Abstract
Degradation of peatlands is an issue of global concern, yet ample knowledge of local conditions is lacking when it comes to determining (1) the impacts of river and floodplain development and (2) how best to conserve peat swamp ecosystems. This chapter documents the relationships between scientific and local names of fishes and recent changes in fish biodiversity in the mid-Kampar River Basin of Sumatra. A questionnaire was administered to 164 householders in the village of Rantau Baru and information on 96 species was triangulated with previous English- and Indonesian-language research. Results indicate the local extinction (defined as caught in the past but not observed during the last five years) of large predatory fishes and the invasion of several exotic species, potentially pointing to the early stage of degradation of the freshwater ecosystems. The potentiality of establishing effective freshwater protected areas in the mid-Kampar Basin is assessed by a narrative review of studies and methods from other developing countries. Local-scale ecosystem conservation that incorporates local perspectives and scientific investigation is of the highest priority to address development pressures on rivers, floodplains, and surrounding communities.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Ecosystem degradation
- Ecosystem linkage
- Fishery management
- Indigenous knowledge
- Invasive species
- Local ecological knowledge
4.1 Introduction
The ecosystems of Southeast Asian rivers are known as some of the largest biodiversity hotspots in the world (Myers et al. 2000; Dudgeon 2011). The species diversity and the vast production of fish provide important food resources to the peoples of Southeast Asian countries (FAO 2018). Despite their significance, however, Southeast Asian rivers are also known as some of the most threatened ecosystems in the world (Dudgeon 2011). This is due to human interventions and the resulting impacts, including dam construction, logging and deforestation, sand and gravel mining, sedimentation, and various types of water pollution (Allen et al. 2012). Without consideration of ecosystem conservation, the rapid development of Southeast Asian economies currently underway presents serious concerns for the food and water security of these countries at both local and national scales.
In Riau Province in eastern Sumatra, natural forest cover has decreased rapidly since the 1980s due to national and international industrial activity (in particular, the oil-palm and pulp industries), as well as agricultural activity of residents and immigrants from northern Sumatra and Java (Miettinen et al. 2016; Mizuno and Kusumaningtyas 2016; Shimamura 2016). In tropical peat swamp ecosystems, forests and grasslands grow on accumulated peatland intersected by permanent and temporal bodies of water, such as river channels, oxbow lakes, and floodplains; these ecosystems are scattered across lowland areas at 0–10° latitudes (Nofrizal et al., Chap. 5). Floodplains and their surrounding peatlands are typically seen as unsuitable for cultivation, and thus have been less developed relative to other forested areas in Indonesia. But clear-cutting in these areas has increased since the 2000s (Masuda et al. 2016). Floodplain habitats, such as riparian forests and swamps that become submerged during the rainy season, provide spawning, rearing, and foraging habitats for river fishes in tropical regions (Amoros and Bornette 2002; Correa and Winemiller 2014). Therefore, floodplain development presents a serious threat to river health, basin ecosystems, and the sustainability of inland fisheries. However, scientific knowledge about how freshwater fish use floodplain forests is lacking, and the potential risks of the loss of floodplain forests in Indonesia to future inland fisheries are largely unknown.
Local ecological knowledge (LEK) is knowledge accumulated over a lifetime through one’s observations and hands-on interactions with ecological systems and natural resources, or a cumulative body of local ecosystem knowledge that transcends generations through cultural transmission (Thornton and Scheer 2012; Berkström et al. 2019). LEK includes taxon-specific information, such as preferred habitat, abundance, behavior, breeding, and these seasonal patterns. Such information greatly increases comprehension of ecosystems, and therefore can be critical to ecosystem conservation, particularly in situations where scientific data are scarce or unavailable, such as in developing countries (see for example Berkström et al. 2019). As holders of LEK, local residents are essential to environmental conservation, and including their LEK in scientific interpretations may advance the success of conservation efforts. Despite this, LEK is rarely shared with village governments or higher administrative organizations and is seldom reflected in development planning or conservation efforts (Glaser et al. 2010; Satria and Adhuri 2010). Thus, identifying how to combine science with local wisdom remains a contemporary challenge to achieving sustainable development for local communities and ecosystems (Osawa, Chap. 6).
In an effort to document LEK in an area experiencing peatland degradation, Nakagawa et al. (2021) focus on the local names of fish used in a fishing village along the middle reaches of the Kampar River in Sumatra, Indonesia. Understanding and cross-referencing local names is necessary when collecting species-specific information from local residents or from existing literature written in a local language (see Ankei 1989; Castillo et al. 2018). The taxonomic description of a species and the determination of its scientific name are guided by international codes (e.g. the International Code of Zoological Nomenclature [ICZN], the International Code of Nomenclature for Algae, Fungi, and Plants [ICN], and the International Code of Nomenclature of Prokaryotes [ICNP]) (ICZN et al. 1999; Turland et al. 2018; Parker et al. 2019). While these codes were developed in the context of conventional natural sciences, the local name for a species is typically determined by its morphological or behavioral character and is based on LEK and the historical and cultural context of the human community where the species is found (Medin and Atran 2004). Therefore, the scientific name of a species often does not directly correspond to its local name. For a speciose taxon, a local name often relates to a scientific name higher than the species level, such as the genus or family (see for example Ankei 1989; Castillo et al. 2018). In addition, a single species may have multiple local names that correspond to its various body sizes or to ontogenetic stages during which a fish is important to a community’s livelihood (see Ankei 1989; Castillo et al. 2018). It is therefore critical to explicitly define the relationships between local and scientific names prior to collecting LEK, particularly when planning and implementing conservation activities at the ecological community and ecosystem levels.
This chapter presents the results of a survey that aims to (1) identify the relationships between scientific and local names of fishes and (2) understand recent changes to the fish biodiversity at the research site, a fishing village along the middle reaches of the Kampar River in Indonesia. The results of the survey are then discussed in the context of risks to ecosystem stability and the potential for ecosystem conservation in the Sumatran peatlands. In addition, the potentiality of establishing effective freshwater protected areas in the mid-Kampar Basin is assessed by a narrative review of studies and methods from other developing countries. Settlement of protected areas may be an effective strategy for biodiversity conservation from the view of the precautionary principle (Lauck et al. 1998) when ecosystem knowledge is limited, as is the case in most Southeast Asian regions.
4.2 Materials and Methods
4.2.1 Research Site
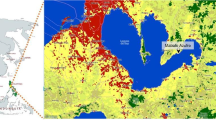
The village of Rantau Baru, Pangkalan Kerinci Sub-district, Pelalawan District, Riau Province is located along the middle reaches of the Kampar River, which flows through eastern Sumatra from west to east (Fig. 4.1a). The village is approximately 200 km from the river mouth and consists of two settlements (Fig. 4.1c). The main, and older, settlement of Rantau Baru is located on the banks of the Kampar River and consists of 116 houses. The newer settlement of Sei Pebadaran was constructed on hinterland peat soil by the district government around 2005; it is 8 km north of the settlement of Rantau Baru and consists of 48 houses. The two settlements are together customarily called Rantau Baru, which is also the name of the administrative village (Osawa and Binawan, Chap. 3). The settlements of Rantau Baru are regarded as having the longest history of any in Pelalawan District. Most villagers recognize that their ancestors lived in the proto settlement upstream on the opposite shore of the Kampar River and moved to the present-day village of Rantau Baru at least a few hundred years ago. In addition, tens of immigrants from Java and northern Sumatra live in the settlement of Sei Pebadaran to work in surrounding oil palm plantations at present (Osawa and Binawan, Chap. 3).
(a) Location of the village of Rantau Baru on the island of Sumatra; (b) Satellite image of the area surrounding the village of Rantau Baru (Google Maps, https://www.google.co.jp/maps/, accessed March 19, 2020). The grey line marks the administrative boundary of the village; the white shaded area indicates the remaining floodplain forested area used by villagers as a fishing ground; (c) Location of the settlements of Rantau Baru and Sei Pebadaran. The shaded area indicates the administrative boundary of the village. (Modified from Nakagawa et al. 2021 with the permission of Center for Southeast Asian Studies)
Rantau Baru village is typical of the fishing villages of the middle reaches of the Kampar River (Nofrizal, Chap. 5). Most households, including those in Sei Pebadaran, fish commercially or for self-consumption in the Kampar River and its tributaries, as well as in the oxbow lakes, canals, and swamps near the river. Typical fishing equipment includes fixed traps, gill nets, casting nets, and long lines (Masuda 2012). The village is surrounded by a floodplain, and riparian areas are typically submerged during the rainy season. Large portions of the riverbanks are covered with peat soils.
The area surrounding the village of Rantau Baru has undergone dramatic changes during the past 20 years. In 1996, a large-scale hydroelectric dam (PLTA Koto Panjang) was constructed on the upper reaches of the Kampar River, which has changed the flood regime downstream (Fitri and Husni 2020). Since the late 1980s, peat swamps in the research area have been drained for the development of acacia and oil-palm plantations (Shimamura 2016). The drained and dried hinterlands, which were covered by forested peat swamps in the past, now experience frequent fire, and burned areas often do not recover to forests or plantations, but instead have become abandoned bush (Shimamura 2016).
4.2.2 Survey by Questionnaire
A questionnaire was administered to all 164 houses in Rantau Baru between January 27 and February 2, 2020. The questionnaire was comprised of 101 questions designed to obtain basic information about the respondents and their households, attitudes toward conservation of peat swamp forests, levels of participation in local community activities, fishery activity, land ownership status, and incomes and assets (Dewi, Chap. 7; Hasegawa, Chap. 8; Prasetyawan, Chap. 9).
Three questions regarding fish names and species sightings were asked of residents who reported fishing for consumption or commercial purposes. The questions were:
-
1.
Tolong tulis jenis-jenis ikan yang Anda tangkap dalam 1 tahun terakhir. (Please write the names of fishes that you have caught during the past year.)
-
2.
Apakah ada jenis ikan yang ditangkap di masa lalu, tetapi dalam 5 tahun terakhir tidak ditemukan lagi? Jika ada, tolong tuliskan nama jenis ikan nya (boleh lebih dari satu). (Is there any type of fish that was caught in the past, but has not been found during the last 5 years? If so, please write down the name of the fish (may be more than one)).
-
3.
Apakah ada jenis ikan yang dulu tidak ada namun sekarang ditemukan? Jika ada, tolong tuliskan nama jenis ikan nya (boleh lebih dari satu). (Is there any type of fish that was not caught in the past but is caught now? If so, please write down the name of the fish (may be more than one)).
Before administration of the questionnaire, respondents were informed of the survey method and the objective of the research. Enumerators were careful not to show respondents fish names to avoid leading questions, and the respondents were free to provide any local name that they knew.
4.2.3 Literature Survey
Two sources from the English-language literature and three sources from the Indonesian-language literature about fish fauna in the middle reaches of the Kampar River were used to obtain a reference species list for known fishes from the area surrounding the village of Rantau Baru (Fauzi 2004; Fithra and Siregar 2010; Efizon et al. 2015; Aryani 2015; Aryani et al. 2020). Misidentifications and synonymous scientific names in the literature were corrected following Nelson et al. (2016) and FishBase (Froese and Pauly 2000). Records of fish that had been identified only to the genus level or higher were removed from the list, except for Tor sp., which could not be resolved to species due to taxonomic uncertainty (Pinder et al. 2019). Lists of Indonesian names of fish recorded from sites upstream and downstream of Rantau Baru, as well as from neighboring rivers (the Rokan, Siak, and Indragiri) were used supplementarily (Siregar et al. 1994; Tjakrawidjaja and Haryono 2000; Iskandar and Dahiyat 2012; Fahmi et al. 2015; Firdaus et al. 2015; Lubis et al. 2016; Purnama and Yolanda 2016; Yustina 2016).
4.2.4 Collation of Local and Scientific Names and Tabulation of Species Sightings
The scientific, English, Indonesian, and local names of fishes found in Rantau Baru were collected, along with all local alternate names. Spelling variations of local fish names that appeared to be caused by a difference in pronunciation or a listening error were first collated (for example, Kayang, Khayangan, Koloso, and Keloso were combined as the local name Kayangan/Arwana (Scleropages formosus)). Using the Indonesian name of fishes as a reference, the local names were then correlated to the scientific names. The resulting comprehensive list is presented in Table 4.1. Hereafter, names in quotation marks refer to the local names obtained from the questionnaires. Note that the spelling of local names provided in Table 4.1 is that as given by the respondents; thus, in several cases, the spelling of a local name does not correspond to Indonesian orthography (for example asin, or “salt” in standard Indonesian, is presented as masin, and kucil, or “small” in standard Indonesian, is presented as kucir). Table 4.1 also includes the number of respondent(s) who reported sightings of each species in response to Question 1 (fishes caught within the last year), Question 2 (fishes caught previously but not observed within the last five years), and Question 3 (fishes caught now that were not caught previously).
Finally, rarefaction curves of the number of local names relative to the number of respondents were drawn to evaluate the effect of the sampling effort on the number of local names identified both before (Fig. 4.2a) and after (Fig. 4.2b) collation of spelling variations. After accounting for spelling variation, a confidence rate of 95% was calculated using bootstrap resampling with 999 iterations.
(a) Rarefaction curve of the number of local names of fishes relative to the number of respondents prior to spelling collation; (b) Rarefaction curve of the number of local names of fishes relative to the number of respondents following spelling collation. (Reprinted from Nakagawa et al. 2021 with the permission of Center for Southeast Asian Studies)
4.3 Results and Discussion
4.3.1 Local Names of Fishes in Rantau Baru
Ninety-four of the 164 respondents provided local fish names. A total of thirty-eight local fish names were recorded following the spelling compilation and the removal of names that clearly did not relate to a specific taxon or were related to marine fish. The number of local names recorded did not reach saturation without compilation (Fig. 4.2a), but it did reach saturation at approximately 70 respondents with spelling compilation (Fig. 4.2b). The comprehensive species list in Table 4.1 was compiled using questionnaire responses and existing literature about the area. The list includes 95 species belonging to 52 genera, 27 families, and 12 orders of fishes and one prawn species. Of the total, twenty-eight local names were related to scientific names of fish taxa from the area (Table 4.1). More than half of the local names were related to a scientific name at the genus level or higher and were also related to multiple scientific names at the species level. For example, “Pantau/Tabingal” was associated with multiple species belonging to the genera Puntioplites or Rasbora. “Baung” referred to multiple species belonging to the genera Hemibagrus or Mystus and included several alternates, such as “Baung kuning” (1/52 cases in Question 1) and “Baung pisane” (2/52 cases in Question 1), although the relationship between these alternates and scientific names could not be verified. “Selais” referred to multiple species belonging to the genera Ompok or Kryptopterus. “Patin” referred to multiple species of the genus Pangasius or Pangasianodon, and respondents often gave several alternates referring to Pangasius spp. or Pangasianodon spp., such as “Juaro” (13/20 cases in Question 1) and “Patin kunyit” (1/20 cases in Question 1). However, these alternates did not correlate to scientific names at the species level.
Four local names, “Kayangan/Arwana,” “Ikan Parang,” “Gadi,” and “Belut,” directly connected to the scientific names of four fish species (Scleropages formosus, Chirocentrus dora, Tor sp., and Monopterus albus, respectively). The existence of these species has not been recorded in previous scientific research of the Rantau Baru area (Aryani 2015; Efizon et al. 2015; Fauzi 2004; Fithra and Siregar 2010). In this study, the first three of these species were reported as fish that had been caught in the past but had not been observed by respondents during the last five years (defined here as extirpated, or locally extinct). Respondents reported a total of 16 local names of fishes that “had been caught previously but have not been observed within the last five years” (Question 2), and 7 local names of fishes that were not caught previously but are caught now, or exotic species (Question 3) (Table 4.1). Excluding the previously mentioned three species (“Kayangan/Arwana,” “Ikan Parang,” and “Gadi”), “Patin” (13 cases), especially its alternates, “Patin kunyit” (3 cases) and “Patin juaro” (2 cases), was frequently recorded in response to Question 2. Local residents also recognized several subgroups within “Patin.” The spelling of several local names compiled as “Patin” resembled the scientific name or a synonym of a species belonging to the genus Pangasius. We suspect that Pangasius juaro (a synonym of Pangasius polyuranodon), Pangasius kunyit, and Pangasius djambal are related to “Juaro,” “Patin kunyit,” and “Patin jambal,” respectively. Interestingly, all three of these species are described as native to Sumatra Island, whereas local respondents defined “Patin kunyit” as the name of an extirpated species and “Patin jambal” as the name of an exotic species. This may reflect temporal changes in the composition of Pangasius spp. during the last several years or a cross-swapping of local and scientific names due to miscommunication among and between residents and scientists.
4.3.2 Decreased Sightings or Local Extinction of Large Predators
Among the species that were reported by respondents as unseen in the previous five years, the most frequently mentioned were Scleropages formosus, Chirocentrus dora, Tor sp., and Pangasius sp., which are all large predators that occupy the higher trophic levels of aquatic ecosystems in peat swamp areas. The absence of such species therefore may indicate that bodies of water in the sampling site are in an early stage of ecosystem degradation. Generally, top predators that have a large body size, are low in abundance, and that have a high number of home-range requirements are particularly vulnerable to habitat fragmentation or destruction (Raffaelli 2004). This vulnerability is explained by their position at the top of an ecological pyramid. Because the pyramid is formed by the constraints of prey–predator mass ratios and that of the transfer of energy from lower to upper trophic levels of the pyramid (Trebilco et al. 2012), a larger ecological pyramid is needed to maintain a larger predator population. The decreased sighting of large predators raises concerns that recent developments in peat swamp ecosystems, such as deforestation, palm plantations, and fire, may be shrinking the pyramid, as depicted in Figs. 4.3a and b. Indeed, in their investigation of trophic positions of stream fishes using stable isotope analysis in Southeastern Sabah, Malaysian Borneo, Wilkinson et al. (2021) demonstrate that while the position of meso-predators does not change in oil-palm plantations versus forests, the trophic positions of apex predators in oil-palm plantations are lower than in forests.
Local residents with LEK about river ecosystems recognize that floodplain forests are vital not only as fishing grounds, but also as spawning areas, and primary and secondary floodplain forests are relatively conserved around settlements compared to other areas around the mid-Kampar River (Nakagawa et al. 2021). However, the range of species dispersal generally correlates with the body length of the fish (Minns 1995; Radinger and Wolter 2014), and the dispersal distance of large river fish (≥ 500 mm in standard length) is often longer than 50 km (Radinger and Wolter 2014). In addition, several fish species in Southeast Asian rivers migrate distances farther than hundreds of kilometers and these fishes are often commercially important (Poulsen et al. 2002). Thus, the remaining forested area of the village, which is smaller than ten square kilometers (Fig. 4.1b), is too small to sustain populations of large fish species.
Furthermore, the loss of top predators often precipitates long-lasting impacts to natural ecosystems, including drastic changes in the species composition of lower trophic levels, ecosystem productivity and other functions, and even changes to landscape characteristics, which typically are not wanted by humans (Raffaelli 2004; Estes et al. 2011). For example, fishing down, or the negative spiral whereby the size of caught fish and the mesh in fish gear progressively decreases as larger individuals and species are successively eliminated, is typical to the collapse of aquatic ecosystems (Allan et al. 2005). Theoretical models predict that the fishing down initially leads to an increase in the weight of the total catch as the number of harvested species and individuals increases, followed by a plateau or slight decline in total catch (Welcomme 2001). If the decrease in the number of sightings and local extinction of large predatory fishes in the survey area indeed reflects the early stage of ecosystem degradation, scientific investigation to assess the effects of development in peat swamp ecosystems is urgently needed.
4.3.3 Exotic Species
In response to Question 3 about sightings of new, or exotic, species, “Sapu-sapu” was the most frequently mentioned (15 cases). “Patin” (4 cases), especially its alternate “Patin jambal” (2 cases), was the second-most frequent response. “Sapu-sapu,” or Pterygoplichthys spp., is a major ornamental fish that has been artificially introduced into tropical and subtropical regions worldwide (Orfinger and Goodding 2018). Several species belonging to the genus Pangasius and Pangasianodon were also artificially moved beyond their native ranges, primarily for the purpose of aquaculture; among these, Pangasianodon hypophthalmus has been the main species introduced to the Indonesian islands (Lazard et al. 2009; Rimmer et al. 2013). Anthropogenic introduction of exotic fishes favors large species that have a high fishery or ornamental value. These species are often top predators that have never been exposed to native prey species throughout their evolutionally history and thus, become invasive (Mack et al. 2000). Therefore, contrasting with the impact of ecosystem fragmentation and destruction, which tends to affect species at higher trophic levels, the effects of exotic species tend to be more obvious at lower trophic levels, where species are directly consumed by an invader (Estes et al. 2011) (Fig. 4.3c). For example, many studies report serious decreases of native aquatic organisms in Asian and North and Central American countries following the invasion of Pterygoplichthys spp. (Orfinger and Goodding 2018). Most reports of the negative effects of Pangasianodon hypophthalmus on ecosystems come indirectly from studies of chemical and nutrient leakage from aquaculture ponds (see for example Rico et al. 2013). However, several studies directly address the negative effects of this catfish on other species via predation and/or competition (see Lazard et al. 2009; Rimmer et al. 2013).
The introduction of exotic fish species has various origins, from intensive ones with commercial purposes to unintentional ones due to deviations and abandonment of farmed or ornamental fishes. Regardless of the origin, however, assessment of the impact on the endemic ecosystem is rarely done in advance (Leprieur et al. 2009; Gozlan et al. 2010), despite these unassessed introductions often causing serious, even catastrophic, degradations of freshwater ecosystems (see for example Gophen et al. 1995; McDowall 2006; Hughes and Herlihy 2012; Matsuzaki and Kadota 2015). In villages along the middle reaches of the Kampar River, a diverse array of native species, from small to large fishes, are useful for self-consumption and commercial purposes. The invasion and increase of exotic species that potentially affect these fishery resources should therefore be observed with the utmost attention.
4.3.4 Potentiality of a Freshwater Protected Area in the Mid-Kampar River Basin
Although studies about the planning, management, and effectiveness of protected areas for sustainable fisheries and biodiversity conservation have mainly investigated marine ecosystems (Lester et al. 2009; Edgar et al. 2014), studies that report the effectiveness of protected areas in river ecosystems are increasing (Acreman et al. 2020). Peatlands, especially in floodplain areas that become seasonally submerged, are less suitable for oil-palm or acacia plantations compared to unsubmerged lands with mineral soils. At the same time, the drying of peatlands by channel construction for plantations results in the increase of fires. Therefore, the development of floodplains often does not improve the economic conditions of residents (see Kasori, Chap. 11). Indeed, if development of floodplains will decrease fishery production, then establishing freshwater protected areas that include surrounding floodplains may be a better choice than agricultural development when considering how best to sustain local economies.
Specifically, what types of planning and management of a protected area would be effective in the research site and areas like it? Acreman et al. (2020) review scientific papers on the effectiveness of protected freshwater areas and offer eight lessons on how to enhance the conservation of freshwater biodiversity. Their lessons about ecosystem monitoring (Lesson 1), size and habitat heterogeneity of the protected area (Lessons 2, 3, and 4), and trade-offs between biodiversity conservation and other human activities (Lessons 5, 7, and 8), are particularly applicable to peat swamp ecosystems in Indonesia today. Here, a perspective on the management of peat swamp freshwater ecosystems is warranted.
Ideally, to examine the effectiveness of a protected area in a freshwater system characterized by high naturally temporal variability, it is necessary to include both quantitative and comparable monitoring, such as before-after control-impact (BACI) design, which arguably requires long time frames (Adams et al. 2015). This type of monitoring also requires highly trained persons to conduct field surveys and continuous budget allocations to pay for their laborious work. Although BACI monitoring may be realized in the future, it is difficult to implement in addressing urgent issues. In this situation, local fishers sharing daily catch records with scientists may be effective. Estimation of stocks using the catch per unit effort (CPUE) is a well-established and widely used method in fishery management. Combining estimates based on the CPUE with other scientific census data can be a powerful means to grasp the conditions of the focal systems as well as their determinant factors (see for example Russ et al. 2004). However, it should be noted that, as suggested by the local fish names presented in this study, in order to gain sufficient practical data with this method, it is necessary to obtain prior coordination between scientists and fishers.
In riverine systems, many fish species use different habitats and parts of the basin at different life stages. In addition, aquatic habitats typically interface with a riparian or littoral zone, where stands of semi-aquatic and terrestrial vegetation regulate shade and water temperature, channel stability, and supplies of nutrients and organic matter to aquatic food webs (Fig. 4.3a). Furthermore, the natural flood regime is a defining feature that governs channel structure and connectivity, and substrate characteristics. Therefore, catchment-scale management is ideal for conserving biodiversity in freshwater ecosystems (Naiman et al. 2005). The Indonesian government uses the KHG (Kesatuan Hidologis Gambut), or peat hydrological unit, as the fundamental unit for managing peat swamp ecosystems (Kasori, Chap. 11). A KHG is an area of peat deposits formed between two rivers, between a river and a sea, and/or in a swamp, which is usually 0.01–10 km2 in size (Ibrahim et al. 2019). A KHG does not contain a river catchment, but catchment-scale management can easily be applied to a KHG, because the centers of two neighboring KHGs usually consist of lateral slopes of a river channel. Although KHG governance at present mainly targets water management and fire prevention, it may be beneficial to incorporate a fisheries management perspective into KHG governance in the future.
Bhutan may have the most advanced case of catchment-scale ecosystem conservation in the world. In this country, Nature Needs Half (NNH), the international conservation movement that aims to protect 50% of the earth by 2050 (Pimm et al. 2018), has already protected terrestrial areas, and additional conservation plans for freshwater ecosystems that explicitly consider catchment-scale management have been suggested (see Dorji et al. 2020). While it may be difficult at present to implement an ecosystem conservation plan like Bhutan’s in Indonesia, the floodplain forests intentionally left undisturbed by the villagers in the research area are clearly too small to sustain a population of large predators, as mentioned above. However, effective ecosystem conservation may be possible, even if individual protected areas are small. For example, Koning et al. (2020) demonstrate the effectiveness of protected areas created by 23 separate local residential communities in Thailand’s Salween Basin. In this case, although the area of each individual protected area is small, the network of the areas works like a meta-community (Leibold et al. 2004) and has markedly increased richness, density, and biomass in fishes relative to adjacent areas. Many fisher villages similar to the research site are scattered along the Kampar River. Establishing a protected area in most of these villages, even on a small scale, could together function as one large protected area, which may in turn produce promising results for the entire basin.
4.3.5 How to Establish and Manage Freshwater Protected Areas in the Mid-Kampar River Basin
Acreman et al. (2020) indicate that biodiversity conservation in a protected area mainly fails due to external factors, such as inappropriate, illegal, or unregulated land and water management of the catchment area, or internal factors, such as human activities other than biodiversity conservation, including recreational uses, within the protected area. They emphasize how laws and regulations associated with protected areas need to be enforced and how regulatory activities should involve local communities. The challenges of local governance and politics vis-à-vis environmental issues are discussed in Chapts. 3, 8, and 11. Here, the potentiality of establishing protected areas in Rantau Baru village and the surrounding local communities is discussed. In the village, while local peoples seem to recognize the importance of floodplain forests to sustaining fishery resources as empirically based LEK (Prasetyawan, Chap. 9), floodplain development is still ongoing, even though it does not necessarily lead to an increase in the villagers’ incomes (see Kasori, Chap. 11). This situation suggests that vague future concerns based on LEK do not work to disincentivize the commodification of the lands they own (see Osawa and Binawan, Chap. 3).
Conversely, the success of ecosystem conservation in this region may be achieved by the verification of local concerns via scientific investigation, sharing investigation results with local governments and peoples, and raising awareness of the importance of ecosystem conservation in the local communities. In practice, the “scenario planning method,” used in the Community-Based Management of Environmental Challenges in Latin America (COMET-LA) project (Waylen et al. 2015), may be applicable in the communities of the middle Kampar. While the method is implemented by facilitators who are mix of interdisciplinary researchers and project-specific civil society organization personnel, its outputs are selected by local stakeholders via a workshop that is comprised of four steps. These steps are: (1) explore how drivers of change may influence the socio-ecosystem using morphological analysis (see Godet 2006 for detailed methods), (2) construct alternative scenarios, (3) identify ‘robust’ response options, and (4) discuss implications and requirements of response options (Waylen et al. 2015). These steps may be implemented in Rantau Baru as follows. First, referencing natural and sociological scientific investigations, facilitators identify the potential impacts of land developments (the drivers) and identify two opposing states, such as “floodplains will remain forested” or “floodplains will be developed to oil-palm or acacia plantations.” These drivers and the contrasting states are then presented to the communities to modify or reselect. Second, alternative scenarios are created that consider balancing increases in local incomes with losses of natural resources. Community participants discuss and amend the scenarios to determine what the acceptable scenario is. Third, community participants discuss what actions, or response options, might be sufficiently relevant and robust to achieve community goals in light of possible future changes. These may include regulating the commodification of their lands and establishing protected areas. Finally, workshop participants discuss what specifically needs to be done, by when, and by whom, identifying specific actions for individuals, the community, and external actors to operationalize the robust response options. For example, the actions and responses may include local governments and stakeholders creating new rules to govern land use and fisheries and fishers recording daily catches to continuously monitor the status of natural resources.
4.4 Recreational Fishing: The Novel Commodification Activity of Fisheries in Rantau Baru
In Chap. 5, Nofrizal discusses the potential of recreational fishing to increase fisher income. Indeed, if managed appropriately, recreational fishing has numerous potential benefits. For example, recreational fishing may provide local people with alternative options for stable livelihoods and may provide national economies of developing countries an alternative revenue stream to that of extractive industries (such as logging or mining) or other activities that transform natural landscapes (such as large-scale agriculture or aquaculture) (Barnett et al. 2016). However, recreational fishing is also known to cause ecosystem conservation failure when it (1) fails to consider the local sociocultural context, (2) does not adequately distribute direct employment and knock-on benefits to local people, and (3) inappropriately regulates the ownership and tenure of natural resources and the space where recreational fishing occurs (Barnett et al. 2016; Acreman et al. 2020). It is common in developing countries, especially in cases where recreational fishing generates significant economic benefits, that very few businesses are owned or operated by indigenous people, and few indigenous people are employed by local businesses; thus, incentivizing sustainable livelihoods is necessary (Barnett et al. 2016). In any case, the implementation of recreational fishing schemes is based on the presumption that fishery production will be maintained at the same or a higher level than at present. Therefore, considering the current conditions of the mid-Kampar River Basin from the perspective of both natural resource sustainability and diversifying the economy, ecosystem conservation is the highest priority.
4.5 Conclusion
The need for ecosystem conservation in peat swamp areas has been repeatedly raised and is obvious in terms of global interests, such as achieving carbon neutrality and biodiversity conservation (Couwenberg et al. 2010; Hooijer et al. 2010; Posa et al. 2010; Miettinen et al. 2016). However, local perspectives based on scientific investigation are equally important when assessing the trade-offs of land development for income on the one hand and ecosystem conservation to sustain traditional—and future—livelihoods on the other. As we see from this chapter, this is particularly important when it comes to addressing development pressures on rivers and floodplains.
References
Acreman M, Hughes KA, Arthington AH et al (2020) Protected areas and freshwater biodiversity: a novel systematic review distils eight lessons for effective conservation. Conserv Lett 13:e12684. https://doi.org/10.1111/conl.12684
Adams VM, Setterfield SA, Douglas MM et al (2015) Measuring benefits of protected area management: trends across realms and research gaps for freshwater systems. Philos Trans R Soc B 370(1681):20140274. https://doi.org/10.1098/rstb.2014.0274
Allan JD, Abell R, Hogan Z et al (2005) Overfishing of inland waters. BioScience 55:1041–1051. https://doi.org/10.1641/0006-3568(2005)055[1041:OOIW]2.0.CO;2
Allen DJ, Darwall WRT, Smith KG (2012) The status and distribution of freshwater biodiversity in Indo-Burma. IUCN, Cambridge, UK; Gland, Switzerland
Amoros C, Bornette G (2002) Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshw Biol 47:761–776. https://doi.org/10.1046/j.1365-2427.2002.00905.x
Ankei Y (1989) Folk knowledge of fish among the Songola and the Bwari: comparative ethnoichthyology of the Lualaba River and Lake Tanganyika fishermen. Afr Study Monogr, Supplementary Issue 9:1–88. https://doi.org/10.14989/68349
Aryani N (2015) Native species in Kampar Kanan River, Riau Province Indonesia. Int J Fish Aquat Stud 2:213–217
Aryani N, Suharman I, Azrita A et al (2020) Diversity and distribution of fish fauna of upstream and downstream areas at Koto Panjang Reservoir, Riau Province, Indonesia. F1000Research 8:1435. https://doi.org/10.12688/f1000research.19679.2
Barnett A, Abrantes KG, Baker R et al (2016) Sportfisheries, conservation and sustainable livelihoods: a multidisciplinary guide to developing best practice. Fish Fish 17:696–713. https://doi.org/10.1111/faf.12140
Berkström C, Papadopoulos M, Jiddawi NS et al (2019) Fishers’ local ecological knowledge (LEK) on connectivity and seascape management. Front Mar Sci 6(130):00130. https://doi.org/10.3389/fmars.2019.00130
Castillo TI, Brancolini F, Saigo M et al (2018) Ethnoichthyology of artisanal fisheries from the lower La Plata River Basin (Argentina). J Ethnobiol 38:406–423. https://doi.org/10.2993/0278-0771-38.3.406
Correa SB, Winemiller KO (2014) Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecol 95:210–224. https://doi.org/10.1890/13-0393.1
Couwenberg J, Dommain R, Joosten H (2010) Greenhouse gas fluxes from tropical peatlands in Southeast Asia. Glob Change Biol 16:1715–1732. https://doi.org/10.1111/j.1365-2486.2009.02016.x
Dorji T, Linke S, Sheldon F (2020) Freshwater conservation planning in the context of nature needs half and protected area dynamism in Bhutan. Biol Conserv 251:108785. https://doi.org/10.1016/j.biocon.2020.108785
Dudgeon D (2011) Asian river fishes in the Anthropocene: threats and conservation challenges in an era of rapid environmental change. J Freshw Fish Biol 79:1487–1524. https://doi.org/10.1111/j.1095-8649.2011.03086.x
Edgar GJ, Stuart-Smith RD, Willis TJ et al (2014) Global conservation outcomes depend on marine protected areas with five key features. Nature 506:216–220. https://doi.org/10.1038/nature13022
Efizon D, Putra RM, Kurnia F et al (2015) Keanekarangaman jenis-jenis ikan di oxbow pinang dalam desa buluh cina Kabupaten Kampar, Riau. In: Ramli Z, Razman MR, Efizon D (eds) Prosiding seminar antarabangsa ke 8: ekologi, habitat manusia dan perubahan persekitaran. Universiti Kebangsaan Malaysia, Langkawi, pp 23–46
Estes JA, Terborgh J, Brashares JS et al (2011) Trophic downgrading of planet Earth. Science 333:301–306. https://doi.org/10.1126/science.1205106
Fahmi MR, Ginanjar R, Kusumah RV (2015) Diversity of ornamental fish in peatlands biosphere reserve Bukit-Batu, Riau Province. Pros Sem Nas Masy Biodiv Indon 1:51–58. https://doi.org/10.13057/psnmbi/m010108
FAO (Food and Agriculture Organization of the United Nations) (2018) The state of fisheries and aquaculture 2018: meeting the sustainable development goals. FAO, Rome
Fauzi M (2004) Struktur komunitas ikan sungai Kampar yang dipengaruhi perubahan massa air akibat bendungan PLTA Koto panjang. J Perikan Kelaut 9:47–60
Firdaus F, Pulungan CP, Efawni E (2015) A study on fish composition in the Air Hitam River, Pekanbaru, Riau Province. JOM FAPERIKA UNRI 2:1–14
Fithra RY, Siregar YI (2010) Keanekaragaman ikan Sungai Kampar inventarisasi dari Sungai Kampar Kanan. J Ilmu Lingkungan 4:139–147. https://doi.org/10.31258/jil.4.02.p.139-147
Fitri AR, Husni D (2020) Disaster education to increase family resilience (community based participatory action research on post flood reconstruction phase). In: Agung S, Nanto D, Adrefiza A et al (eds) ICEMS 2019. Proceedings of the 5th international conference on education in Muslim society, Jakarta, September–October 2019. EAI, Gent. https://doi.org/10.4108/eai.30-9-2019.2291125
Froese R, Pauly D (eds) (2000) FishBase 2000: concepts, design and data sources. ICLARM, Los Baños
Glaser M, Baitoningsih W, Ferse SCA et al (2010) Whose sustainability? Top-down participation and emergent rules in marine protected area management in Indonesia. Mar Policy 34:1215–1225. https://doi.org/10.1016/j.marpol.2010.04.006
Godet M (2006) Creating futures: scenario planning as a strategic management tool, 2nd edn. Economica, Paris
Gophen M, Ochumba PBO, Kaufman LS (1995) Some aspects of perturbation in the structure and biodiversity of the ecosystem of Lake Victoria (East Africa). Aquat Living Resour 8:27–41. https://doi.org/10.1051/alr:1995003
Gozlan RE, Britton JR, Cowx I et al (2010) Current knowledge on non-native freshwater fish introductions. J Fish Biol 76:751–786. https://doi.org/10.1111/j.1095-8649.2010.02566.x
Hooijer A, Page S, Canadell JG et al (2010) Current and future CO2 emissions from drained peatlands in Southeast Asia. Biogeosciences 7:1505–1514. https://doi.org/10.5194/bg-7-1505-2010
Hughes RM, Herlihy AT (2012) Patterns in catch per unit effort of native prey fish and alien piscivorous fish in 7 Pacific Northwest USA rivers. Fisheries 37:201–211. https://doi.org/10.1080/03632415.2012.676833
Ibrahim FK, Suryadi Y, Soekarno I et al (2019) Water management system of peatlands for palawija plants on KHG Pulang Pisau, Central of Kalimantan. MATEC Web Conf 270:04006. https://doi.org/10.1051/matecconf/201927004006
ICZN (International Commission on Zoological Nomenclature) (1999) International code of zoological nomenclature, 4th edn. International Trust for Zoological Nomenclature, London
Iskandar J, Dahiyat Y (2012) Keaneka ragaman ikan di sungai Siak Riau. Bionatura 14:51–58
Koning AA, Pelales KM, Fluet-Chouinard E et al (2020) A network of grassroots reserves protects tropical river fish diversity. Nature 588:631–635. https://doi.org/10.1038/s41586-020-2944-y
Lauck T, Clark CW, Mangel M et al (1998) Implementing the precautionary principle in fisheries management through marine reserves. Ecol Appl 8:S72–S78. https://doi.org/10.1890/1051-0761(1998)8[S72:ITPPIF]2.0.CO;2
Lazard J, Cacot P, Slembrouck J et al (2009) Fish farming of Pangasiids. Cah Agric 18:164–173
Leibold MA, Holyoak M, Mouquet N et al (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Leprieur F, Brosse S, García-Berthou E et al (2009) Scientific uncertainty and the assessment of risks posed by non-native freshwater fishes. Fish Fish 10:88–97. https://doi.org/10.1111/j.1467-2979.2008.00314.x
Lester SE, Halpern BS, Grorud-Colvert K et al (2009) Biological effects within no-take marine reserves: a global synthesis. Mar Ecol Prog Ser 384:33–46. https://doi.org/10.3354/meps08029
Lubis AY, Efawani F, Windarti W (2016) Re-inventarisation of fish in the Sali River, Pekanbaru Regency, Riau province. JOM Faperi 3:1–10
Mack RN, Simberloff D, Lonsdale WM et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Masuda K (2012) Indoneshia mori no kurashi to kaihatsu: tochi wo meguru ‘tsunagari’ to ‘semegiai’ no shakaishi (Livelihoods and developments in Indonesian forest: sociohistory of cooperation and conflict for land use). Akashi Shoten, Tokyo
Masuda K, Mizuno K, Sugihar K (2016) A socioeconomic history of the peatland region: From trade to land development, and then to conservation. In: Mizuno K, Fujita MS, Kawai S (eds) Catastrophe and regeneration in Indonesia’s peatlands: ecology, economy and society, Kyoto CSEAS series on Asian studies, vol 15. NUS Press; Kyoto University Press, Singapore; Kyoto, pp 148–184
Matsuzaki SS, Kadota T (2015) Trends and stability of inland fishery resources in Japanese lakes: introduction of exotic piscivores as a driver. Ecol Appl 25:1420–1432. https://doi.org/10.1890/13-2182.1
McDowall RM (2006) Crying wolf, crying foul, or crying shame: alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev Fish Biol Fish 16:233–422. https://doi.org/10.1007/s11160-006-9017-7
Medin DL, Atran S (2004) The native mind: biological categorization and reasoning in development and across cultures. Psychol Rev 111:960–983. https://doi.org/10.1037/0033-295x.111.4.960
Miettinen J, Shi C, Liew SC (2016) Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob Ecol Conserv 6:67–78. https://doi.org/10.1016/j.gecco.2016.02.004
Minns CK (1995) Allometry of home range size in lake and river fishes. Can J Fish Aquat Sci 52:1499–1508. https://doi.org/10.1139/f95-144
Mizuno K, Kusumaningtyas R (2016) Land and forest policy in Southeast Asia. In: Mizuno K, Fujita MS, Kawai S (eds) Catastrophe and regeneration in Indonesia’s peatlands: ecology, economy and society, Kyoto CSEAS series on Asian studies, vol 15. NUS Press; Kyoto University Press, Singapore; Kyoto, pp 19–68
Myers N, Mittermeier RA, Mittermeier CG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Naiman RJ, Décamps H, McClain ME et al (2005) Riparia: ecology, conservation, and management of streamside communities. Academic Press, Cambridge. https://doi.org/10.1016/B978-0-12-663315-3.X5000-X
Nakagawa H, Osawa T, Binawan A et al (2021) Local names of fishes in a fishing village on the bank of the middle reaches of the Kampar River, Riau, Sumatra Island, Indonesia. Southeast Asian Stud 10:435–454. https://doi.org/10.20495/seas.10.3_435
Nelson JS, Grande TC, Wilson MCH (2016) Fishes of the world, 5th edn. Wiley, Hoboken
Orfinger AB, Goodding DD (2018) The global invasion of the suckermouth armored catfish genus Pterygolichthys (Siluriformes: Loricariidae): annotated list of species, distributional summary, and assessment of impacts. Zool Stud 57:7. https://doi.org/10.6620/ZS.2018.57-07
Parker CT, Tindall BJ, Garrity GM (2019) International code of nomenclature of prokaryotes: prokaryotes code (2008 revision). Int J Syst Evol Microbiol 69:S1–S111. https://doi.org/10.1099/ijsem.0.000778
Pimm SL, Jenkins CN, Li BV (2018) How to protect half of Earth to ensure it protects sufficient biodiversity. Sci Adv 4:eaat2616. https://doi.org/10.1126/sciadv.aat2616
Pinder AC, Britton JR, Harrison AJ et al (2019) Mahseer (Tor spp.) fishes of the world: status, challenges and opportunities for conservation. Rev Fish Biol Fish 29:417–452. https://doi.org/10.1007/s11160-019-09566-y
Posa MRC, Wijedasa LS, Corlett RT (2010) Biodiversity and conservation of tropical peat swamp forests. BioScience 61:49–57. https://doi.org/10.1525/bio.2011.61.1.10
Poulsen AF, Poeu O, Viravong S et al (2002) Fish migrations of the lower Mekong River Basin: implications for development, planning and environmental management. MRC Technical Paper no. 8, Mekong River Commission, Phnom Penh, p 62
Purnama AA, Yolanda R (2016) Diversity of freshwater fish (Pisces) in Kumu River, Rokan Hulu Distinct, Riau Province, Indonesia. Aquac Aquar Conserv Legis 9:785–789
Radinger J, Wolter C (2014) Patterns and predictors of fish dispersal in rivers. Fish Fish 15:456–473. https://doi.org/10.1111/faf.12028
Raffaelli D (2004) How extinction patterns affect ecosystems. Science 306:1141–1142. https://doi.org/10.1126/science.1106365
Rico A, Phu TM, Satapornvanit K et al (2013) Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 412–413:231–243
Rimmer MA, Sugama K, Rakhmawati D et al (2013) A review and SWOT analysis of aquaculture development in Indonesia. Rev Aquac 5:255–279. https://doi.org/10.1111/raq.12017
Russ GR, Alcala AC, Maypa AP et al (2004) Marine reserve benefits local fisheries. Ecol Appl 14:597–606. https://doi.org/10.1890/03-5076
Satria A, Adhuri DS (2010) Pre-existing fisheries management systems in Indonesia, focusing on Lombok and Maluku. In: Ruddle K, Satria A (eds) Managing coastal and inland waters: pre-existing aquatic management systems in Southeast Asia. Springer, Dordrecht, pp 31–55. https://doi.org/10.1007/978-90-481-9555-8_2
Shimamura T (2016) An overview of tropical peat swamps. In: Mizuno K, Fujita MS, Kawai S (eds) Catastrophe and regeneration in Indonesia’s peatlands: ecology, economy and society, Kyoto CSEAS series on Asian studies, vol 15. NUS Press; Kyoto University Press, Singapore; Kyoto, pp 123–147
Siregar S, Putra RM, Sukendi (1994) Fauna ikan di perairan sekitar bukit Tigapuluh Seberida, Sumatera. In: Sandbukt O, Wiriadinata H (eds) Rain forest and resource management: proceeding of the NORINDRA seminar, Jakarta, May 1993. Indonesian Institute of Science, Jakarta, pp 67–70
Thornton TF, Scheer AM (2012) Collaborative engagement of local and traditional knowledge and science in marine environments: a review. Ecol Soc 17:8. https://doi.org/10.5751/ES-04714-170308
Tjakrawidjaja AH, Haryono (2000) Keanekaragaman jenis ikan di areal penambangan gambut Perawang dan sekitarnya, kabupaten Bengkalis-Riau. In: Sjafei DS et al (eds) Prosiding Seminar Nasional Keanekaragaman Hayati Ikan, Bogor, June 2000. Pusat Studi Ilmu Hayat Institut Pertanian Bogor, Bogor, pp 55–60
Trebilco R, Baum JK, Salomon AK et al (2012) Ecosystem ecology: size-based constraints on the pyramids of life. Trends Ecol Evol 28:423–431. https://doi.org/10.1016/j.tree.2013.03.008
Turland NJ, Wiersema JH, Barrie FR et al (eds) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159 [Online]. Koeltz Botanical Books, Glashütten. https://doi.org/10.12705/Code.2018
Waylen KA, Martin-Ortega J, Blackstock KL et al (2015) Can scenario-planning support community-based natural resource management? Experiences from three countries in Latin America. Ecol Soc 20:28. https://doi.org/10.5751/ES-07926-200428
Welcomme RL (2001) Inland fisheries: ecology and management. Fishing News Books, Oxford
Wilkinson CL, Chua KWJ, Fiala R et al (2021) Forest conservation to oil palm compresses food chain length in tropical streams. Ecology 102:e03199. https://doi.org/10.1002/ecy.3199
Yustina (2016) The impact of forest and peatland exploitation towards decreasing biodiversity of fishes in Rangau River, Riau-Indonesia. Int J Appl Bus Econ Res 14:1043–1055
Acknowledgements
We thank villagers in the Rantau Baru village for the warm understanding and the kind cooperation to our research. This research was supported by Research Institute for Humanity and Nature (RIHN: a constituent member of NIHU) Project No. 14200117. Figures 4.1 and 4.2, and Table 4.1 are reproduced from Nakagawa et al. (2021), Southeast Asian Studies Vol. 10, No. 3, under the permission of the Center for Southeast Asian Studies.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Nakagawa, H. (2023). Inferring Recent Changes in Fish Fauna in the Middle Reaches of the Kampar River: Survey Results from the Fishing Village of Rantau Baru. In: Okamoto, M., Osawa, T., Prasetyawan, W., Binawan, A. (eds) Local Governance of Peatland Restoration in Riau, Indonesia. Global Environmental Studies. Springer, Singapore. https://doi.org/10.1007/978-981-99-0902-5_4
Download citation
DOI: https://doi.org/10.1007/978-981-99-0902-5_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-0901-8
Online ISBN: 978-981-99-0902-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)