Abstract
The major global problems are the prevalence rate of Mycobacterium tuberculosis (Mtb) and processes of resistance against continuing therapy. The shortage of possible drug candidates and consumer recognition along with unhygienic procedures are the key explanations for MDR, TDR and XDR Mtb strains in rapid emergence. Mtb’s powerful molecular structure and drug resistance pathways, demands expertise to develop new anti-tuberculosis therapies. Eventually, the synthesis of modern genomic knowledge of drug resistance mechanisms in Mtb will offer a new path for combinatorial drug development and provide considerable support for highly successful anti-tubercular drugs.
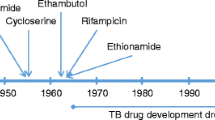
After a time of relative lack of interest in cell envelope targeting inhibitors, the immediate necessity of new therapeutic approaches has motivated renewed work towards a deeper understanding of the cell envelope, its biogenesis and function during Mtb’s stages of proliferation, survival and reactivation. As a result, new appealing goals for drugs were identified and followed, with varying effects, in the form of target-based scanning and other target-based strategies. The challenge of detecting compounds whose inhibitory action against filtered targets translated into entire Mtb cell activity prompted several field investigators to go back to cell-based screens. This strategy leads to several new groups of inhibitors being identified, and some of those are now in preclinical research.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM et al (2014) Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 58:470–480
Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR et al (2014) REMox TB consortium. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 371:1577–1587
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6:29–40
Sukuru SC, Jenkins JL, Beckwith RE, Scheiber J, Bender A, Mikhailov D et al (2009) Plate-based diversity selection based on empirical HTS data to enhance the number of hits and their chemical diversity. J Biomol Screen 14:690–699
Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129
https://www.nap.edu/read/9144/chapter/4 (Last assessed on 08 September 2020)
http://www.tbonline.info/diagnostics/ (Last assessed on 08 September 2020)
https://www.treatmentactiongroup.org/wp-content/uploads/2017/04/TB-Diagnostics-Guide.pdf (Last assessed on 08 September 2020)
Goswam A, Chakraborty U, Bhattacharya B, Pal NK (2016) Association of generation time with anti-tubercular drug(s) resistance pattern of Mycobacterium tuberculosis isolates among treatment failure pulmonary tuberculosis patients. Asian J Pharm Clin Res 9(1):258–261
Nguyen L (2016) Antibiotic resistance mechanisms in M. tuberculosis: an update. Arch Toxicol 90(7):1585–1604
Somoskovi A, Parsons LM, Salfinger M (2001) The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir Res 2(3):164–168
Timmins GS, Deretic V (2006) Mechanisms of action of isoniazid. Mol Microbiol 62:1220–1227
Sensi P (1983) History of the development of rifampin. Rev Infect Dis 5:S402–S406
Campbell EA, Korzheva N, Mustaev A et al (2001) Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104:901–912
Pourakbari B, Mamishi S, Mohammadzadeh M, Mahmoudi S (2016) First-line anti-tubercular drug resistance of Mycobacterium tuberculosis in IRAN: a systematic review. Front Microbiol 28:1139
Forbes M, Kuck NA, Peets EA (1962) Mode of action of ethambutol. J Bacteriol 84:1099–1103
Konno K, Feldmann FM, McDermott W (1967) Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am Rev Respir Dis 95:461–469
Zhang S, Che J, Shi W et al (2013) Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg Microbes Infect e34:2
Honore N, Cole ST (1994) Streptomycin resistance in mycobacteria. Antimicrob Agent Chemother 38:238–242
Dookie N, Rambaran S, Padayatchi N et al (2018) Evolution of drug resistance in Mycobacterium tuberculosis: a review on the molecular determinants of resistance and implications for personalized care. J Antimicrob Chemother 73:1138–1151
Yano T, Kassovska-Bratinova S, Teh JS, Winkler J, Sullivan K, Isaacs A, Schechter NM, Rubin H (2011) Reduction of clofazimine by mycobacterial type 2 NADH: quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem 286:10276–10287
Grosset JH, Tyagi S, Almeida DV, Converse PJ, Li SY, Ammerman NC et al (2013) Assessment of clofazimine activity in a second-line regimen for tuberculosis in mice. Am J Respir Crit Care Med 188:608–612
Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE III (2004) The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 279:40174–40184
Weinstein EA, Yano T, Li LS, Avarbock D, Avarbock A, Helm D, McColm AA, Duncan K, Lonsdale JT, Rubin H (2005) Inhibitors of type II NADH: menaquinone oxidoreductase represent a class of antitubercular drugs. Proc Natl Acad Sci U S A 102:4548–4553
Kristiansen JE, Dastidar SG, Palchoudhuri S, Roy DS, Das S, Hendricks O, Christensen JB (2015) Phenothiazines as a solution for multidrug resistant tuberculosis: from the origin to present. Int Microbiol 18:1–12
Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S et al (2013) Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157–1160
Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R et al (2007) Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol 3:323–324
Tantry SJ, Markad SD, Shinde V, Bhat J, Balakrishnan G, Gupta AK et al (2017) Discovery of imidazo[1,2-α]pyridine ethers and squaramides as selective and potent inhibitors of mycobacterial adenosine triphosphate (ATP) synthesis. J Med Chem 60:1379–1399
Zhang Y, Wade MM, Scorpio A, Zhang H, Sun Z (2003) Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother 52:790–795
Zhang Y, Shi W, Zhang W, Mitchison D (2014) Mechanisms of pyrazinamide action and resistance. Microbiol Spectr 2:MGM2-0023-2013
Li K, Schurig-Briccio LA, Feng X, Upadhyay A, Pujari V, Lechartier B et al (2014) Multi-target drug discovery for tuberculosis and other infectious diseases. J Med Chem 57:3126–3139
Reddy VM, Einck L, Andries K, Nacy CA (2010) In vitro interactions between new antitubercular drug candidates SQ109 and TMC207. Antimicrob Agents Chemother 54:2840–2846
Geetha VR (2014) MmpL3 a potential new target for development of novel anti-tuberculosis drugs. Expert Opin Ther Targets 18(3):247–256
Mary J, Michael RM, Patrick JB (2013) Progress in targeting cell envelope biogenesis in Mycobacterium tuberculosis. Future Microbiol 8(7):855–875
Ishizaki Y, Hayashi C, Inoue K, Igarashi M, Takahashi Y, Pujari V et al (2013) Inhibition of the first step in synthesis of the mycobacterial cell wall core, catalyzed by the GlcNAc-1-phosphate transferase WecA, by the novel caprazamycin derivative CPZEN-45. J Biol Chem 288:30309–30319
Hayashi T, Yamamoto O, Sasaki H, Kawaguchi A, Okazaki H (1983) Mechanism of action of the antibiotic thiolactomycin inhibition of fatty acid synthesis of Escherichia coli. Biochem Biophys Res Commun 115:1108–1113
Sergio S, Pirali G, White R, Parenti F (1975) Lipiarmycin, a new antibiotic from Actinoplanes III. Mechanism of action. J Antibiot (Tokyo) 28:543–549
Xie Y, Chen R, Si S, Sun C, Xu H (2007) A new Nucleosidyl-peptide antibiotic, Sansanmycin. J Antibiot 60:158–161
Lee H, Suh JW (2016) Anti-tuberculosis lead molecules from natural products targeting Mycobacterium tuberculosis ClpC1. J Ind Microbiol Biotechnol 43:205–212
Kling A, Lukat P, Almeida DV, Bauer A, Fontaine E, Sordello S et al (2015) Targeting DnaN for tuberculosis therapy using novel griselimycins. Science 348:1106–1112
Iwatsuki M, Uchida R, Takakusagi Y, Matsumoto A, Jiang C-L, Takahashi Y et al (2007) Lariatins, novel anti-mycobacterial peptides with a lasso structure, produced by Rhodococcus jostii K01-B0171. J Antibiot 60:357–363
Pruksakorn P, Arai M, Liu L, Moodley P, Jacobs WR Jr, Kobayashi M (2011) Action mechanism of trichoderin a, an anti-dormant mycobacterial aminolipopeptide from marine sponge-derived Trichoderma sp. Biol Pharm Bull 34:1287–1290
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, L., Dua, K., Kumar, S., Kumar, D., Majhi, S. (2021). Targeting Molecular and Cellular Mechanisms in Tuberculosis. In: Dua, K., Löbenberg, R., Malheiros Luzo, Â.C., Shukla, S., Satija, S. (eds) Targeting Cellular Signalling Pathways in Lung Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-33-6827-9_14
Download citation
DOI: https://doi.org/10.1007/978-981-33-6827-9_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6826-2
Online ISBN: 978-981-33-6827-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)