. 2016). There are no reports of clinical trials concerning the administration of in vitro prepared Breg cells.
Among the mentioned regulatory lymphocytes types, the greatest and possibly the sole role in cell therapies is attributed to Tregs. The therapies based on Tregs have not been initiated until recently and are associated with a range of uncertainties. The number of cells sufficient for effective therapy, the duration of treatment, and the number of doses still remain unknown. The unanswered questions include the following: Whether patient’s own tTregs are more efficient than alloantigen-stimulated pTregs? When should the preparation procedure begin before administration? May they be frozen and stored? Moreover, it cannot be clearly determined whether the pro-inflammatory signaling in the patient’s body would not cause the administered Tregs to convert into effector T cells. Many questions emerge concerning the safety of cell therapy, its efficacy, and finally its cost. The importance of immunologic monitoring of patients undergoing cell therapy is also being emphasized (Singer et al. 2014; van der Net et al. 2016). However, it should be presumed that due to a large demand, we will continue to observe the dynamic development of such therapies.
Abbreviations
- Breg:
-
Regulatory B cell
- CCL:
-
Chemokine ligand
- CCR:
-
Chemokine receptor
- CTL:
-
Cytotoxic T cell
- CTLA-4:
-
Cytotoxic T lymphocyte antigen-4
- DC:
-
Dendritic cell
- FasL:
-
Fas ligand
- Foxp3:
-
Forkhead box P3
- GITR:
-
Glucocorticoid-induced TNF receptor
- IL:
-
Interleukin
- LAG-3:
-
Lymphocyte-activation gene 3
- M:
-
Macrophage
- MDSC:
-
Myeloid-derived suppressor cell
- PD-1:
-
Programmed cell death protein-1
- PD-L:
-
Programmed death-ligand
- STAT:
-
Signal transducer and activator of transcription 5
- TGF-β:
-
Transforming growth factor β
- TIM-3:
-
Mucin domain containing molecule-3
- Tregs:
-
Regulatory T cell
References
Abbas AK, Benoist C, Bluestone JA et al (2013) Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol 14:307–308
Aerts JG, Hegmans JP (2013) Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 73:2381–2388
Akdis CA, Akdis M (2011) Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 127:18–27
Akdis M, Verhagen J, Taylor A et al (2004) Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific Tregulatory 1 and T helper 2 cells. J Exp Med 199:1567–1575
Avogadri F, Yuan J, Yang A et al (2011) Modulation of CTLA-4 and GITR for cancer immunotherapy. Curr Top Microbiol Immunol 344:211–244
Baecher-Allan C, Viglietta V, Hafler DA (2004) Human CD4+CD25+ regulatory T cells. Semin Immunol 16:89–98
Battaglia M, Stabilini A, Roncarolo MG (2005) Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 05:4743–4748
Biragyn A, Lee-Chang C, Bodogai M (2014) Generation and identification of tumor-evoked regulatory B cells. Methods Mol Biol 1190:271–289
Bocian K, Borysowski J, Wierzbicki P et al (2010) Rapamycin, unlike cyclosporine A, enhances suppressive functions of in vitro induced CD4+CD25+ Tregs. Nephrol Dial Transplant 25:710–717
Braza F, Chesne J, Castagnet S et al (2014) Regulatory functions of B cells in allergic diseases. Allergy 69:1454–1463
Burkholder B, Huang RY, Burgess R et al (2014) Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta 1845:182–201
Calvo-Turrubiartes M, Romano-Moreno S, García-Hernandez M et al (2009) Quantitative analysis of regulatory T cells in kidney graft recipients: a relationship with calcineurin inhibitor level. Transpl Immunol 21:43–49
Chaput N, Darrasse-Jeze G, Bergot AS et al (2007) Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol 179:4969–4978
Chen BJ, Morris RE, Chao NJ (2000) Graft-versus-host disease prevention by rapamycin: cellular mechanisms. Biol Blood Marrow Transplant 6:529–536
Chesneau M, Michel L, Degauque N et al (2013) Regulatory B cells and tolerance in transplantation: from animal models to human. Front Immunol 4:497
Chesneau M, Pallier A, Braza F et al (2014) Unique B cell differentiation profile in tolerant kidney transplant patients. Am J Transplant 14:144–155
Clatworthy MR, Watson CJ, Plotnek G et al (2009) B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med 360:2683–2685
Coenen JJ, Koenen HJ, van Rijssen E et al (2006) Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 107:1018–1023
De Rosa V, Procaccini C, Cali G et al (2007) A key role of leptin in the control of regulatory T cell proliferation. Immunity 26:241–255
Dieckmann D, Plottner H, Berchtold S et al (2001) Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 193:1303–1310
Ding Q, Yeung M, Camirand G et al (2011) Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 121:3645–3656
Doedens AL, Stockmann C, Rubinstein MP et al (2010) Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70:7465–7475
Domagala-Kulawik J, Hoser G, Dabrowska M et al (2011) CD4+/CD25+ cells in systemic inflammation in COPD. Scand J Immunol 73:59–65
Draghiciu O, Lubbers J, Nijman HW et al (2015) Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology 4:e954829
Durand J, Chiffoleau E (2015) B cells with regulatory properties in transplantation tolerance. World J Transplant 5:196–208
Feng Q, Liang S, Jia H et al (2015) Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun 6:6528
Ferrer IR, Hester J, Bushell A et al (2014) Induction of transplantation tolerance through regulatory cells from mice to men. Immunol Rev 258:102–116
Fujimura T, Yonekura S, Horiguchi S et al (2011) Increase of regulatory T cells and the ratio of specific IgE to total IgE are candidates for response monitoring or prognostic biomarkers in 2-year sublingual immunotherapy (SLIT) for Japanese cedar pollinosis. Clin Immunol 139:65–74
Gagliani N, Magnani CF, Huber S et al (2013) Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 19:739–746
Gallimore AM, Simon AK (2008) Positive and negative influences of regulatory T cells on tumour immunity. Oncogene 27:5886–5893
Galluzzi L, Senovilla L, Zitvogel L et al (2012) The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 11:215–233
Gao W, Lu Y, El Essawy B et al (2007) Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant 7:1722–1732
Geissler EK (2012) The ONE Study compares cell therapy products in organ transplantation: introduction to a review series on suppressive monocyte-derived cells. Transplant Res 1:11
Geraldes L, Morgado J, Almeida A et al (2010) Expression patterns of HLA-DR+ or HLADR–on CD4+/CD25++/CD127low regulatory T cells in patients with allergy. J Investig Allergol Clin Immunol 20:201–209
Göbel C, Breitenbuecher F, Kalkavan H et al (2014) Functional expression cloning identifies COX-2 as a suppressor of antigen-specific cancer immunity. Cell Death Dis 5:e1568
Gregori S, Goudy KS, Roncarolo MG (2012) The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol 3:30
Grindebacke H, Wing K, Andersson AC et al (2004) Defective suppression of Th2 cytokines by CD4+CD25+ regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy 34:1364–1372
Grygorowicz MA, Biernacka M, Bujko M et al (2016) Human regulatory T cells suppress proliferation of B lymphoma cells. Leuk Lymphoma 57:1903–1920
Guery L, Hugues S (2015) Th17 cell plasticity and functions in cancer immunity. Biomed Res Int 2015:314620
Hartl D, Koller B, Mehlhorn AT et al (2007) Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol 119:1258–1266
He Y, Qian H, Liu Y et al (2014) The roles of regulatory B cells in cancer. J Immunol Res 2014:215471
Hoser G, Wasilewska D, Domagala-Kulawik J (2004) Expression of Fas receptor on peripheral blood lymphocytes from patients with non-small cell lung cancer. Folia Histochem Cytobiol 42:249–252
Juvet SC, Whatcott AG, Bushell AR et al (2014) Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant 14:750–763
Kamekura R, Shigehara K, Miyajima S et al (2015) Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol 158:204–211
Karagiannidis C, Akdis M, Holopainen P (2004) Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol 114:1425–1433
Katoh H, Watanabe M (2015) Myeloid-derived suppressor cells and therapeutic strategies in cancer. Mediators Inflamm 2015:159269
Kim EH, Bird JA, Kulis M et al (2011) Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 127(640–646):e1
Kim JI, Rothstein DM, Markmann JF (2015) Role of B cells in tolerance induction. Curr Opin Organ Transplant 20:369–375
Kim AS, Doherty TA, Karta MR et al (2016) Regulatory B cells and T follicular helper cells are reduced in allergic rhinitis. J Allergy Clin Immunol 138(1192–1195):e5
Kitchens WH, Adams AB (2016) Nonhuman primate models of transplant tolerance: closer to the holy grail. Curr Opin Organ Transplant 21:59–65
Kleer IM, Wedderburn LR, Taams LS et al (2004) CD4+ CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol 172:6435–6443
Komohara Y, Fujiwara Y, Ohnishi K et al (2016) Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev 99(Pt B):180–185
Kong F, Gao F, Li H et al (2016) CD47: a potential immunotherapy target for eliminating cancer cells. Clin Transl Oncol 18:1051–1055
Korczak-Kowalska G, Wierzbicki P, Bocian K et al (2007) The influence of immunosuppressive therapy on the development of CD4+CD25+ T cells after renal transplantation. Transplant Proc 39:2721–2723
Korecka-Polak A, Bocian K, Pachówka M et al (2016) Suppressor properties of human CD8+CD28− T cells in mixed leukocyte reaction are not affected by CsA and RAPA. Arch Immunol Ther Exp 64:409–416
Kowal K, Silver R, Slawinska E et al (2011) CD163 and its role in inflammation. Folia Histochem Cytobiol 49:365–374
Krummey SM, Ford ML (2014) Braking bad: novel mechanisms of CTLA-4 inhibition of T cell responses. Am J Transplant 14:2685–2690
Kurose K, Ohue Y, Wada H et al (2015) Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin Cancer Res 21:4327–4336
Kwiecien I, Stelmaszczyk-Emmel A, Polubiec-Kownacka M et al (2017) Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol Immunother 66:161–170
Labiano S, Palazon A, Melero I (2015) Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol 42:378–386
Lambrecht BN, Hammad H (2013) Asthma: the importance of dysregulated barrier immunity. Eur J Immunol 43:3125–3137
Landwehr-Kenzel S, Issa F, Luu SH et al (2014) Novel GMP-compatible protocol employing an allogeneic B cell bank for clonal expansion of allospecific natural regulatory T cells. Am J Transplant 14:594–606
Lazar GA, Dang W, Karki S et al (2006) Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA 103:4005–4010
Lee JH, Yu HH, Wang LC et al (2007) The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol 148:53–63
Lee KM, Stott RT, Zhao G et al (2014) TGF-β-producing regulatory B cell induce regulatory T cells and promote transplantation tolerance. Eur J Immunol 44:1728–1736
Ligocki AJ, Niederkorn JY (2015) Advances on non-CD4+Foxp3+ T regulatory cells: CD8+, Type 1, and double negative T regulatory cells in organ transplantation. Transplantation 99:1553–1559
Ling EM, Smith T, Nguyen XD et al (2004) Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T cell activation to atopic status and expression of allergic disease. Lancet 363:608–615
Liu B, Qu L, Yan S (2015) Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int 15:106
Lou W, Wang C, Wang Y et al (2012) Responses of CD4+CD25+Foxp3+ and IL-10-secreting type I T regulatory cells to cluster-specific immunotherapy for allergic rhinitis in children. Pediatr Allergy Immunol 23:141–149
Maenhout SK, Thielemans K, Aerts JL (2014) Location, location, location: functional and phenotypic heterogeneity between tumor-infiltrating and non-infiltrating myeloid-derived suppressor cells. Oncoimmunology 3:e956579
Marcen R (2009) Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs 69:2227–2243
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13
Meszaros G, Szalay B, Toldi G et al (2009) FoxP3+ regulatory T cells in childhood allergic rhinitis and asthma. J Investig Allergol Clin Immunol 19:238–240
Mocellin S, Nitti D (2013) CTLA-4 blockade and the renaissance of cancer immunotherapy. Biochim Biophys Acta 1836:187–196
Moed H, Gerth Van Wijk R, Hendriks RW et al (2013) Evaluation of clinical and immunological responses: a 2-year follow-up study in children with allergic rhinitis due to house dust mite. Mediators Inflamm 2013:345217
Moingeon P (2013) Update on immune mechanisms associated with sublingual immunotherapy: practical implications for the clinician. J Allergy Clin Immunol Pract 1:228–241
Mougiakakos D, Johansson CC, Trocme E et al (2010) Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer 116:2224–2233
Muranski P, Restifo NP (2013) Essentials of Th17 cell commitment and plasticity. Blood 121:2402–2414
Nadig SN, Wieckiewicz J, Wu DC et al (2010) In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med 16:809–813
Newell KA, Asare A, Kirk AD et al (2010) Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120:1836–1847
Nguyen KD, Fohner A, Booker JD et al (2008) XCL1 enhances regulatory activities of CD4+CD25highCD127low/− T cells in human allergic asthma. J Immunol 181:5386–5395
Nguyen KD, Vanichsarn C, Fohner A et al (2009) Selective deregulation in chemokine signaling pathways of CD4+CD25hiCD127lo/(−) regulatory T cells in human allergic asthma. J Allergy Clin Immunol 123(933–939):e10
Nishikawa H, Sakaguchi S (2010) Regulatory T cells in tumor immunity. Int J Cancer 127:759–767
Noh J, Noh G, Kim HS et al (2012) Allergen-specific responses of CD19(+)CD5(+)Foxp3(+) regulatory B cells (Bregs) and CD4(+)Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cell Immunol 274:109–114
Nouel A, Simon Q, Jamin C et al (2014) Regulatory B cells: an exciting target for future therapeutics in transplantation. Front Immunol 5:11
Novak N, Bieber T, Allam JP (2011) Immunological mechanisms of sublingual allergen-specific immunotherapy. Allergy 66:733–739
Nywening TM, Wang-Gillam A, Sanford DE et al (2016) Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 17:651–662
O’Callaghan G, Houston A (2015) Prostaglandin E2 and the EP receptors in malignancy: possible therapeutic targets? Br J Pharmacol 172:5239–5250
Orentas RJ, Kohler ME, Johnson BD (2006) Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol 16:137–149
Osinska I, Wolosz D, Domagala-Kulawik J (2014) Association between M1 and M2 macrophages in bronchoalveolar lavage fluid and tobacco smoking in patients with sarcoidosis. Pol Arch Med Wewn 124:359–364
Pallier A, Hillion S, Danger R et al (2010) Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 78:503–513
Palomares O, Yaman G, Azkur AK et al (2010) Role of Treg in immune regulation of allergic diseases. Eur J Immunol 40:1232–1240
Peters JH, Preijers FW, Woestenenk R et al (2008) Clinical grade Treg: GMP isolation, improvement of purity by CD127 depletion, Treg expansion, and Treg cryopreservation. PLoS One 3:e3161
Petersen RP, Campa MJ, Sperlazza J et al (2006) Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 107:2866–2872
Pierini A, Alvarez M, Negrin RS (2016) NK cell and CD4+FoxP3+ regulatory T cell based therapies for hematopoietic stem cell engraftment. Stem Cells Int 2016:9025835
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982
Punt S, Langenhoff JM, Putter H et al (2015) The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review. Oncoimmunology 4:e984547
Quatromoni JG, Suzuki E, Okusanya O et al (2013) The timing of TGF-beta inhibition affects the generation of antigen-specific CD8+ T cells. BMC Immunol 14:30
Radulovic S, Jacobson MR, Durham SR et al (2008) Grass pollen immunotherapy induces Foxp3-expressing CD4+CD25+ cells in the nasal mucosa. J Allergy Clin Immunol 121:1467–1472
Ring J, Gutermuth J (2011) 100 years of hyposensitization: history of allergen-specific immunotherapy (ASIT). Allergy 66:713–724
Robb RJ, Lineburg KE, Kuns RD et al (2012) Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood 119:5898–5908
Romagnani S (2008) Human Th17 cells. Arthritis Res Ther 10:206
Romano E, Kusio-Kobialka M, Foukas PG et al (2015) Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA 112:6140–6145
Safa K, Chandran S, Wojciechowski D (2015) Pharmacologic targeting of regulatory T cells for solid organ transplantation: current and future prospects. Drugs 75:1843–1852
Safinia N, Scotta C, Vaikunthanathan T et al (2015) Regulatory T cells: serious contenders in the promise for immunological tolerance in transplantation. Front Immunol 6:438
Sagoo P, Ali N, Garg G et al (2011) Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med 3:83ra42
Scadding GW, Shamji MH, Jacobson MR et al (2010) Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy 40:598–606
Scalea JR, Tomita Y, Lindholm CR et al (2016) Transplantation tolerance induction: cell therapies and their mechanisms. Front Immunol 7:87
Schubert R, Eickmeier O, Garn H et al (2009) Safety and immunogenicity of a cluster specific immunotherapy in children with bronchial asthma and mite allergy. Int Arch Allergy Immunol 148:251–260
Segundo DS, Lopez-Hoyos M, Arias M (2013) Regulatory B-cells in transplantation. Antibodies 2:587–597
Shi HZ, Li S, Xie ZF et al (2004) Regulatory CD4+CD25+ T lymphocytes in peripheral blood from patients with atopic asthma. Clin Immunol 113:172–178
Singer BD, King LS, D’Alessio FR (2014) Regulatory T cells as immunotherapy. Front Immunol 5:46
Solito S, Marigo I, Pinton L et al (2014) Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 1319:47–65
Sørensen AE, Johnsen CR, Dalgaard LT (2013) Human leukocyte antigen-G and regulatory T cells during specific immunotherapy for pollen allergy. Int Arch Allergy Immunol 162:237–252
Srivastava MK, Andersson A, Zhu L et al (2012) Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 4:291–304
Stanic B, van de Veen W, Wirz OF et al (2015) IL-10-overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol 135:771–780
Stelmaszczyk-Emmel A, Zawadzka-Krajewska A, Szypowska A et al (2013) Frequency and activation of CD4+ CD25 FoxP3+ regulatory T cells in peripheral blood from children with atopic allergy. Int Arch Allergy Immunol 162:16–24
Stelmaszczyk-Emmel A, Zawadzka-Krajewska A, Głodkowska-Mrówka E et al (2015) FoxP3 Tregs response to sublingual allergen specific immunotherapy in children depends on the manifestation of allergy. J Immunol Res 2015:731381
Suárez-Fueyo A, Ramos T, Galán A et al (2014) Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol 133(130–138):e1–e2
Swamy RS, Reshamwala N, Hunter T et al (2012) Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol 130:215–224
Syed A, Garcia MA, Lyu SC et al (2014) Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 133:500–510
Tai X, Van LF, Pobezinsky L et al (2012) Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 119:5155–5163
Tamura K, Shimizu C, Hojo T et al (2011) FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol 22:1302–1307
Tartour E, Zitvogel L (2013) Lung cancer: potential targets for immunotherapy. Lancet Respir Med 1:551–563
Tebbe B, Wilde B, Ye Z et al (2016) Renal transplant recipients treated with calcineurin-inhibitors lack circulating immature transitional CD19+CD24hiCD38hi regulatory B-lymphocytes. PLoS One 11:e0153170
Thunberg S, Gafvelin G, Nord M et al (2010) Allergen provocation increases TH2-cytokines and FOXP3 expression in the asthmatic lung. Allergy 65:311–318
Toh B, Wang X, Keeble J et al (2011) Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 9:e1001162
Trzonkowski P, Bieniaszewska M, Juscińska J et al (2009) First-In Man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol 133:22–26
Tzankov A, Meier C, Hirschmann P et al (2008) Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 93:193–200
van de Veen W, Stanic B, Yaman G et al (2013) IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 131:1204–1212
Van der Net JB, Bushell A, Wood KJ, Harden PN (2016) Regulatory T cells: first steps of clinical application in solid organ transplantation. Transpl Int 29:3–11
van der Vlugt LE, Mlejnek E, Ozir-Fazalalikhan A et al (2014) CD24(hi)CD27(+) B cells from patients with allergic asthma have impaired regulatory activity in response to lipopolysaccharide. Clin Exp Allergy 44:517–528
Walker LS, Sansom DM (2015) Confusing signals: recent progress in CTLA-4 biology. Trends Immunol 36:63–70
Wang WW, Yuan XL, Chen H et al (2015) CD19+CD24hiCD38hi Bregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget 6:33486–33499
Weiskopf K, Weissman IL (2015) Macrophages are critical effectors of antibody therapies for cancer. MAbs 7:303–310
Weng WK, Levy R (2003) Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 21:3940–3947
Wolf D, Sopper S, Pircher A et al (2015) Treg(s) in cancer: friends or foe? J Cell Physiol 230:2598–2605
Woo EY, Yeh H, Chu CS et al (2002) Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 168:4272–4276
Wood KJ, Bushell A, Hester J (2012) Regulatory immune cells in transplantation. Nat Rev Immunol 12:417–430
Xu G, Mou Z, Jiang H et al (2007) A possible role of CD4+CD25+ T cells as well as transcription factor Foxp3 in the dysregulation of allergic rhinitis. Laryngoscope 117:876–880
Yan Y, van der Putten K, Bowen DG et al (2002) Postoperative administration of donor B cells induces rat kidney allograft acceptance: lack of association with Th2 cytokine expression in long-term accepted grafts. Transplantation 73:1123–1130
Zdanov S, Mandapathil M, Abu ER et al (2016) Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol Res 4:354–365
Zeng H, Zhang R, Jin B et al (2015) Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 12:566–571
Zhang Q, Qian FH, Liu H et al (2008) Expression of surface markers on peripheral CD4+CD25high T cells in patients with atopic asthma: role of inhaled corticosteroid. Chin Med J 121:205–212
Zhou J, Min Z, Zhang D et al (2014) Enhanced frequency and potential mechanism of B regulatory cells in patients with lung cancer. J Transl Med 12:304
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Korczak-Kowalska, G., Stelmaszczyk-Emmel, A., Bocian, K. et al. Expanding Diversity and Common Goal of Regulatory T and B Cells. II: In Allergy, Malignancy, and Transplantation. Arch. Immunol. Ther. Exp. 65, 523–535 (2017). https://doi.org/10.1007/s00005-017-0471-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-017-0471-9