Abstract
The investigation of the magnitude of residues after application of a pesticide is important to ensure consumer safety and is also a regulatory requirement to grant authorization. To address those issues, the behavior of trifloxystrobin residues was investigated in outdoor strawberry and cucumber cultivations, following the recommended and more critical agricultural practices under Egyptian dry climatic conditions. Fruits were collected at several pre-harvest intervals and analyzed with the Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) extraction protocol followed by liquid chromatography-tandem mass spectrometry. The limit of quantitation of the method was 0.001 mg kg−1. When trifloxystrobin was applied on the field, the half-lives were 2.4 days in cucumbers and 6.2 days in strawberries. Risk assessment showed that chronic and acute dietary exposure to residues following the investigated agricultural patterns are of no concern to consumers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
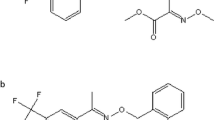
Trifloxystrobin (Fig. 1) belongs to the strobilurin class of broad spectrum fungicides widely used to control mildew by inhibiting mitochondrial respiration of fungi (Bartlett et al. 2002). The diseases harm fruiting vegetables and berries such as cucumber and strawberries, which are crops of worldwide interest cultivated under various climatic conditions. In Egypt, strawberry and cucumber cultivation is of high economic importance. Along with citrus, garlic and onion (FAO 2015), strawberry and cucumber are important export goods. Thus, in compliance with international standards concerning pesticide residues, maximum residue levels (MRLs) are crucial for international trading.
Residues are a consequence of pesticide use by farmers in order to produce the yields for the high demand of agricultural products, and to simultaneously keep the quality of these products at a high commercial level. Although pesticides are applied according to the specific application patterns or good agricultural practices (EC 2009) authorized in each country, improper use e.g., of higher doses, failure to respect the pre-harvest intervals (PHI) or off-label use on a not label-conform crop (Aktar et al. 2009) could lead to MRL violations.
For the protection of strawberry and cucurbits with edible peel cultivations, several highly effective fungicides are available, with trifloxystrobin being one of the most effective and widely used active substances for protection of fruits and vegetables. To the best of our knowledge, similar studies in other countries with dryland climatic conditions are not available. In literature, few references are available investigating the residue dissipation and risk assessment of trifloxystrobin for strawberries, with work limited to the climatic conditions of China (Song et al. 2020). Since climate conditions in outdoor cultivations affect the residue behavior of a pesticide (EC 2013a, b, 2019; OECD 2009), from both a scientific and regulatory point of view, these data cannot be considered representative for countries with dry climate conditions like in the mediterranean part of Africa or Arabic countries. Studies under EU conditions were not available in open literature, however information on the residue behavior in these crops are available from regulatory studies used to set EU MRLs (EFSA 2017) or Codex Maximum Residue Limits (CXLs) (JMPR 2012).
The objectives of this study are (a) to investgate the residue behaviour of trifloxystrobin via estimating the degradation kinetics, and terminal residues at the recommended (authorized) and 4 more agricultural patterns in Egypt, and (b) to estimate the health risks for the consumers arising from the residues in fruits. The results of the this investigation could contribute to a safer use of trifloxystrobin in cucumbers or other cucurbits with edible peel and strawberries from Egypt and other countries of similar climatic conditions.
2 Materials and methods
2.1 Chemicals and reagents
The commercial formulation used in the field trials was a plant protection product (PPP) containing trifloxystrobin at 25% water dispersible granules (NATIVO) purchased from a local market (Giza, Egypt). Deionized water was obtained from a Milli-Q water purification system (Millipore, USA). Organic membrane filters (0.22 μm) were purchased from Agilent Technologies (Santa Clara, CA, USA). Materials for the QuEChERS method, i.e. anhydrous magnesium sulfate, sodium chloride, disodium hydrogen citrate sesquihydrate, and trisodium citrate dihydrate, were purchased from Merck (Darmstadt, Germany). Neat analytical standard (99% purity) of trifloxystrobin was purchased from Dr. Ehrenstorfer (Augsburg, Germany). A stock solution of trifloxystrobin at 1000 mg L−1 was prepared in acetonitrile and with further dilutions, calibration standards of 0.001, 0.005, 0.01, 0.025, 0.05 and 0.1 mg L−1 in acetonitrile and matrix-matched with strawberry and cucumber were freshly prepared at the day of the validation experiments or sample analysis.
2.2 Field experimental design
The study was conducted at Matshtul district, El Sharkiya Governorate, in the Mediterranean part of Egypt during the growing season 2018/2019.
The experimental field was divided into 6 plots of 50 m2 each, i.e. a blank control plot and 5 test plots. A buffer zone of 15 m2 separated adjacent plots. For the strawberry experiment, seedlings (Fragaria × ananassa Selva variety), were transplanted in September 2018 and for the cucumber experiment, cucumber seedlings (Cucumis sativus L.) were transplanted in March 2019. The air temperature of the experimental area ranged between 11–29 °C during the strawberry and 25–35 °C during the cucumber cultivation period.
Representative samples of the whole plot were collected according to guidelines (Codex 1993) at several PHI. For the estimation of the dissipation rate, trifloxystrobin was applied according to the recommended dosage (1 × 150 g a.i ha−1) and samples were taken 0 (2 h), 1, 3, 7, 10, and 14 days after the last treatment. For the estimation of terminal residues, trifloxystrobin was applied 2 or 3 × at 150 or 300 g a.i ha−1, and samples were taken at 3 and 7 days after the last treatment. In all cases, the applications were performed after the consumable part of the fruit is formed (BBCH 81-89). As mentioned, improper or unauthorized use might be observed in practice, thus the investigation of more critical application patterns was conducted. In addition, this information might be useful for authorization holders or regulatory bodies in case, additional applications are needed for the control of the disease.
Each sample weighing at least 2 kg (12 fruits from 12 separate plants) was immediately transported to the laboratory, where it was homogenized (Food Chopper, Hobart, Troy, OH, USA) and stored at - 18 °C until analysis. For strawberries, stems were removed (EC 2018). The period between sampling and analysis was up to one week, thus degradation of trifloxystrobin during storage is assumed to be negligible (OECD 2007).
2.3 Sample preparation and liquid chromatographic-tandem mass spectrometric analysis
For the determination of trifloxystrobin residues the, QuEChERS (BSI 2008; Anastassiades et al. 2003; Anagnostopoulos et al. 2012) was performed in combination with high-performance liquid chromatography-tandem mass spectrometry (LC–MS/MS). The methodology was previously used and validated in line with European guidelines (EC 2017) by the authors for the determination of thiophanate methyl, picoxystrobin and chromafenozide in strawberries (Malhat et al. 2014, 2020, 2021; Saber et al. 2020).
Briefly, a 50 mL centrifuge tube was used containing 10 g of the homogenized sample and 10 mL acetonitrile. After vortexing for 2 min, 4.0 g anhydrous MgSO4, 1.0 g NaCl, 1.0 g sodium citrate tribasic dehydrate, and 0.5 g citric acid disodium salt sesquihydrate were added. After manual shaking for 2 min, the tube was centrifuged at 4800 rpm for 5 min. An aliquot of 200 µL supernatant was collected and 800 µL acetonitrile was added. The final extract was filtered with a PTFE (0.22 μm) syringe filter before LC–MS/MS analysis.
LC–MS/MS analysis was conducted using an Exion HPLC system coupled to a 6500+ QTRAP (AB Sciex, Foster City, CA). A Synergi 2.5 µm Fusion-RP 100 Å, 50 × 3.0 mm (Phenomenex, Torrance, CA, USA) column operating at 40 ºC and with a consistent flow rate of 0.4 mL min−1 was used. The mobile phase consisted of 10 mmol L−1 HCOONH4 (pH 4) in 90:10 water/methanol (v/v) (phase A) and methanol (phase B). The elution program was: 0 min, 80% A; 1–8 min from 80 to 5% A; 8–10 min 80% A, resulting in a total run time of 10 min with the analyte eluting at 7.2 min. Ionization was performed using electrospray (ESI) in positive mode. MS/MS operated at multiple reaction monitoring (MRM) mode with two transitions 409 > 186 and 409 > 206 for quantification and qualification of trifloxystrobin, respectively. Source and MS/MS setting are presented in Table 1. A typical LC–MS/MS chromatogram of a blank strawberry or cucumber sample, a matrix matched standard and a fortified sample at the limit of quantitation (LOQ), are presented Figs. 2 and 3. The Analyst software (Version 1.7.1, Applied Biosystems) was used for data acquisition and processing.
Regarding the identification of the analytes, the ion ratio from sample extracts was within ± 30% (relative) to the average of matrix matched calibration standards from the same sequence, and the retention time of the analyte in the extract, corresponded to that of the matrix matched calibration standards with a tolerance of ± 0.1 min (EC 2017).
2.4 Models and statistical analysis
Dissipation rate was evaluated by subjecting the data to a first-order kinetic model, by plotting the concentration of the pesticide against the PHI (Malhat et al. 2014, 2020, 2021). The half-life (t1/2) is calculated according to the following equation (Eq. 1):
The dietary exposure to the residues was calculated using 2 different approaches. In the first approach, the risk quotient approach (Wang et al. 2015; Malhat et al. 2020, 2021) was used. In the second approach, the deterministic European Food Safety Authority (EFSA) PRIMo revision 3.1 model (EFSA 2019) was employed.
3 Results and discussion
3.1 Method validation
The method showed an excellent linear response (r2 > 0.997) for trifloxystrobin over the investigated concentration range (0.001–0.1 mg L−1) in both solvent and matrix extracts. Satisfactory results were also obtained for accuracy and precision, with recovery values ranging from 86 to 117%, and inter-day relative standard deviation (RSDr) values up to 11%, the highest being at the LOQ. Reproducibility (daily for 4 days) was also estimated with RSDR values up to 7% for cucumbers and up to 5% for strawberries showing excellent day to day precision. A detailed overview of the validation data shows Table 2. Matrix effect was also calculated as follows (Eq. 2):
where %ME is the % estimation of the matrix effect, Mmatrix and Msolvent are the slopes of calibration curves in the matrix extract (strawberry or cucumber) and in acetonitrile, respectively.
The calculated matrix effect from the strawberry extract was 12% and from the cucumber extract 8% which is below 20%, and thus can be considered as not significant (Ferrer et al. 2011).
Based on the generated validation data, the LOQ was estimated to be 0.001 mg kg−1 which is 10 × lower that the default LOQ of 0.01 mg kg−1 that is mostly used in pesticide residue analysis covering the already established MRLs from the EU (2019) at international level which are 0.3 mg kg−1 for cucumbers and 1 mg kg−1 for strawberries.
3.2 Decline of trifloxystrobin residues in strawberry and cucumber
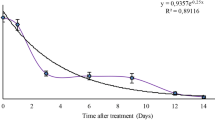
The dissipation rate for trifloxystrobin showed first-order kinetics with good correlation coefficients for both strawberries (R2 > 0.908) and cucumbers (R2 > 0.967) (Table 3). This is consistent with several reports in the literature on trifloxystrobin in various commodities (Sharma et al. 2019). At 0.6 mg kg−1, a 14% degradation was observed in strawberries within one day, in contrast to cucumber where residues declined almost by 30% at 0.5 mg kg−1. The residue concentration at each level along with a graphical presentation of the dissipation pattern shows Fig. 4a/b. For strawberries, the agricultural pattern of the EU is one application of 150 g a.i ha−1 with a one day PHI. The residues were between 0.04–0.15 mg kg−1 (n = 9) in North Europe, 0.06–0.23 mg kg−1 (n = 9) in South Europe and 0.08–0.41 mg kg−1 (n = 8) in indoor conditions. Those residue levels are lower compared to the results of this study, with residue levels of 0.6 mg kg−1 after 1 day.
The compliance with the MRL were achieved after 3 days (0.12 mg kg−1) in cucumber (MRL 0.3 mg kg−1) in contrast to strawberries in MRL of 1.0 mg kg−1 after day 0.
The calculated half-life (t1/2) of trifloxystrobin in strawberry and cucumber varied significantly and was 6.2 and 2.4 days, respectively. Half-lives differentiate in other crops, varying up to 19.38–24.93 days in apples (Patyal et al. 2013), 2.8–3.3 days in green tea (Mariappan et al. 2017), 9.0 days in mango, 6.0–6.2 days in onions (Mohapatra 2014; Badal et al. 2016), and 19.4–28.9 days in gherkin, a commodity similar with cucumbers in terms of cultivation and plant protection needs (Mariappan et al. 2015).
3.3 Terminal trifloxystrobin residues in strawberry and cucumbers
Terminal residues were investigated for all 4 agricultural patterns at PHIs of 3 and 7 days. A tabular presentation of the results along with a comparison of the residue levels among the agricultural patterns with an increased dose rate and/or number of applications are summarised in Table 4.
In cucumbers at a PHI of 3 days, the residue levels were 0.4 ± 0.027 mg kg−1 after twice a dose of 150 g a.i. ha−1, and 0.47 ± 0.01 mg kg−1 at double dose rate, showing similar residues in both cases. Likewise, with a 3 application pattern instead of 2, the residue degradation between the two sampling points was 43–48% except for the 2 × 150 g a.i. ha−1 pattern, with a more extensive degradation of 91%.
In strawberries, at a PHI of 3 days, the residue levels were 0.37 ± 0.038 mg kg−1 after twice a dose of 150 g a.i. ha−1 and 0.63 ± 0.058 mg kg−1 after double dose rate, showing a 63% increase in residues due to the higher dose. However, with 28% this increase is lower at higher dose (300 g a.i. ha−1). Between the two sampling points, the degradation of the compound differs as the number of applications increases and with that the total seasonal burden. When trifloxystrobin is applied twice (seasonal burden: 300 or 600 g a.i. ha−1), the decrease is 22–38%, and 61–67% after 3 applications (seasonal burden: 450 or 900 g a.i. ha−1).
3.4 Dietary risk assessment
For trifloxystrobin, an acceptable daily intake (ADI) and acute reference dose (ARfD) of 0.1 and 0.5 mg kg−1 bw day−1, respectively (EFSA 2017; EC 2020) were established. The calculated exposure was compared with the ADI to estimate the long-term exposure and with the ARfD for the acute exposure. According to the residue definition for risk assessment set by EFSA (2017) and JMPR (2012), the contribution of the metabolite CGA 321113 should be considered. To calculate the additional contribution of the metabolite, a conversion factor of 1.4 was used. Note that for the EU, the EFSA proposed to include 3 isomers of trifloxystrobin (CGA357262, CGA 357261, CGA 331409). However, since the toxicity assesment of the isomers is still pending, they were not considered for the current work.
Following the risk quotient (RQ) method, long term exposure was estimated for all PHI values (Table 5). All national estimated daily intake (mg kg−1, bw, NEDI) values were far below the ADI with RQ < 1% (0.004–0.042% for cucumbers and 0.023–0.813% for strawberries). By applying the EFSA PRIMo model revision 3.1, the estimated chronic exposure was up to 1.4% of the ADI for cucumbers and 0.6% for strawberries. Short term exposure was up to 12.6% of the ARfD for cucumbers and 4.2% for strawberries. Based on the above calculations, even at more critical agricultural patterns in terms of number or dose rate per application, a chronic and acute risk for consumers was not identified.
Regarding a potential regulatory use of these results, it should be noted that the the results above are indicative, since the calculations are based on exposure data from a single region, thus further independent trials are necessary for a more robust exposure estimation.
4 Conclusion
The present work, investigated the residue behavior of trifloxystrobin in strawberries and cucumber. A residue decline following a first-order decay was observed with a half-life (t1/2) of 2.38 days for cucumbers and 6.18 for strawberries. The highest terminal residues in fruits were up to 0.71 mg kg−1 for cucumbers and 0.77 mg kg−1 for strawberries, with a dietary exposure < 2% of the ADI and 12.6% of the ARfD using FAO/WHO and EFSA models, indicating no health risks for consumers. As these residue levels derive from more critical agricultural patterns, it shows that even at misuse, a dietary health risk is unlikely. When the present work was carried out, EU and Codex MRLs were set at 0.3 mg kg−1 for cucumbers and 1 mg kg−1 for strawberries. Therefore, if trifloxystrobin is applied according to the recommended agricultural pattern, a PHI of 3 days for cucumbers and 1 day for strawberries is sufficient to respect the MRL and consequently, to avoid trade problems related to non-compliances. For cucumbers and in contrast to the dietary exposure calculations, an application with more critical agricultural patterns might lead to an MRL exceedance.
Availability of data and material
The datasets used and/or analysed during the current study are available from Dr. Farag Malhat (farag_malhat@yahoo.com) on reasonable request.
Code availability
Not applicable.
References
Aktar W, Dwaipayan S, Ashim C (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2(1):1–12. https://doi.org/10.2478/v10102-009-0001-7
Anagnostopoulos CJ, Liapis K, Haroutounian S, Paspatis E (2012) Simultaneous determination of different classes of plant growth regulator in high water content agricultural products by liquid chromatography tandem mass spectrometry and time of flight mass spectrometry. J Liq Chromatogr Relat Technol 36(3):315–335. https://doi.org/10.1080/10826076.2012.657730
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multi-residue method employing acetonitrile extraction/partitioning and “dispersive solid phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431. https://doi.org/10.1093/jaoac/86.2.412
Badal VP, Suchi C, Hetal G, Payal U, Kaushik DP, Anil RP, Paresh GS (2016) Residue decline and risk assessment of fluopyram+tebuconazole (400SC) in/on onion (Allium cepa). Environ Sci Pollut Res 23(20):20871–20881
Bartlett D, Clough J, Godwin J, Hall A, Hamer M, Parr-Dobrzanski B (2002) The strobilurin fungicides. Pest Manag Sci 58:649–662. https://doi.org/10.1002/ps.520
BSI (British Standards Institution) (2008) Foods of plant origin—determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and clean-up by dispersive SPE –QuEChERS-Method EN 15662:2008
Codex (1993) Guidelines on good laboratory practice in pesticide residue analysis. CAC/GL 40:1–36
EC (European Commission) (2009) Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p 1–50
EC (European Commission) (2013a). Commission Regulation (EU) No 283/2013a of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market Text with EEA relevance. Official Journal of the European Union 93, 3.4.2013:1–84
EC (European Commission) (2013b) Commission Regulation (EU) No 283/2013b of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. L 93, 3.4.2013, OJ L 184, p 61–66: 1–84
EC (European Commission) (2017) Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. SANTE/11813/2017
EC (European Commission) (2018) Regulation (EU) 2018/62 of 17 January 2018 Replacing Annex I to Regulation (EC) No 396/2005 of the European Parliament and of the Council. Official Journal of the European Union L 18/1, 23.1.2018:1–73
EC (European Commission) (2019) Regulation (EU) 2019/1791 of 17 October 2019 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 1-decanol, 2,4-D, ABE-IT 56, cyprodinil, dimethenamid, fatty alcohols, florpyrauxifen-benzyl, fludioxonil, fluopyram, mepiquat, pendimethalin, picolinafen, pyraflufen-ethyl, pyridaben, S-abscisic acid and trifloxystrobin in or on certain products. Official Journal of the European Union 277, 29.10.2.019:1–65
EC (European Commission) (2020) EU Pesticide Database. https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN. Accessed 1 Feb 2020
EFSA (European Food Safety Authority) (2017) Conclusion on the peer review of the pesticide risk assessment of the active substance trifloxystrobin. EFSA J 15(10:4989):29
EFSA (European Food Safety Authority) (2019) Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1. EFSA Supporting Publications 16:3. https://doi.org/10.2903/sp.efsa.2019.EN-1605
FAO (Food and Agriculture Organization of the United Nations) (2015) FAOSTAT: Food and agriculture data
Ferrer C, Lozano A, Aguera A, Giron AJ, Fernandez-Alba AR (2011) Overcoming matrix effects using thedilution approach in multiresidue methods for fruits and vegetables. J Chromatogr A 1218(42):7634–7639
JMPR (Joint Meeting on Pesticide Residues) (2012) Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues in Rome, Italy FAO
Malhat F, Badawy HMA, Barakat DA, Saber AN (2014) Residues, dissipation and safety evaluation of chromafenozide in strawberry under open field conditions. Food Chem 152:18–22. https://doi.org/10.1016/j.foodchem.2013.11.110
Malhat F, El Saber S, Sayed Amin A, Anagnostopoulos C, Abdelsalam-Shokr S (2020) Magnitude of picoxystrobin residues in strawberry under Egyptian conditions: dissipation pattern and consumer risk assessment. Food AdditContam Part A 2020:1–10. https://doi.org/10.1080/19440049.2020.1736342
Malhat F, Abdallah O, Ahmed F, Abdel Salam S, Anagnostopoulos C, Tawfic Ahmed M (2021) Dissipation behavior of thiophanate-methyl in strawberry under open field condition in Egypt and consumer risk assessment. Environ Sci Pollut Res 28:1029–1039. https://doi.org/10.1007/s11356-020-10186-4
Mariappan P, Chellamuthu S, Manthirachalam D, Samiyannan J, Subramanian C (2015) Simultaneous determination of tebuconazole, trifloxystrobin, and its metabolite trifloxystrobin acid residues in gherkin under field conditions. Separat Sci 38:958–964
Mariappan P, Manthirachalam D, Chellamuthu S, Subramanian C (2017) Dissipation kinetics, safety evaluation of tebuconazole and trifloxystrobin in tea under tropical field condition. Food Addit Contam Part A 34(12):2155–2163
Mohapatra S (2014) Persistence and dissipation kinetics of trifloxystrobin and tebuconazole in onion and soil. J Environ Sci Health B 49(7):513–520
OECD (Organisation for Economic Co-operation and Development) (2007) Test No 506: Stability of Pesticide Residues in Stored Commodities. Edited by OECD, Guidelines for the Testing of Chemicals, Paris
OECD (Organisation for Economic Co-operation and Development) (2009) Test No. 509: crop field trial. Edited by OECD. Vol. Section 5, guidelines for the testing of chemicals. OECD Publishing, Paris
Patyal SK, Sharma ID, Chandel RS, Dubey JK (2013) Dissipation kinetics of trifloxystrobin and tebuconazole on apple (Malus domestica) and soil—a multi location study from north western Himalayan region. Chemosphere 92(8):949–954
Saber AN, Malhat F, Anagnostopoulos C, Kasiotis KM (2020) Evaluation of dissipation, unit–unit-variability and terminal residue of etoxazole residues in strawberries from two different parts in Egypt. J Consumer Protect Food Saf. https://doi.org/10.1007/s00003-019-01266-w
Sharma KK, Tripathy V, Rao CS, Bhushan VS, Reddy KN, Jyot G, Sahoo SK (2019) Persistence, dissipation, and risk assessment of a combination formulation of trifloxystrobin and tebuconazole fungicides in/on tomato. Regul Toxicol Pharmacol 108:104471. https://doi.org/10.1016/j.yrtph.2019.104471
Song L, Zhong Z, Han Y, Zheng Q, Qin Y, Wu Q, He X, Pan C (2020) Dissipation of sixteen pesticide residues from various applications of commercial formulations on strawberry and their risk assessment under greenhouse conditions. Ecotoxicol Environ Saf 188:109842. https://doi.org/10.1016/j.ecoenv.2019.109842
Wang Z, Cang T, Qi P, Zhao X, Xu H, Wang X, ZhangWang HX (2015) Dissipation of four fungicides on greenhouse strawberries and an assessment of their risks. Food Control 55:215–220. https://doi.org/10.1016/j.foodcont.2015.02.050
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Part of the current study was funded by king Khalid university under grand no GRP/6/42.
Author information
Authors and Affiliations
Contributions
FM designed and coordinated the study, developed the analytical method, analyzed the samples, interpreted the results, edited, and wrote the manuscript. CA wrote the manuscript, bibliography search, performed statistical analysis, interpreted the results, and performed the risk assessment. E-SS conducted the experimental design, field trial, and analysis of the samples. SAS conducted the experimental design, field trial, analysis of the samples. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to Publish
The authors consent on the publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Malhat, F., Saber, ES., Anagnostopoulos, C. et al. Dissipation rate and exposure risk of trifloxystrobin in dry climatic field environments. J Consum Prot Food Saf 17, 353–361 (2022). https://doi.org/10.1007/s00003-022-01392-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00003-022-01392-y