Abstract
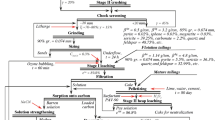
In this study, cyanidation experiments were carried out on a massive sulfide ore sample, whose grades of the precious metals gold and silver were found to be 4.2 g/t and 311.9 g/t, respectively. Other elements in the ore were arsenic with average grade of 1,751.6g/t; antimony, 619.7 g/t; zinc, 781.4 g/t; copper, 454.6 g/t; lead, 101.8 g/t; mercury, 324.3 g/t; and bismuth, 0.6 g/t. The first objective of test workfocused on determining the best conditions for extracting goldfrom the ore, ground to less than 75 microns. Different influential parameters such as particle size fraction, cyanide concentration, pH and leaching time were investigated. The optimum parameters were found to be particle size fraction of less than 25 microns, cyanide concentration of 2,000 mg/L, pH of 11 and cyanidation time of 24 hours. In these optimum conditions, the highest gold and silver leaching efficiencies were 76.88 and 59.63 percent, respectively. In addition, roasting as pretreatment at a temperature of 700°C over a period of two hours was evaluated. Cyanidation testing of the roasted ore in the optimum conditions found increases in the gold and silver leaching efficiencies to 85.97 and 67.57 percent, respectively. Moreover, antimony extraction rose to 43.10 percent, from 23.73 percent without roasting.
Similar content being viewed by others
References
Adams, M.D., 2005, Advances in Gold Ore Processing, Developments in Mineral Processing, Elsevier, Amsterdam, 1,028 pp.
Aghamirian, M.M., and Yen, W.T., 2005, “Mechanism of galvanic interactions between gold and sulfide minerals in cyanide solution,” Minerals Engineering, Vol. 18, pp. 393–407.
Akcil, A., 2002, “First application of cyanidation process in Turkish gold mining and its environmental impacts,” Minerals Engineering, Vol. 15, pp. 695–699.
Akcil, A., and Ciftci, H., 2009, “Pretreatments applied to refractory gold ores,” Journal of the Chamber of Mining Engineers of Turkey (Madencilik), Vol. 48, No. 1, pp. 17–30 (in Turkish).
Angelidis, T.N., and Kydros, K.A., 1995, “Selective gold dissolution from a roasted auriferous pyrite-arsenopyrite concentrate,” Hydrometallurgy, Vol. 37, pp. 75–88.
Azizi, A., Petre, C.F., Olsen, C., and Farachi, F., 2010, “Electrochemical behavior of gold cyanidation in the presence of a sulfide-rich industrial ore versus its major constitutive sulfide minerals,” Hydrometallurgy, Vol. 101, pp. 108–119.
Celep, O., Alp, I., Deveci, H., and Vicil, M., 2009, “Characterization of refractory behavior of complex gold/silver ore by diagnostic leaching,” Transactions of Nonferrous Metals Society of China, Vol. 19, pp. 707–713.
Celep, O., Aslan, N., Alp, I., and Tasdemir, G., 2011, “Optimization of some parameters of stirred mill for ultra-fine grinding of refractory Au/Ag ores,” Powder Technology, Vol. 208, pp. 121–127.
Ciftci, H., and Akcil, A., 2010, “Effect of biooxidation conditions on cyanide consumption and gold recovery from a refractory gold concentrate,” Hydrometallurgy, Vol. 104, pp. 142–149.
Ciftci, H., and Akcil, A., 2013, “Biohydrometallurgy in Turkish gold mining: First shake flaskand bioreactor studies,” Minerals Engineering, Vol. 46–47, pp. 25–33.
Dai, X., and Jeffrey, M.I., 2006, “The effect of sulfide minerals on the leaching of gold in aerated cyanide solutions,” Hydrometallurgy, Vol. 82, pp. 118–125.
Deng, T., and Fiao, M., 2002, “Gold recovery enhancement from a refractory flotation concentrate by sequential bioleaching and thiourea leach,” Hydrometallurgy, Vol. 63, pp. 249–255.
Ellis, S., and Senanayake, G., 2004, “The effects of dissolved oxygen and cyanide dosage on gold extraction from a pyrrhotite-rich ore,” Hydrometallurgy, Vol. 72, pp. 39–50.
Fink, C.G., and Putnam, G.F., 1950, “The action of sulphide ion and of metal salts on the dissolution of gold in cyanide solutions,” Mining Engineering, Vol. 187, pp. 952–955.
Guzman, F., Segarra, M., Chimenos, J.M., Fernandez, M.A., and Espiell, F., 1999, “Gold cyanidation using hydrogen peroxide,” Hydrometallurgy, Vol. 52, pp. 21–35.
Habashi, F., 1967, “Kinetics and mechanism of gold and silver dissolution in cyanide solution,” Montana Bureau of Mines and Geology, Vol. 59, pp. 1–42.
Habashi, F., 1970, Principles of Extractive Metallurgy. Hydrometallurgy, Vol. 2, Gordon and Breach, New York.
Hedley, N., and Tabachnick, H., 1968, “Mineral Dressing Notes No. 23. Chemistry of Cyanidation,” American Cyanamid Company, New Jersey.
Jeffrey, M.I., and Breuer, P.L., 2000, “The cyanide leaching of gold in solutions containing sulfide,” Minerals Engineering, Vol. 13, pp. 1097–1106.
Karimi, P., Abdollahi, H., Amini, A., Noaparast, M., Shafaei, S.Z., and Habashi, F., 2010, “Cyanidation of gold ores containing copper, silver, lead, arsenic and antimony,” International Journal of Mineral Processing, Vol. 95, pp. 68–77.
Komnitsas, C., and Pooley, F.D., 1990, “Bacterial oxidation of a refractory gold sulphide concentrate from Olympias, Greece,” Minerals Engineering, 3(3/4), pp. 295–306.
Komnitsas, C., and Pooley, F.D., 1991, “Optimization of the bacterial oxidation of an arsenical gold sulphide concentrate from Olympias, Greece,” Minerals Engineering, Vol. 4, Issue 12, pp. 1297–1303.
Kondos, P.D., Deschenes, G., and Morrison, R.M., 1995, “Process optimization studies in gold cyanidation,” Hydrometallurgy, Vol. 39, pp. 235–250.
Kuyucak, N., and Akcil, A., 2013, “Cyanide and removal options from effluents in gold mining and metallurgical processes,” Minerals Engineering, Vol. 50–51, pp. 13–29.
Kyle, J.H., Breuer, P.L., Bunney, K.G., and Pleysier, R., 2012, “Review of trace toxic elements (Pb, Cd, Hg, As, Sb, Bi, Se, Te) and their deportment in gold processing Part II: Deportment in gold ore processing by cyanidation,” Hydrometallurgy, Vol. 111–112, pp. 10–21.
La Brooy, S.R., Finge, H.G., and Walker, G.S., 1994, “Review of gold extraction from ores,” Minerals Engineering, Vol. 7, pp. 1213–1241.
Fawrence, R.W., and Bruynesteyn, A., 1983, “Biological pre-oxidation to enhance gold and silver recovery from refractory ores and concentrates,” CIM Bulletin, Vol. 76, pp. 107–110.
Fi, Q., Li, D., and Qian, F., 2009, “Pre-oxidation of high-sulfur and high-arsenic refractory gold concentrate by ozone and ferric ions in acidic media,” Hydrometallurgy, Vol. 97, pp. 61–66.
Forenzen, F., and van Deventer, J.S.J., 1992, “Electrochemical interactions between gold and its associated minerals during cyanidation,” Hydrometal- lurgy, Vol. 30, pp. 177–194.
Marsden, J.O., and House, C., 2006, The Chemistry of Gold Extraction, 2nd Edition, Society for Mining, Metallurgy and Exploration, Englewood, CO.
Mason, P.G., 1990, “Energy requirements for the pressure oxidation of gold- bearing sulphides,” Journal of the Minerals, Metals & Materials Society (TMS), Vol. 42, pp. 15–18.
Murthy, D.S.R., Dey, M.L., Roychowdhury, S.K., Mathur, S.B., and Akerkar, D.D., 1992, “Studies on extraction of refractory gold silverthrough thiourea leaching” International Conference on Extractive Metallurgy of Gold and Base Metals, V.N. Misra, D. Halbe, and D.J. Spottiswood, eds., pp. 329–332.
Nicol, M.J., Fleming, C.A., and Paul, R.L., 1987, “The chemistry of the extraction of gold,” The Extractive Metallurgy of Gold, Vol. 2, G.G. Stanley, ed., South African Institute of Mining and Metallurgy, Johannesburg, pp. 831–905.
Rees, K.L., and van Deventer, J.S.J., 1999, “The role of metal-cyanide species in leaching gold from a copper concentrates” Minerals Engineering, Vol. 12, pp. 877–892.
Rubisov, D.H., Papengelakis, V.G., and Kondos, P.D., 1996, “Fundamental kinetic models for gold ore cyanide leaching,” Canadian Metallurgical Quarterly, Vol. 35, pp. 353–361.
Senanayake, G., 2008, “A review of effects of silver, lead, sulfide and carbonaceous matter on gold cyanidation and mechanistic interpretation,” Hydrometallurgy, Vol. 90, pp. 46–73.
Tan, H., Feng, D., Luckey, G.C., and van Deventer, J.S.J., 2005, “The behavior of carbonaceous matter in cyanide leaching of gold,” Hydrometallurgy, Vol. 78, pp. 226–235.
Weichselbaum, J., Tumilty, J.A., and Schmidt, C.G., 1989, “The effect of sulphide and lead on the rate of gold cyanidation” AusIMM Annual Conference, Perth-Kalgoorlie, pp. 221–224.
Yannopoulos, J.C., 1991, The Extractive Metallurgy of Gold, Van Nostrand Reinhold, New York.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper number MMP-13-094.
Discussion of this peer-reviewed and approved paper is invited and must be submitted to SME Publications Dept. prior to Feb. 29, 2015.
Rights and permissions
About this article
Cite this article
Abdollahi, H., Karimi, P., Amini, A. et al. Direct cyanidation and roasting combination of a semi-refractory massive sulfide ore. Mining, Metallurgy & Exploration 32, 161–169 (2015). https://doi.org/10.1007/BF03402284
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402284