Abstract
Indonesia created a breakthrough in peatland management by establishing the Peatland Restoration Agency in early 2016 with the aim of restoring 2.67 million ha of degraded peatlands. This effort is intended to accelerate the recovery of peatlands and return of its hydrological functions after extensive damage by fire, drainage canals, and other external factors. This paper highlights the potential biomass and carbon resources in various land-use covers located in the Riau Biosphere Reserve. It discusses the results of restoration experiments conducted in severely degraded peatlands, and estimates carbon emission reductions in targeted priority areas. The total estimated emission reduction in natural forests was higher than in logged-over forests and disturbed forests: 207.36 CO2 Mg h−1, 161.48 CO2 Mg h−1, and 65.87 CO2 Mg h−1, respectively. The restoration of 2.3 million ha of targeted peatland ecosystems was estimated to have reduced carbon emissions by 98.77–153.53 Mt CO2e. The value of carbon from peatlands is considered important for maintaining ecological function while optimizing economic benefits. We have confirmed that above ground carbon storage can be restored even in severely degraded peatlands. Avoiding vegetation loss is an important aspect of restoration activity, but recovery of vegetation in degraded areas depends on below-ground carbon stocks, as these are indicative of fertile soils in various kinds of land cover and use.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Communities around the world are increasingly aware of the fact that the steady rise of anthropogenic greenhouse gas (GHG) emissions in the atmosphere is a major cause of contemporary climate change. Mitigating GHG emissions to prevent a global rise in temperature above 1.5–2 °C is a major challenge, especially GHG emissions until 2030 are projected to be 52–58 Gt CO2e. Increasing global temperatures have already begun to damage human life through extreme heat events, increasing sea levels, increases in drought, reduction of crop yields, and the destruction of the coral reef.
Agriculture and forestry, including changes in land use, are responsible for a quarter of the global GHG emissions (Masson-Delmotte et al. 2019). In Indonesia, almost half of total national emissions are related to forestry and peatland issues (MoEF 2018). Peatlands are huge deposits of organic carbon, and the alteration of their natural condition can lead to the release of stored carbon in amounts that may affect the global climate. Addressing global climate change requires transformative actions from multiple stakeholders and targeted programs that can be implemented consistently, transparently, and collaboratively.
Tropical peat swamp forests play an important role in the global carbon cycle, have tangible and intangible ecological value, and provide numerous environmental services (Yu et al. 2010; Page et al. 2011; Novita et al. 2020). A wise and sustainable management system is essential to optimize the economic, social, and ecological value of tropical peatlands (Rieley et al. 2005). Good peatlands management involves harvesting renewable resources sustainably while conserving nonrenewable resources and maintaining the attributes and functions of the peatlands.
Nearly half of the tropical peatlands in Southeast Asia, as much as 14.9 million ha, are found in Indonesia. Peat management requires improved knowledge of the carbon cycle in tropical peatlands and how it responds to land-use and land-cover change. Optimum carbon storage in tropical peatlands requires a combination of high vegetation biomass (carbon sequestration potential), a water table that is near to or above the peat surface for most of the year, and a slow rate of organic matter decomposition. Drainage of peatlands and other disturbances lead to increased aeration, decomposition, and carbon losses in surface peat (Hooijer et al. 2012; Page et al. 2011). Furthermore, peat fires related to land clearing occur frequently, releasing significant amounts of carbon from the forest vegetation and soil. Conversion of peatlands for agriculture and plantations requires radical changes in the vegetation cover and permanent drainage. These changes lower the peat water table, reducing or, in most cases, eliminating the natural capacity of the peatland system to sink carbon. Aeration leads to continuous aerobic decomposition of organic matter and increased peat temperatures, which then increases aerobic heterotrophic respiration and peat surface CO2 emissions (Hooijer et al. 2009; Hooijer et al. 2008).
Even though the importance of peat swamp forest for carbon sequestration and other ecosystem functions is widely recognized they are one of the most rapidly disappearing forest types in the world (Hooijer et al. 2008). In a natural state, peat swamp forests are characterized by dense forest vegetation, thick peat deposits (up to 20 m), and a groundwater table that is above or close to the peat surface throughout the year (Hirano et al. 2009; Page et al. 2004; Takahashi et al. 2002). Peat has low bulk density (approximately 0.1 g cm−3) as it is composed of approximately 10% tree remains and 90% water (Hooijer et al. 2010) and is 50–60% carbon by dry weight (Page et al. 2011; Neuzil 1997). Peat swamp forests also store large amounts of carbon in living plant biomass, with typical values ranging from 100 t C ha−1 to 250 t C ha−1 (Novita et al. 2020; Page et al. 2011).
Peatlands with 2772 t C ha−1 are based on an average peat thickness of 5.5 m (Page et al. 2011). The carbon reserve of these peatlands is very high, ranging from 30 kg C m−3 to 70 kg C m−3 (Agus et al. 2009), equivalent to 300–700 Mg C m−1 of soil depth. Initial estimates show that on average, peat in Sumatra is thicker and has a carbon content of approximately 3000 Mg ha−1 (Wahyunto et al. 2010). Data on biomass and carbon storage in the remaining peat swamp forests are scarce, however (van der Meer and Verwer 2011).
One of the main problems related to sustainable management of peat swamp forests is their current state of severe degradation. In the Riau Biosphere Reserve, land conversion and poor management has caused the loss of around 300,000 ha of natural peat swamp forest in the last 17 years. The uniqueness of this biosphere reserve is that it is a vast landscape consisting of a hydrological network of small lakes and streams and peat swamp forests. The dominant natural ecosystems are peat swamp forests, which are surrounded by land that is being used in various ways, e.g., production forests, degraded/abandoned lands, industrial plantations (timber and oil palm), and agricultural lands and settlements. The remaining natural peat swamp forest, located in the core area, consists of 84,967 ha in the Giam Siak Kecil Wildlife Reserve and 21,500 ha in the Bukit Batu Wildlife Reserve. The function of the core area is to conserve biodiversity, while the buffer zone functions to protect this area. The outer and largest area of the biosphere reserve functions as a transition area.
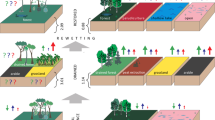
Levels of land degradation can be classified as severe, moderate, and heavy, with “severely degraded” characterized as the absence of trees or dead trees and open areas colonized by fern or grass. A “heavily degraded” forest has lost much of its original biodiversity and most of its structure (bintangur stands). These areas are colonized by grass, fern, and Melastoma sp. after an earlier succession. A “moderately degraded” forest regenerates naturally, and some residual trees still remain (e.g., Palaquium sumatranum). Natural regeneration of tree species in the peat swamp forests in the Riau Biosphere Reserve is vigorous, but some of the typical upper-story species (i.e., Shorea spp., Gonystylus bancanus, Tetramerista glabra, Durio carinatus, Dyera lowii, and Calophyllum lowii) show limited or even no regeneration. Rehabilitation is therefore an urgent ecological matter, particularly for maintaining and preserving species populations (Gunawan et al. 2012). Additionally, in the case of biosphere reserves, conservation and sustainable restoration efforts should be promoted in order to restore large areas of degraded peatlands and forests due to high fire intensity, poor canal drainage, and illegal logging activities; to reduce forest encroachment and conversion; to protect the livelihoods of local people; and to restore carbon sequestration and protect the remaining peat carbon stocks.
Restoration activities have attracted extensive attention in Southeast Asia in the last decade due to their potential to restore the multiple functions of the peat swamp ecosystem. There is limited information regarding the extent of these activities, however. Peatland restoration is still in its early stages and has been limited to experimental rehabilitation projects that aim to restore the main functions of peat ecosystems, especially the hydrological and carbon cycles (Page et al. 2008; Giesen 2004). The Government of Indonesia established the Peatland Restoration Agency (Badan Restorasi Gambut, BRG) in 2016 to reduce conversion of peatlands to other uses and accelerate the recovery and return of hydrological functions of peat that have been mainly damaged by fire and drying.
Protecting and managing peatland ecosystems can be done by avoiding the conversion of peatland to other land uses and enhancing peat soils capacity to storck carbon. This paper highlights potential biomass and carbon resources in various land-use covers located in the Riau Biosphere Reserve and current results of restoration experiments in severely degraded peat swamp ecosystems.
2 Methods
2.1 Data Collection
2.1.1 Above-Ground Carbon Storage
Our study observed above-ground biomass in 0.5 ha from a 25 × 25 m2 plot for natural forest (plot 1, plot 2, plot 3), logged-over forest (plot 4), and wind-disturbed forest (plot 5, plot 6). For each plot, the authors determined the structure and species composition of trees that had a diameter at breast height (dbh; 1.3 m) of over 3 cm. Allometric equations were applied to estimate biomass from dbh as a dependent variable.
Natural peat swamp forests, which are mostly found in biosphere reserves where there have been no tree-felling activities and no drainage canals, can be classified into two main forest types: mixed-peat swamp forest and bintangur (Callophylum lowii) forest. These forest types are distinguished by dominant tree species, vegetation communities, floristic composition, and basal areas. We found high species richness, tree diversity, and unique environmental characteristics in both types of peat swamp forest. Mixed-peat swamp forest located in the upstream river basin of the conservation area was identified by different environmental characteristics, such as the water situation, floristic composition, basal areas, and density. The bintangur forest was located far away from the river basin; Callophylum lowii (local name: bintangur) is the dominant species. Bintangur trees have unique vegetation formations and exist in very deep peat (>10 m).
In logged-over forest these dominant tree species of the peat swamp forests have been felled. Natural regeneration does not occur for almost 10 years after logging activities end. In 2005, a large peat swamp forest area was burned and then colonized by ferns and Melastoma spp. The landscape became more open, allowing the wind to attack the forest stands. Wind-disturbed forest areas are surrounded by an acacia timber plantation and have been designated a forest conservation area by the forest company. At the same time, however, the open landscape has become more accessible to people, and the population of the nearby village has increased dramatically since 2008.
Improved rehabilitation management is needed to reestablish the peat swamp forest ecosystem dominant tree species and vegetation communities. The main causes of degradation in logged-over forest were logging and forest conversion. Since the early 2000s, illegal logging activities have occurred in those areas, and approximately 3 km on both sides of the Bukit Batu river basin were degraded forest areas. The level of degradation varies between severely, heavily, and moderately degraded. The severely degraded areas were characterized as being mostly covered by grass and fern. Heavily degraded areas were covered by Macaranga sp. or woody pioneer species, and moderately degraded areas had regenerating woody species that formed the main vegetation of peat swamp forests.
Our study found two main differences in the logged-over forest and wind-disturbed forest. First, in relation to the regeneration performance of the upper-story tree species, we found that Palaquium sumatranum and Callophlum lowii have vigorous recovery capacity. Our study therefore indicates that recovery is possible in both logged and wind-disturbed peat swamp forests in the Riau Biosphere Reserve, where Palaquium sumatranum and Callophylum lowii are the dominant species. We found secondary forest recovery even when most of the upper story was absent due to limited or even no regeneration.
2.1.2 Below-Ground Carbon Storage
At each site, natural forest (NF), logged-over forest (LOF), wind-disturbed forest, oil palm plantations, and rubber garden soil were sampled through the use of a random sampling design. A peat auger was used to determine peat depth. Peat samples were taken from each corner and center of a square sampling area (25 m2) from the surface to a depth of 40–60 cm to determine the percentage of carbon content and bulk density. Following the bulking of samples from each area, the samples were transported to the Soil Laboratory of Bogor Agriculture University, where they underwent analysis upon arrival.
2.2 Analysis
2.2.1 Biomass and Carbon Storage
Total above-ground biomass in each plot was estimated using an allometric equation (Hiratsuka et al. 2006; Brown 1997):
where Y is total above-ground biomass in kg tree−1 and D is diameter at breast height (dbh in cm).
For comparison, we used the allometric equation from the following studies below:
Equations (8.4, 8.5, and 8.6) were used to calculate the biomass of trees planted in experimental plots in forested or burned areas.
Above-ground carbon storage was calculated by assuming that carbon storage was 0.5 of the total above-ground biomass (Brown and Lugo 1982). The team used other secondary data sources to estimate the amount of above-ground carbon storage in acacia, rubber, and oil palm plantations. These data were used to determine the total amount of above- and below-ground carbon storage for all different land uses in the Riau Biosphere Reserve.
2.2.2 Below-Ground Carbon Storage
The estimation of below-ground carbon storage (Mg C ha−1) involved determining the bulk density of the soil’s organic matter content. Bulk density was determined by the tube core or clod method with the following formula (Murdiyarso et al. 2005):
The below-carbon storage was estimated using the following equation:
where
C: total carbon (Mg C)
A: area (ha)
D: average depth (meter)
BD: bulk density (g cm−3)
CC: carbon content in peat sample (%)
3 Results and Discussion
Above-ground biomass was calculated for the different forest types to indicate the relative proportions of carbon storage and carbon sequestration (see Fig. 8.1). Mean carbon storage per hectare varied from 10.6 Mg C ha−1 to 60.8 Mg C ha−1. Comparisons using other equations showed that carbon storage was high in all of the sampling plots (Figs. 8.1 and 8.2). The highest rates of carbon storage was found in the natural forest (plots 1, 2, 3), followed by logged-over forest (plot 4) and wind-disturbed forest (plots 5 and 6). The highest amount of stored carbon ranged from 89.6 Mg C ha−1 to 98.2 Mg C ha−1 in natural forest (plots 1, 2, 3). The estimated above-ground carbon stock in the natural forest plots was slightly lower than in the forest sampled in the same area by Istomo (2002), who reported mean above-ground C stocks of 131 Mg C ha−1 in Riau Province. We found logged-over forest stocks 71.5 Mg C ha−1, while wind-disturbed forest carbon stock (plots 5 and 6) ranged from 15.7 Mg C ha−1 to 37.8 Mg C ha−1.
The above-ground biomass ranged from 108.9 Mg ha−1 to 121.6 Mg ha−1 for natural forest and dropped to 88.1 Mg ha−1 for logged-over forest and 21.2 Mg ha−1 for wind-disturbed forest. A sharp drop in biomass occurred in disturbed forests, especially those affected by wind and fires. This shows that natural disturbances negatively impact the protection of the biomass and carbon storage of the remaining peat swamp forests in the Riau Biosphere Reserve. Land-cover changes from natural vegetation to industrial forest plantations also have a negative effect. Wind-disturbed forest is one of the remaining natural forest types in the buffer zone of the Riau Biosphere Reserve.
Based on the below-ground carbon stock analysis of peatlands in the Riau Biosphere Reserve, logged-over forest has the highest value (5981 Mg C ha−1), followed by acacia plantations (5460 Mg C ha−1), and natural forest (4200 Mg C ha−1; Fig. 8.3). The lowest below-ground carbon stocks were found in oil palm plantations (3960 Mg C ha−1).
The results show higher below-ground peatland carbon storage in both forested and developed peatland areas compared to the results of Agus et al. (2009) and Wahyunto et al. (2010). High variation in below-ground carbon is also reported, with amounts ranging from 39 kg C m−3 to 66 kg C m−3, which is equivalent to 390–660 Mg C ha−1 m−1 of soil depth. Initial estimates show that on average, peat in Sumatra is thicker and has a carbon content of approximately 3000 Mg C ha−1, while the peat in Kalimantan is thinner and contains about 2000 Mg C ha−1. In comparison, the carbon content of mineral soil is usually concentrated in the first several centimeters of the surface layer and rarely exceeds 250 Mg C ha−1 (Wahyunto et al. 2010).
Above-ground biomass in tropical forests may vary considerably in response to differences in climate and soil parameters. For example, a study in Rondonia, Western Brazil, found that above-ground biomass in primary forest was 290–495 Mg ha−1 and about 40–60% less in young secondary forests in the same area. Another study in Sumatra showed that dipterocarp hill forest had an above-ground biomass ranging from 271 Mg ha−1 to 478 Mg ha−1, whereas the above-ground biomass of other lowland dipterocarp forest was 509 Mg ha−1. According to values published in various sources, the above-ground biomass carbon stored in undisturbed peat swamp forest ranges from 73 Mg C ha−1 to 323 Mg C ha−1, while that of logged-over and secondary peat swamp forest was between 65 Mg C ha−1 and 167 Mg C ha−1 (van der Meer and Verwer 2011).
In Central Kalimantan, the mean total ecosystem carbon stock of primary and secondary peat swamp forests was 1770 ± 123 Mg C ha−1 and 533 ± 49 Mg C ha−1, respectively (Novita et al. 2020). Another study from West Kalimantan reported that the mean total ecosystem carbon stock was 4021 Mg C ha−1, with peat thickness ranging from 650 cm to 1050 cm (Basuki 2017). The estimated amount of above-ground biomass was 252 Mg ha−1 in the Riau natural peat swamp forest, 111.4 Mg ha−1 in the logged-over forest, and 65 Mg ha−1 in the secondary forest. In comparison with other types of forest, logging forest in Jambi, Sumatra, resulted in a biomass decrease of 158.8 Mg ha−1. The total above-ground biomass can be influenced by soil fertility, and the occurrence of different forest types depends on the hydrology, chemistry, and organic content of the peat. Tropical peat swamp forests are characterized by high acidity (pH < 4), low nutrient quality, and a low rate of litter input (Neuzil 1997).
The total amount of above-ground carbon storage in lowland peat swamp forest in Indonesia was around 230 Mg C ha−1, with below-ground carbon storage of 2425 Mg C ha−1. Peat swamp forest stores large amounts of carbon in plant biomass, with typical values ranging from 100 Mg C ha−1 to 250 Mg C ha−1 (Page et al. 1999). Below-ground carbon storage on peatlands with 2772 Mg C ha−1 is based on an average peat thickness of 5.5 m (Page et al. 2011). Peat in Sumatra is thicker and has carbon storage of approximately 3000 Mg ha−1 (Wahyunto et al. 2010).
The results of this study show that the amount of above-ground carbon storage varies according to forest type and land use. The intensity and different causes of forest degradation affect how the vegetation stores and sequesters carbon. Forest degradation caused by humans could have less impact compared to natural disturbances, such as frequent fires and wind, which slow down the natural recovery of forests. As Binkley et al. (1997) stated, disturbances in natural forests may cause regeneration delays because viable seed sources are not available and the site is not suitable for the recovery of forest vegetation. The above-ground carbon storage in the peat swamp forests that remain today may vary greatly due to disturbances and natural variation (Verwer and van der Meer 2010).
The decrease in the amount of above-ground carbon between natural forest and logged-over and wind-disturbed forest highlights a decrease or malfunction in carbon sequestration following land-cover and land-use changes as a result of fire or the conversion to agricultural land. The same pattern is observed when comparing the amount of biomass in the different types of peat swamp forest.
Most of the research area in the Riau Biosphere Reserve is covered by deep peat ranging from 4.5 m to 10 m in depth that has carbon stock of 3960–5981 Mg C ha−1. This is higher than the amount of below-ground carbon stored in other peat areas in other Southeast Asian countries, including other areas in Indonesia such as Kalimantan (Novita et al. 2020) and even Sumatra (Page et al. 2011; Wahyunto et al. 2010; see Table 8.1).
Despite differences in biomass and carbon sequestration capacity, these forest areas play a crucial role as an essential pool of carbon. In terms of reducing carbon losses from land-cover changes and degradation, the rehabilitation of degraded peat swamp forests was recommended as the preferred land-use policy for future development in the Riau Biosphere Reserve. The results of this study suggest that degraded peat swamp forests currently contain between 15.7 Mg C ha−1 and 37.8 Mg C ha−1 and have the potential to increase their above-ground carbon content to at least 89.6–98.2 Mg C ha−1. Consequently, conservation and restoration of the peat swamp forests is crucial for conserving and even improving the amount of carbon stored in the Riau Biosphere Reserve.
Some typical canopy species of peat swamp forest are preferred in the rehabilitation of logged-over forest areas. For example, Tetrameristra glabra, Palaquium burckii, and Xylophia havilandii grow well in more open-gap plots. In addition, keeping wet conditions on the forest floor will encourage continuous growth. These results show that level degradation of the peat swamp forest ecosystem and selection of suitable tree species would influence the success of vegetation rehabilitation efforts. Table 8.2 shows that the amount of carbon is generally much higher below ground for all types of land use.
Table 8.2 highlights the importance of the peat swamp forest ecosystem for all carbon storage, not just the carbon stored in trees. This should be taken into consideration when evaluating management options for the Riau Biosphere reserve. The largest amount of below-ground carbon of 5981 Mg C ha−1 was found in logged-forest areas, followed by peatlands under acacia plantations (5775 Mg C ha−1). The lowest amount of below-ground carbon was 3960 Mg C ha−1 and was found in oil palm plantations.
Natural forest had the highest amount of above-ground carbon, followed by logged-over forest. The lowest was wind-disturbed forest. In developed peatlands, the highest amount of carbon was measured under rubber trees and was 68 Mg C ha−1, while Acacia crassicarpa had 42.57 Mg C ha−1 and Elaeis guineensis had 42.3 Mg C ha−1. These differences could be useful for addressing concerns about maintaining carbon storage in the peatland ecosystem when managing these plantations or choosing land uses.
The percentage of carbon composition is similar in forested areas and developed peatland areas. In contrast, bulk density was the highest in rubber gardens compared to other the sample plots and lowest in disturbed forest areas. Bulk density can be determined by the degree of decomposition, and the generally low bulk density values indicate low decomposition in peat. Rubber gardens had the highest bulk density because the peat had decomposed in advance compared to the other plot areas.
The bulk density of peat soil is likely the most important parameter because it varies considerably across peat types and even within peat types. Fibric tropical forest peats have a bulk density of less than 0.1 g cm−3, while highly decomposed sapric peats have values in excess of 0.2 g cm−3. Higher values are common in areas with topogenous clayey peats near the fringes of dome-shaped peat formations in areas with intensive agriculture management, such as paddy fields and oil palm plantations. The bulk density values of surface soils in the central dome areas under peat forests are generally lower than those peats under fringe domes of mixed swamp forest vegetation. In shallow fringe areas of peat domes where peat soils commonly contain clay, bulk density values are higher at certain depths than in the surface soils (Driessen and Rochimah 1976).
The bulk density of peatland under natural forest, logged-over forest, and disturbed forest means it can be classified as fibric and hemic, with bulk densities ranging from 0.083 g cm−3 to 0.145 g cm−3. Meanwhile, the carbon density of these same forest types ranges from 4200 Mg C ha−1 to 5981 Mg C ha−1. In developed peatland areas, the bulk density ranges from 0.12 g cm−3 to 0.16 g cm−3, classifying them as hemic. The carbon density of peatland under development in rubber gardens, acacia plantations, and oil palm plantations ranges from 3960 Mg C ha−1 to 5460 Mg C ha−1.
Generally, there are patterns in the carbon storage of peat in the Riau Biosphere Reserve. For example, forested areas have higher carbon compared to developed peatland areas, mainly due to peat decomposition processes. In contrast, developed peatland areas that had a continuously managed hydrology regime (e.g., forested areas and acacia plantations) showed similar amounts of below-ground carbon storage. Insufficient hydrology management can lead to lower amounts of below-ground carbon, however (e.g., oil palm plantations and rubber gardens). Therefore, management of peatlands significantly influences bulk density and the rate of the decomposition process.
3.1 Biomass Restoration and Carbon Reduction
Increase in biomass, carbon storage, and carbon sequestration in forested areas occurs through natural regeneration processes. Restoration activities with revegetation and natural forest regeneration approaches in tropical peatland ecosystems are effective and natural climate solutions for emissions reduction. The potential for emissions reduction through revegetation and natural regeneration shown in this study provides evidence that avoiding vegetation loss is an important part of restoration activities. The total estimated emission reduction provided by natural forest was higher than that estimated for logged-over forest and wind-disturbed forest: 207.36 CO2 Mg h−1, 161.48 CO2 Mg h−1, and 65.87 CO2 Mg h−1, respectively.
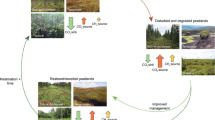
The Research and Development Division of the Peatland Restoration Agency conducted action research between 2016 and 2020 that was based on robust science and technology. A pilot project of revegetation with native tree species, or paludiculture, was mixed with the agroforestry model to maximize land utilization and improve the livelihoods of local people. The pilot project and paludiculture research were developed in degraded peatlands, and the results showed that revegetation can turn degraded peatlands into cultivation areas that improve the livelihoods of local people while mitigating peat fires and improving carbon sequestration.
Based on the authors’ study in South Sumatra, revegetation in burned peat areas can have a positive impact on the sequestering of carbon in belangeran (Shorea balangeran), jelutung (Dyera lowii), and ramin (Gonystylus bancanus) stands. A combination of jelutung (Dyera lowii) and ramin (Gonystylus bancanus) has a potential carbon sequestration of about 35.82 t CO2 ha−1 year−1 at 6 years of age and 56.5 t CO2 ha−1 year−1 at 8 years of age in belangeran (Shorea balangeran) stands (Bastoni 2019). The success of above-ground biomass recovery by doing a participatory restoration action research is in 2.5 ha plot of degraded peatland areas during almost 14 years in Tanjung Leban Village, Riau Province (Fig. 8.4). This plot has been extended by an integrated restoration actions were rewetting, revegetation, and revitalization of livelihood in a total of 2400 ha of water zones. The above-ground carbon sequestration has been restored even in heavily degraded peatlands. The Tanjung Leban plot is a landmark in tropical peatland restoration and has the potential to become a model for the permanent restoration of the peatland landscape and promote peatland green economy in the near future.
3.2 Potential Carbon Credit in the Peatlands Ecosystem
Based on the Indonesia Peatland Reference Emissions Level, the historical annual emissions are estimated to be 10.8 Mt CO2 year−1 and will reach up to 612 Mt CO2 by 2030. The Peatland Restoration Agency (Stage 1) has applied rewetting to degraded peatlands while improving community livelihoods in an effort to reduce carbon emissions. This program is expected to reduce emissions to 305.6 MtCO2 in 11.6 million ha located in prioritized restoration areas. The main restoration target will be 2.3 million ha that will contribute up to 54–74% or 98.77–153.53 MtCO2e of the emissions reduction (CCROM IPB 2021). The value of carbon from peatlands is important for maintaining ecological function while optimizing economic benefits. Community awareness regarding the importance of conserving the remaining peat forests and restoring degraded peatlands should be improved as these lands act as frontiers for this rich carbon ecosystem. Payment for Ecosystem Services (PES) through paludiculture may be key to increasing carbon sequestration and reducing economic pressure.
4 Conclusion
In this study, revegetation with native species contributed to climate change mitigation strategies by enhancing carbon sequestration, so reducing the amount of CO2 in the atmosphere. Avoiding peat deforestation and degradation during revegetation activities would increase potential emissions reduction provided by the peatland ecosystem. Human activities and natural disturbances reduce the ability of the peat swamp forest ecosystem to sequester CO2, as shown by our analysis of logged-over and wind-disturbed forest. Improved management of secondary forest must be achieved through rehabilitation, ceasing forest conversion, and reducing the impact of wind and fire disturbances. Natural regeneration is very important to improving the condition of secondary degraded peat swamp forest, but is not sufficient in itself for recovery of forest vegetation and associated biodiversity. At this point, some form of human-assisted regeneration is needed.
The indigenous tree species of the peat swamp forest used in restoration were Gonystylus bancanus, Callophylum lowii, Dyera lowii, Cratoxylon arborescens, Shorea spp., and Shorea balangeran. Total estimated emission reduction was higher in natural forest than in logged-over forest and wind-disturbed forest: 207.36 CO2 Mg h−1, 161 CO2 Mg h−1, and 65.87 CO2 Mg h−1, respectively. This study confirmed that above-ground peat swamp carbon sequestering can be restored even in severely degraded peatlands. Avoiding vegetation loss is an important part of restoration activities and is needed to continuously maintain the below-ground carbon stock in various types of land use. Restoration of biomass requires a longer time, and integrated restoration actions are needed to achieve economic, ecological, and social success. The utilization of local trees in peat swamp forests is a preferred restoration strategy for enhancing carbon stock and reducing the amount of carbon released into the atmosphere. In addition to carbon sequestration, natural regeneration in the peat ecosystem reduces peat decomposition caused by canal drainage.
Peat restoration must be included in the national mitigation strategy to achieve Indonesia’s national commitment to reducing its carbon emissions by 29–41% by 2030. This study provides strong evidence that peat restoration is an effective and natural solution for peat-rich countries, particularly Indonesia.
References
Agus F, Runtunuwu E, June T et al (2009) Carbon budget in land use transitions to plantation. Indones J Agric Res Dev 29(4):119–126
Bastoni (2019) Pengembangan Agro-Silvo-Fishery (Wana-Mina-Tani) untuk restorasi gambut berbasis KHG. Pertemuan ilmiah restorasi gambut. Kedeputian Penelitian dan Pengembangan Badan Restorasi Gambut, Jakarta
Basuki I (2017) Carbon stocks and emissions factors of tropical peat swamp forest in response to land cover changes in West Kalimantan, Indonesia. Oregon State University, Corvallis
Binkley CS, Apps MJ, Dixon RK et al (1997) Sequestering carbon in natural forests. Crit Rev Environ Sci Technol 27:S23–S45
Brown S (1997) Estimating biomass and biomass change of tropical forests: a primer. FAO Forestry Paper 134, Rome, p 87
Brown S, Lugo AE (1982) The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica 14(3):161–187
CCROM IPB (2021) Laporan akhir pendugaan emisi GRK setelah intervensi restorasi gambut. Laporan Internal Kedeputian Penelitian dan Pengembangan Badan Restorasi Gambut, Jakarta
Driessen PM, Rochimah L (1976) Physical properties of lowland Peats from Kalimantan. In: Peat and podzolic soils and their potential for agriculture in Indonesia. Proceedings ATA 106 Midterm Seminar, Tugu, October 1976. Soil Research Institute, Bogor
Giesen W (2004) Introduction. In: Causes of peat swamp forest degradation in Berbak NP, Indonesia, and recommendation for restoration. ARCADIS Euroconsult, Arnhem, pp 11–19
Gunawan H, Kobayashi S, Mizuno K et al (2012) Peat swamp forest types and their regeneration in Giam Siak Kecil-Bukit Batu biosphere reserve, Riau, East Sumatra, Indonesia. Mires Peat 10:5
Hirano T, Jauhiainen J, Inoue T et al (2009) Controls on the carbon balance of tropical peatlands. Ecosystems 12(6):873–887. https://doi.org/10.1007/s10021-008-9209-1
Hiratsuka M, Toma T, Diana R et al (2006) Biomass recovery of naturally regenerated vegetation after the 1998 forest fire in East Kalimantan, Indonesia. JARQ 40(3):277–282. https://doi.org/10.6090/jarq.40.277
Hooijer A, Haasnoot M, van der Vat M et al (2008) Master plan for the conservation and development of the ex-mega rice project area in Central Kalimantan. Technical report no 2, Euroconsult Mott MacDonald/Deltares, Jakarta/Delft
Hooijer A, Page SE, Jauhiainen J (2009) Summary interim report, 2007–2008: first tentative findings on hydrology, water management, carbon emissions and landscape ecology. Delft Hydraulics, Delft
Hooijer A, Page SE, Canadell JG et al (2010) Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 7:1505–1514. https://doi.org/10.5194/bg-7-1505-2010
Hooijer A, Page SE, Jauhiainen J et al (2012) Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 9(3):1053–1071. https://doi.org/10.5194/bg-9-1053-2012
Istomo (2002) Phosphorus and calcium contents in the soil and biomass of peat swamp forest: a case study at the concession area of PT. Diamond Raya Timber, Bagan Siapi-api, Riau Province. Dissertation, Bogor Agricultural University
Masripatin N, Ginoga K, Pari G et al (2010) Carbon stocks on various types of forest and vegetation in Indonesia. Research and Development of Forestry Department, Bogor
Masson-Delmotte V, Zhai P, Pörtner HO et al (eds) (2019) Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. IPCC, Geneva
MoEF (Ministry of Environment and Forestry) (2018) Indonesia second biennial update report: under the United Nations framework convention on climate change. Directorate General of Climate Change, Ministry of Environment and Forestry, Jakarta
Murdiyarso D, Herawati H, Iskandar H (2005) Carbon sequestration and sustainable livelihoods: a workshop synthesis. CIFOR, Bogor
Murdiyarso D, Hergoualc’h K, Verchot LV (2010) Opportunities for reducing greenhouse gas emissions in tropical peatlands. PNAS 107(46):19655–19660. https://doi.org/10.1073/pnas.0911966107
Neuzil SG (1997) Onset and rate of peat and carbon accumulation in four domed ombrogenous peat deposits, Indonesia. In: Rieley JO, Page SE (eds) Biodiversity and sustainability of tropical peatlands, Proceedings of the International symposium on biodiversity, environmental importance and sustainability of tropical peat and peatlands, Palangkaraya, September 1995. Samara Publishing, Cardigan, pp 55–72
Novita N, Kauffman JB, Hergoualc’h K et al (2020) Carbon stocks from peat swamp forest and oil palm plantation in Central Kalimantan, Indonesia. In: Djalante R, Jupesta J, Aldrian E (eds) Climate change research, policy and actions in Indonesia: science, adaptation and mitigation. Springer Climate. Springer, Cham, pp 203–227. https://doi.org/10.1007/978-3-030-55536-8_10
Page SE, Rieley JO, Shotyk OW et al (1999) Interdependence of peat and vegetation in a tropical peat swamp forest. Philos Trans R Soc B 354(1391):1885–1897. https://doi.org/10.1098/rstb.1999.0529
Page SE, Wüst RAJ, Weiss D et al (2004) A record of Late Pleistocene and Holocene carbon accumulation and climate change from an equatorial peat bog (Kalimantan, Indonesia): implications for past, present and future carbon dynamics. J Quat Sci 19(7):625–635. https://doi.org/10.1002/jqs.884
Page SE, Hosciło A, Wösten H et al (2008) Restoration ecology of lowland tropical peatlands in Southeast Asia: current knowledge and future research directions. Ecosystems 12(6):888–905. https://doi.org/10.1007/s10021-008-9216-2
Page SE, Rieley JO, Banks JC (2011) Global and regional importance of the tropical peatland carbon pool. Glob Change Biol 17(2):798–818. https://doi.org/10.1111/j.1365-2486.2010.02279.x
Palm C, Tomich T, van Noordwijk M et al (2004) Mitigating GHG emissions in the humid tropics: case studies from the alternatives to Slash-and-Burn Program (ASB). Environ Dev Sustain 6(1–2):145–162. https://doi.org/10.1023/B:ENVI.0000003634.50442.ca
Rieley JO, Page SE, Jauhiainen (eds) (2005) Wise use of tropical peatlands: focus on Southeast Asia. Alterra, Wageningen
Takahashi H, Shimada S, Ibie BF et al (2002) Annual changes of water balance and a drought index in a tropical peat swamp forest of Central Kalimantan, Indonesia. In: Rieley JO, Page SE, Setiadi B (eds) Peatlands for people: natural resource functions and sustainable management. Proceedings of the International symposium on tropical peatlands, Jakarta
van der Meer PJ, Verwer CC (2011) Towards a reference carbon value for peat swamp forest in Southeast Asia based on historical inventory data. In: Workshop on tropical wetland ecosystems of Indonesia: science needs to address climate change adaptation and mitigation. Sanur Beach Hotel, Bali
Verwer CC, van der Meer PJ (2010) Carbon pools in tropical peat forest: towards a reference value for forest biomass carbon in relatively undisturbed peat swamp forests in Southeast Asia. Alterra report 2108. Alterra, Wageningen
Wahyunto, Dariah A, Agus F (2010) Distribution, properties, and carbon stock of Indonesian peatland. In: Chen ZS, Agus F (eds) Proceedings of International workshop on evaluation and sustainable management of soil carbon sequestration in Asian countries, Bogor, September 2010. Indonesian Soil Research Institute, Bogor; Food and Fertilizer Technology Center for the Asian and Pacific Region, Taipei. National Institute for Agro-Environmental Sciences, Tsukuba
Yu Z, Loisel J, Brosseau DP et al (2010) Global peatland dynamics since the last glacial maximum. Geophys Res Lett 37(13):L13402. https://doi.org/10.1029/2010GL043584
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Gunawan, H., Tryanto, D.H., Mizuno, K., Kozan, O. (2023). Toward Climate Change Mitigation: Restoration of the Indonesian Peat Swamp. In: Mizuno, K., Kozan, O., Gunawan, H. (eds) Vulnerability and Transformation of Indonesian Peatlands. Global Environmental Studies. Springer, Singapore. https://doi.org/10.1007/978-981-99-0906-3_8
Download citation
DOI: https://doi.org/10.1007/978-981-99-0906-3_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0905-6
Online ISBN: 978-981-99-0906-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)