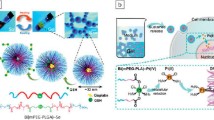
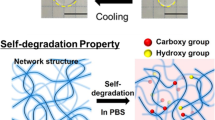
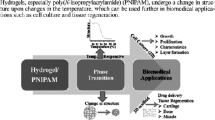
Abstract
Poly(organophosphazenes) as a new type of biodegradable polymers have been exploited as carriers for various drug delivery systems due to versatility of molecular structures and easily modulated physico-chemical properties. Thus, biodegradable thermogelling poly(organophosphazenes) are expected to be very promising biomaterials as injectable systems with minimal surgical intervention for drug delivery and tissue engineering applications. The key advantage of thermosensitive hydrogels based on poly(organophosphazenes) over other thermosensitive polymers is the ease of tuning the hydrogel properties by use of different compositions of side groups or through variations in co-substituent ratios. A variety of poly(organophosphazene) thermogels have been developed with desirable hydrophobic-hydrophilic balance, controllable degradation rate and suitable mechanical properties with respect to different applications. This chapter covers a comprehensive summary of the recent developments in this field of study, including polymer design, property assessment and potential biomedical applications.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hoffman, A.S.: Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 54(1), 3–12 (2002). doi:10.1016/s0169-409x(01)00239-3
Bromberg, L.E., Ron, E.S.: Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv. Drug Deliv. Rev. 31(3), 197–221 (1998). doi:10.1016/s0169-409x(97)00121-x
Gil, E.S., Hudson, S.M.: Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 29(12), 1173–1222 (2004). doi:10.1016/j.progpolymsci.2004.08.003
Jeong, B., Kim, S.W., Bae, Y.H.: Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 54(1), 37–51 (2002). doi:10.1016/s0169-409x(01)00242-3
Miyata, T., Uragami, T., Nakamae, K.: Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 54(1), 79–98 (2002). doi:10.1016/s0169-409x(01)00241-1
Schmaljohann, D.: Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 58(15), 1655–1670 (2006). doi:10.1016/j.addr.2006.09.020
Kwon, I.K., Matsuda, T.: Photo-iniferter-based thermoresponsive block copolymers composed of poly(ethylene glycol) and poly(N-isopropylacrylamide) and chondrocyte immobilization. Biomaterials 27(7), 986–995 (2006). doi:10.1016/j.biomaterials.2005.07.038
Li, C.M., Buurma, N.J., Haq, I., Turner, C., Armes, S.P., Castelletto, V., Hamley, I.W., Lewis, A.L.: Synthesis and characterization of biocompatible, thermoresponsive ABC and ABA triblock copolymer gelators. Langmuir 21(24), 11026–11033 (2005). doi:10.1021/la0515672
Li, C.M., Tang, Y.Q., Armes, S.P., Morris, C.J., Rose, S.F., Lloyd, A.W., Lewis, A.L.: Synthesis and characterization of biocompatible thermo-responsive gelators based on ABA triblock copolymers. Biomacromolecules 6(2), 994–999 (2005). doi:10.1021/bm049331k
Lin, H.H., Cheng, Y.L.: In situ thermoreversible gelation of block and star copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) of varying architectures. Macromolecules 34(11), 3710–3715 (2001). doi:10.1021/ma001852m
Tang, T., Castelletto, V., Parras, P., Hamley, I.W., King, S.M., Roy, D., Perrier, S., Hoogenboom, R., Schubert, U.S.: Thermo-responsive poly(methyl methacrylate)-block-poly(N-isopropylacrylamide) block copolymers synthesized by RAFT polymerization: micellization and gelation. Macromol. Chem. Phys. 207(19), 1718–1726 (2006). doi:10.1002/macp.200600309
Glatter, O., Scherf, G., Schillen, K., Brown, W.: Characterization of a poly(ethylene oxide) poly(propylene oxide) triblock copolymer (eo(27)-po39-eo(27)) in aqueous-solution. Macromolecules 27(21), 6046–6054 (1994). doi:10.1021/ma00099a017
Jorgensen, E.B., Hvidt, S., Brown, W., Schillen, K.: Effects of salts on the micellization and gelation of a triblock copolymer studied by rheology and light scattering. Macromolecules 30(8), 2355–2364 (1997). doi:10.1021/ma9616322
Mortensen, K., Brown, W.: Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous-solution - the influence of relative block size. Macromolecules 26(16), 4128–4135 (1993). doi:10.1021/ma00068a010
Song, M.J., Lee, D.S., Ahn, J.H., Kim, D.J., Kim, S.C.: Dielectric behavior during sol-gel transition of PEO-PPO-PEO triblock copolymer aqueous solution. Polym. Bull. 43(6), 497–504 (2000). doi:10.1007/s002890050007
Sosnik, A., Cohn, D.: Reverse thermo-responsive poly(ethylene oxide) and poly(propylene oxide) multiblock copolymers. Biomaterials 26(4), 349–357 (2005). doi:10.1016/j.biomaterials.2004.02.041
Wanka, G., Hoffmann, H., Ulbricht, W.: The aggregation behavior of poly-(oxyethylene)-poly-(oxypropylene)-poly-(oxyethylene)-block-copolyme rs in aqueous-solution. Colloid Polym. Sci. 268(2), 101–117 (1990). doi:10.1007/bf01513189
Bae, S.J., Suh, J.M., Sohn, Y.S., Bae, Y.H., Kim, S.W., Jeong, B.: Thermogelling poly(caprolactone-b-ethylene glycol-b-caprolactone) aqueous solutions. Macromolecules 38(12), 5260–5265 (2005). doi:10.1021/ma050489m
Fujiwara, T., Mukose, T., Yamaoka, T., Yamane, H., Sakurai, S., Kimura, Y.: Novel thermo-responsive formation of a hydrogel by stereo-complexation between PLLA-PEG-PLLA and PDLA-PEG-PDLA block copolymers. Macromol. Biosci. 1(5), 204–208 (2001). doi:10.1002/1616-5195(20010701)1:5<204:aid-mabi204>3.0.co;2-h
Jeong, B., Bae, Y.H., Lee, D.S., Kim, S.W.: Biodegradable block copolymers as injectable drug-delivery systems. Nature 388(6645), 860–862 (1997)
Lee, J., Bae, Y.H., Sohn, Y.S., Jeong, B.: Thermogelling aqueous solutions of alternating multiblock copolymers of poly(L-lactic acid) and poly(ethylene glycol). Biomacromolecules 7(6), 1729–1734 (2006). doi:10.1021/bm0600062
Li, F., Li, S.M., El Ghzaoui, A., Nouailhas, H., Zhuo, R.X.: Synthesis and gelation properties of PEG-PLA-PEG triblock copolymers obtained by coupling monohydroxylated PEG-PLA with adipoyl chloride. Langmuir 23(5), 2778–2783 (2007). doi:10.1021/la0629025
Loh, X.J., Goh, S.H., Li, J.: New biodegradable thermogelling copolymers having very low gelation concentrations. Biomacromolecules 8(2), 585–593 (2007). doi:10.1021/bm0607933
Jeong, B., Gutowska, A.: Lessons from nature: stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 20(7), 305–311 (2002). doi:10.1016/s0167-7799(02)01962-5
Loh, X.J., Li, J.: Biodegradable thermosensitive copolymer hydrogels for drug delivery. Expert Opin. Ther. Pat. 17(8), 965–977 (2007). doi:10.1517/13543776.17.8.965
Allcock, H.R.: Polyphosphazenes as New Biomedical and Bioactive Materials. In: Langer, R., Chasin, M. (eds.) Biodegradable Polymers as Drug Delivery System, pp. 163–193. Marcel Dekker, New York (1990)
Allcock, H.R.: Rational design and synthesis of polyphosphazenes for tissue engineering. In: Atala, A., Lanza, R. (eds.) Methods of Tissue Engineering, pp. 597–607. Academic Press, New York (2001)
Scopelianos, A.G.: Polyphosphazenes as new biomaterials. In: Shalaby, S. (ed.) Biomedical Polymers, pp. 153–171. Hanser Publishers, Munich and New York (1994)
Allcock, H.R., Kugel, R.L.: Synthesis of high polymeric alkoxy- and aryloxphosphonitriles. J. Am. Chem. Soc. 87(18), 4216 (1965). doi:10.1021/ja01096a056
Allcock, H.R., Kugel, R.L.: Phosphonitrilic compounds. 7. High molecular weight poly(diaminophosphazenes). Inorg. Chem. 5(10), 1716 (1966). doi:10.1021/ic50044a017
Allcock, H.R., Kugel, R.L., Valan, K.J.: Phosphonitrilic compounds.6. High molecular weight poly(alkoxy- and arylox-phosphazenes). Inorg. Chem. 5(10), 1709 (1966). doi:10.1021/ic50044a016
Gettleman, L., Farris, C.L., Rawls, H.R., LeBouef, R.: Soft and firm fluoroalkoxyphosphazene rubber denture linear for a composite denture (1984)
Wade, C.W.R., Gourlay, S., Rice, R., Hegyeli, A., Singler, R., White, J.: Biocompatibility of Eight Poly(organophosphazenes). In: Carraher, C.E., Sheats, J.E., Pittman, C.U. (eds.) Organometallic Polymers, p. 298. Academic Press, New York (1978)
Welle, A., Grunze, M., Tur, D.: Plasma protein adsorption and platelet adhesion on poly bis(trifluoroethoxy)phosphazene and reference material surfaces. J. Colloid Interface Sci. 197(2), 263–274 (1998). doi:10.1006/jcis.1997.5238
Bostman, O., Pihlajamaki, H.: Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials 21(24), 2615–2621 (2000). doi:10.1016/s0142-9612(00)00129-0
Allcock, H.R., Fuller, T.J., Mack, D.P., Matsumura, K., Smeltz, K.M.: Synthesis of poly [(amino acid alkyl ester)phosphazenes]. Macromolecules 10(4), 824–830 (1977). doi:10.1021/ma60058a020
Allcock, H.R., Fuller, T.J., Matsumura, K.: Hydrolysis pathways for aminophosphazenes. Inorg. Chem. 21(2), 515–521 (1982). doi:10.1021/ic00132a009
Allcock, H.R., Pucher, S.R., Scopelianos, A.G.: Poly (amino-acid-ester)phosphazenes—synthesis, crystallinity, and hydrolytic sensitivity in solution and the solid-state. Macromolecules 27(5), 1071–1075 (1994). doi:10.1021/ma00083a001
Allcock, H.R., Pucher, S.R., Scopelianos, A.G.: Poly (amino acid ester)phosphazenes as substrates for the controlled-release of small molecules. Biomaterials 15(8), 563–569 (1994). doi:10.1016/0142-9612(94)90205-4
Hindenlang, M.D., Soudakov, A.A., Imler, G.H., Laurencin, C.T., Nair, L.S., Allcock, H.R.: Iodine-containing radio-opaque polyphosphazenes. Polym. Chem. 1(9), 1467–1474 (2010). doi:10.1039/c0py00126k
Deng, M., Nair, L.S., Nukavarapu, S.R., Jiang, T., Kanner, W.A., Li, X.D., Kumbar, S.G., Weikel, A.L., Krogman, N.R., Allcock, H.R., Laurencin, C.T.: Dipeptide-based polyphosphazene and polyester blends for bone tissue engineering. Biomaterials 31(18), 4898–4908 (2010). doi:10.1016/j.biomaterials.2010.02.058
Deng, M., Kumbar, S.G., Nair, L.S., Weikel, A.L., Allcock, H.R., Laurencin, C.T.: Biomimetic structures: biological implications of dipeptide-substituted polyphosphazene-polyester blend nanofiber matrices for load-bearing bone regeneration. Adv. Funct. Mater. 21(14), 2641–2651 (2011). doi:10.1002/adfm.201100275
Kim, J.I., Jun, Y.J., Seong, J.Y., Jun, M.J., Sohn, Y.S.: Synthesis and characterization of nanosized poly (organophosphazenes) with methoxy-poly(ethylene glycol) and dipeptide ethyl esters as side groups. Polymer 45(21), 7083–7089 (2004). doi:10.1016/j.polymer.2004.08.031
Weikel, A.L., Krogman, N.R., Nguyen, N.Q., Nair, L.S., Laurencin, C.T., Allcock, H.R.: Polyphosphazenes that contain dipeptide side groups: synthesis, characterization, and sensitivity to hydrolysis. Macromolecules 42(3), 636–639 (2009). doi:10.1021/ma802423c
Crommen, J.H.L., Schacht, E.H., Mense, E.H.G.: Biodegradable polymers.1. Synthesis of hydrolysis-sensitive poly (organo)phosphazenes. Biomaterials 13(8), 511–520 (1992). doi:10.1016/0142-9612(92)90102-t
Crommen, J.H.L., Schacht, E.H., Mense, E.H.G.: Biodegradable polymers. 2. Degradation characteristics of hydrolysis-sensitive poly (organo)phosphazenes. Biomaterials 13(9), 601–611 (1992). doi:10.1016/0142-9612(92)90028-m
Schacht, E., Vandorpe, J., Dejardin, S., Lemmouchi, Y., Seymour, L.: Biomedical applications of degradable polyphosphazenes. Biotechnol. Bioeng. 52(1), 102–108 (1996). doi:10.1002/(sici)1097-0290(19961005)52:1<102:aid-bit10>3.0.co;2-q
Allcock, H.R., Scopelianos, A.G.: Synthesis of sugar-substituted cyclic and polymeric phosphazenes and their oxidation, reduction and acetylation reactions. Macromolecules 16(5), 715–719 (1983). doi:10.1021/ma00239a001
Allcock, H.R., Singh, A., Ambrosio, A.M.A., Laredo, W.R.: Tyrosine-bearing polyphosphazenes. Biomacromolecules 4(6), 1646–1653 (2003). doi:10.1021/bm030027i
Krogman, N.R., Hindenlang, M.D., Nair, L.S., Laurencin, C.T., Allcock, H.R.: Synthesis of purine- and pyrimidine-containing polyphosphazenes: physical properties and hydrolytic behavior. Macromolecules 41(22), 8467–8472 (2008). doi:10.1021/ma8008417
Krogman, N.R., Weikel, A.L., Nguyen, N.Q., Nair, L.S., Laurencin, C.T., Allcock, H.R.: Synthesis and characterization of new biomedical polymers: serine- and threonine-containing polyphosphazenes and poly(L-lactic acid) grafted copolymers. Macromolecules 41(21), 7824–7828 (2008). doi:10.1021/ma801961m
Morozowich, N.L., Weikel, A.L., Nichol, J.L., Chen, C., Nair, L.S., Laurencin, C.T., Allcock, H.R.: Polyphosphazenes containing vitamin substituents: synthesis, characterization, and hydrolytic sensitivity. Macromolecules 44(6), 1355–1364 (2011). doi:10.1021/ma1027406
Weikel, A.L., Owens, S.G., Fushimi, T., Allcock, H.R.: Synthesis and characterization of methionine- and cysteine-substituted phosphazenes. Macromolecules 43(12), 5205–5210 (2010). doi:10.1021/ma1007013
Weikel, A.L., Owens, S.G., Morozowich, N.L., Deng, M., Nair, L.S., Laurencin, C.T., Allcock, H.R.: Miscibility of choline-substituted polyphosphazenes with PLGA and osteoblast activity on resulting blends. Biomaterials 31(33), 8507–8515 (2010). doi:10.1016/j.biomaterials.2010.07.094
Ahn, S., Ahn, S.W., Song, S.C.: Thermosensitive amphiphilic polyphosphazenes and their interaction with ionic surfactants. Colloids Surf. A 330(2–3), 184–192 (2008). doi:10.1016/j.colsurfa.2008.07.059
Cho, Y.W., An, S.W., Song, S.C.: Effect of inorganic and organic salts on the thermogelling behavior of poly(organophosphazenes). Macromol. Chem. Phys. 207(4), 412–418 (2006). doi:10.1002/macp.200500483
Kang, G.D., Heo, J.Y., Jung, S.B., Song, S.C.: Controlling the thermosensitive gelation properties of poly(organophosphazenes) by blending. Macromol. Rapid Commun. 26(20), 1615–1618 (2005). doi:10.1002/marc.200500472
Lee, S.B., Song, S.C., Jin, J.I., Sohn, Y.S.: A new class of biodegradable thermosensitive polymers. 2. Hydrolytic properties and salt effect on the lower critical solution temperature of poly(organophosphazenes) with methoxypoly(ethylene glycol) and amino acid esters as side groups. Macromolecules 32(23), 7820–7827 (1999). doi:10.1021/ma990645n
Lee, S.B., Song, S.C., Jin, J.I., Sohn, Y.S.: Thermosensitive cyclotriphosphazenes. J. Am. Chem. Soc. 122(34), 8315–8316 (2000). doi:10.1021/ja001542j
Lee, B.H., Lee, Y.M., Sohn, Y.S., Song, S.C.: Thermosensitive and hydrolysis-sensitive poly(organophosphazenes). Polym. Int. 51(7), 658–660 (2002). doi:10.1002/pi.1019
Lee, B.H., Lee, Y.M., Sohn, Y.S., Song, S.C.: A thermosensitive poly(organophosphazene) gel. Macromolecules 35(10), 3876–3879 (2002). doi:10.1021/ma012093q
Song, S.C., Lee, S.B., Jin, J.I., Sohn, Y.S.: A new class of biodegradable thermosensitive polymers. I. Synthesis and characterization of poly(organophosphazenes) with methoxy-poly(ethylene glycol) and amino acid esters as side groups. Macromolecules 32(7), 2188–2193 (1999). doi:10.1021/ma981190p
Chun, C., Lee, S.M., Kim, C.W., Hong, K.Y., Kim, S.Y., Yang, H.K., Song, S.C.: Doxorubicin-polyphosphazene conjugate hydrogels for locally controlled delivery of cancer therapeutics. Biomaterials 30(27), 4752–4762 (2009). doi:10.1016/j.biomaterials.2009.05.031
Chun, C., Lee, S.M., Kim, S.Y., Yang, H.K., Song, S.C.: Thermosensitive poly(organophosphazene)-paclitaxel conjugate gels for antitumor applications. Biomaterials 30(12), 2349–2360 (2009). doi:10.1016/j.biomaterials.2008.12.083
Chun, C., Lim, H.J., Hong, K.Y., Park, K.H., Song, S.C.: The use of injectable, thermosensitive poly(organophosphazene)-RGD conjugates for the enhancement of mesenchymal stem cell osteogenic differentiation. Biomaterials 30(31), 6295–6308 (2009). doi:10.1016/j.biomaterials.2009.08.011
Lee, S.M., Chun, C.J., Heo, J.Y., Song, S.C.: Injectable and Thermosensitive Poly(organophosphazene) Hydrogels for a 5-Fluorouracil Delivery. J. Appl. Polym. Sci. 113(6), 3831–3839 (2009). doi:10.1002/app.30397
Park, M.R., Chun, C., Ahn, S.W., Ki, M.H., Cho, C.S., Song, S.C.: Cationic and thermosensitive protamine conjugated gels for enhancing sustained human growth hormone delivery. Biomaterials 31(6), 1349–1359 (2010). doi:10.1016/j.biomaterials.2009.10.022
Lee, B.H., Song, S.C.: Synthesis and characterization of biodegradable thermosensitive poly(organophosphazene) gels. Macromolecules 37(12), 4533–4537 (2004). doi:10.1021/ma0305838
Park, M.R., Chun, C.J., Ahn, S.W., Ki, M.H., Cho, C.S., Song, S.C.: Sustained delivery of human growth hormone using a polyelectrolyte complex-loaded thermosensitive polyphosphazene hydrogel. J. Controlled Release 147(3), 359–367 (2010). doi:10.1016/j.jconrel.2010.07.126
Seong, J.Y., Jun, Y.J., Jeong, B., Sohn, Y.S.: New thermogelling poly (organophosphazenes) with methoxypoly(ethylene glycol) and oligopeptide as side groups. Polymer 46(14), 5075–5081 (2005). doi:10.1016/j.polymer.2005.04.024
Ahn, S., Ahn, S.W., Song, S.C.: Polymer structure-dependent ion interaction studied by amphiphilic nonionic poly(organophosphazenes). J. Polym. Sci., Part B: Polym. Phys. 46(19), 2022–2034 (2008). doi:10.1002/polb.21537
Jiang, H.L., Kim, Y.K., Lee, S.M., Park, M.R., Kim, E.M., Jin, Y.M., Arote, R., Jeong, H.J., Song, S.C., Cho, M.H., Cho, C.S.: Galactosylated chitosan-g-PEI/DNA complexes-loaded poly(organophosphazene) hydrogel as a hepatocyte targeting gene delivery system. Arch. Pharm. Res. 33(4), 551–556 (2010). doi:10.1007/s12272-010-0409-9
Park, K.H., Song, S.C.: A thermo-sensitive poly(organophosphazene) hydrogel used as an extracellular matrix for artificial pancreas. J. Biomater. Sci. Polym. Ed. 16(11), 1421–1431 (2005). doi:10.1163/156856205774472272
Park, M.R., Cho, C.S., Song, S.C.: In vitro and in vivo degradation behaviors of thermosensitive poly(organophosphazene) hydrogels. Polym. Degrad. Stab. 95(6), 935–944 (2010). doi:10.1016/j.polymdegradstab.2010.03.024
Ahn, S., Ahn, S.W., Song, S.C.: Thermothickening modification of the poly(ethylene glycol) and amino acid ester grafted polyphosphazenes by monomethyl end-capped poly(ethylene glycol) addition. Colloids Surf. A 333(1–3), 82–90 (2009). doi:10.1016/j.colsurfa.2008.09.045
Al-Abd, A.M., Hong, K.Y., Song, S.C., Kuh, H.J.: Pharmacokinetics of doxorubicin after intratumoral injection using a thermosensitive hydrogel in tumor-bearing mice. J. Controlled Release 142(1), 101–107 (2010). doi:10.1016/j.jconrel.2009.10.003
Cho, J.K., Hong, K.Y., Park, J.W., Yang, H.K., Song, S.C.: Injectable delivery system of 2-methoxyestradiol for breast cancer therapy using biodegradable thermosensitive poly(organophosphazene) hydrogel. J. Drug Target. 19(4), 270–280 (2011). doi:10.3109/1061186x.2010.499461
Cho, J.K., Park, J.W., Song, S.C.: Injectable and biodegradable poly(organophosphazene) gel containing silibinin: its physicochemical properties and anticancer activity. J. Pharm. Sci. 101(7), 2382–2391 (2012). doi:10.1002/jps.23137
Il Kim, J., Kim, B., Chun, C., Lee, S.H., Song, S.C.: MRI-monitored long-term therapeutic hydrogel system for brain tumors without surgical resection. Biomaterials 33(19), 4836–4842 (2012). doi:10.1016/j.biomaterials.2012.03.048
Kang, G.D., Cheon, S.H., Song, S.C.: Controlled release of doxorubicin from thermosensitive poly(organophosphazene) hydrogels. Int. J. Pharm. 319(1–2), 29–36 (2006). doi:10.1016/j.ijpharm.2006.03.032
Kim, J.I., Chun, C., Kim, B., Hong, J.M., Cho, J.K., Lee, S.H., Song, S.C.: Thermosensitive/magnetic poly(organophosphazene) hydrogel as a long-term magnetic resonance contrast platform. Biomaterials 33(1), 218–224 (2012). doi:10.1016/j.biomaterials.2011.09.033
Kim, J.I., Lee, B.S., Chun, C., Cho, J.K., Kim, S.Y., Song, S.C.: Long-term theranostic hydrogel system for solid tumors. Biomaterials 33(7), 2251–2259 (2012). doi:10.1016/j.biomaterials.2011.11.083
Kim, J.H., Lee, J.H., Kim, K.S., Na, K., Song, S.C., Lee, J., Kuh, H.J.: Intratumoral delivery of paclitaxel using a thermosensitive hydrogel in human tumor xenografts. Arch. Pharm. Res. 36(1), 94–101 (2013). doi:10.1007/s12272-013-0013-x
Kwak, M.K., Hur, K., Yu, J.E., Han, T.S., Yanagihara, K., Kim, W.H., Lee, S.M., Song, S.C., Yang, H.K.: Suppression of in vivo tumor growth by using a biodegradable thermosensitive hydrogel polymer containing chemotherapeutic agent. Invest. New Drugs 28(3), 284–290 (2010). doi:10.1007/s10637-009-9253-5
Park, M.R., Seo, B.B., Song, S.C.: Dual ionic interaction system based on polyelectrolyte complex and ionic, injectable, and thermosensitive hydrogel for sustained release of human growth hormone. Biomaterials 34(4), 1327–1336 (2013). doi:10.1016/j.biomaterials.2012.10.033
Seo, B.B., Park, M.R., Chun, C., Lee, J.Y., Song, S.C.: The biological efficiency and bioavailability of human growth hormone delivered using injectable, ionic, thermosensitive poly(organophosphazene)-polyethylenimine conjugate hydrogels. Biomaterials 32(32), 8271–8280 (2011). doi:10.1016/j.biomaterials.2011.07.033
Yu, J., Lee, H.J., Hur, K., Kwak, M.K., Han, T.S., Kim, W.H., Song, S.C., Yanagihara, K., Yang, H.K.: The antitumor effect of a thermosensitive polymeric hydrogel containing paclitaxel in a peritoneal carcinomatosis model. Invest. New Drugs 30(1), 1–7 (2012). doi:10.1007/s10637-010-9499-y
Ahn, S., Monge, E.C., Song, S.C.: Ion and pH effect on the lower critical solution temperature phase behavior in neutral and acidic poly(organophosphazene) counterparts. Langmuir 25(4), 2407–2418 (2009). doi:10.1021/la802815u
Cho, J.K., Lee, S.M., Kim, C.W., Song, S.C.: Synthesis and characterization of biodegradable thermosensitive neutral and acidic poly(organophosphazene) gels bearing carboxylic acid group. J. Polym. Res. 18(4), 701–713 (2011). doi:10.1007/s10965-010-9466-5
Lee, B.B., Song, S.C.: Synthesis and characterization of thermosensitive poly(organophosphazene) gels with an amino functional group. J. Appl. Polym. Sci. 120(2), 998–1005 (2011). doi:10.1002/app.33181
Allcock, H.R.: Chemistry and applications of polyphosphazenes. Wiley, New York (2003)
Chen, C., Liu, X., Tian, Z.C., Allcock, H.R.: Trichloroethoxy-substituted polyphosphazenes: synthesis, characterization, and properties. Macromolecules 45(22), 9085–9091 (2012). doi:10.1021/ma301822m
Liu, X., Breon, J.P., Chen, C., Allcock, H.R.: Substituent Exchange Reactions of Linear Oligomeric Aryloxyphosphazenes with Sodium 2,2,2-Trifluoroethoxide. Inorg. Chem. 51(21), 11910–11916 (2012). doi:10.1021/ic301808v
Liu, X., Breon, J.P., Chen, C., Allcock, H.R.: Substituent exchange reactions with high polymeric organophosphazenes. Macromolecules 45(22), 9100–9109 (2012). doi:10.1021/ma302087a
Liu, X., Tian, Z.C., Chen, C., Allcock, H.R.: Synthesis and Characterization of Brush-Shaped Hybrid Inorganic/Organic Polymers Based on Polyphosphazenes. Macromolecules 45(3), 1417–1426 (2012). doi:10.1021/ma202587z
Tian, Z.C., Liu, X., Chen, C., Allcock, H.R.: Synthesis and micellar behavior of novel amphiphilic poly bis(trifluoroethoxy)phosphazene -co-poly (dimethylamino)ethyl methacrylate block copolymers. Macromolecules 45(5), 2502–2508 (2012). doi:10.1021/ma300139z
Chen, C., Hess, A.R., Jones, A.R., Liu, X., Barber, G.D., Mallouk, T.E., Allcock, H.R.: Synthesis of new polyelectrolytes via backbone quaternization of poly(aryloxy- and alkoxyphosphazenes) and their small molecule counterparts. Macromolecules 45(3), 1182–1189 (2012). doi:10.1021/ma202619j
Liu, X., Zhang, H., Tian, Z.C., Sen, A., Allcock, H.R.: Preparation of quaternized organic-inorganic hybrid brush polyphosphazene-co-poly 2-(dimethylamino)ethyl methacrylate electrospun fibers and their antibacterial properties. Polym. Chem. 3(8), 2082–2091 (2012). doi:10.1039/c2py20170d
Allcock, H.R., Morozowich, N.L.: Bioerodible polyphosphazenes and their medical potential. Polymer Chemistry 3(3), 578–590 (2012). doi:10.1039/c1py00468a
Rose, S.H.: Synthesis of phosphonitrilic fluoroelastomers. Journal of Polymer Science Part B-Polymer Letters 6(12PB), 837 (1968). doi:10.1002/pol.1968.110061203
Tate, D.P.: Polyphosphazene elastomers. J. Polym. Sci., Part C: Polym. Symp. 48, 33–45 (1974)
Liu, X., Tian, Z.C., Chen, C., Allcock, H.R.: UV-cleavable unimolecular micelles: synthesis and characterization toward photocontrolled drug release carriers. Polym. Chem. 4(4), 1115–1125 (2013). doi:10.1039/c2py20825c
Cho, Y.W., Lee, J.R., Song, S.C.: Novel thermosensitive 5-fluorouracil-cyclotriphosphazene conjugates: synthesis, thermosensitivity, degradability, and in vitro antitumor activity. Bioconjug. Chem. 16(6), 1529–1535 (2005). doi:10.1021/bc049697u
Cho, Y.W., Choi, M., Lee, K., Song, S.C.: Cyclotriphosphazene-Pt-DACH conjugates with dipeptide spacers for drug delivery systems. J. Bioact. Compatible Polym. 25(3), 274–291 (2010). doi:10.1177/0883911509356377
Lee, S.B., Song, S.C., Jin, J.I., Sohn, Y.S.: Structural and thermosensitive properties of cyclotriphosphazenes with poly(ethylene glycol) and amino acid esters as side groups. Macromolecules 34(21), 7565–7569 (2001). doi:10.1021/ma010648b
Song, S.C., Lee, S.B., Lee, B.H., Ha, H.W., Lee, K.T., Sohn, Y.S.: Synthesis and antitumor activity of novel thermosensitive platinum(II)-cyclotriphosphazene conjugates. J. Controlled Release 90(3), 303–311 (2003). doi:10.1016/s0168-3659(03)00199-8
Allcock, H.R., Dudley, G.K.: Lower critical solubility temperature study of alkyl ether based polyphosphazenes. Macromolecules 29(4), 1313–1319 (1996). doi:10.1021/ma951129+
Allcock, H.R., Pucher, S.R., Turner, M.L., Fitzpatrick, R.J.: Poly(organophosphazenes) with poly(alkyl ether) side groups—a study of their water solubility and the swelling characteristics of their hydrogels. Macromolecules 25(21), 5573–5577 (1992). doi:10.1021/ma00047a002
Allcock, H.R., Pucher, S.R., Visscher, K.B.: Activity of urea amidohydrolase immobilized within poly di(methoxyethoxyethoxy)phosphazene hydrogels. Biomaterials 15(7), 502–506 (1994). doi:10.1016/0142-9612(94)90015-9
Zhang, J.X., Qiu, L.Y., Zhu, K.J., Jin, Y.: Thermosensitive micelles self-assembled by novel N-isopropylacrylamide oligomer grafted polyphosphazene. Macromol. Rapid Commun. 25(17), 1563–1567 (2004). doi:10.1002/marc.200400180
Zhang, J.X., Qiu, L.Y., Wu, X.L., Jin, Y., Zu, K.J.: Temperature-triggered nanosphere formation through self-assembly of amphiphilic polyphosphazene. Macromol. Chem. Phys. 207(14), 1289–1296 (2006). doi:10.1002/macp.200600139
Zhang, R.X., Li, X.J., Qiu, L.Y., Li, X.H., Yan, M.Q., Jin, Y., Zhu, K.J.: Indomethacin-loaded polymeric nanocarriers based on amphiphilic polyphosphazenes with poly (N-isopropylacrylamide) and ethyl tryptophan as side groups: Preparation, in vitro and in vivo evaluation. J. Controlled Release 116(3), 322–329 (2006). doi:10.1016/j.jconrel.2006.09.013
Lee, S.B., Song, S.C.: Hydrolysis-improved thermosensitive polyorganophosphazenes with alpha-amino-omega-methoxy-poly(ethylene glycol) and amino acid esters as side groups. Polym. Int. 54(9), 1225–1232 (2005). doi:10.1002/pi.1702
Lee, S.B., Song, S.C., Jin, J.I., Sohn, Y.S.: Surfactant effect on the lower critical solution temperature of poly(organophosphazenes) with methoxy-poly(ethylene glycol) and amino acid esters as side groups. Colloid Polym. Sci. 278(11), 1097–1102 (2000). doi:10.1007/s003960000368
Lee, S.B., Song, S.C., Jin, J.I., Sohn, Y.S.: Solvent effect on the lower critical solution temperature of biodegradable thermosensitive poly(organophosphazenes). Polym. Bull. 45(4–5), 389–396 (2000). doi:10.1007/s002890070012
Allcock, H.R., Fuller, T.J.: Phosphazene high polymers with steroidal side groups. Macromolecules 13(6), 1338–1345 (1980). doi:10.1021/ma60078a003
Allcock, H.R., Pucher, S.R.: Polyphosphazenes with glucosyl and methylamino, trifluoroethoxy, phenoxy, or (methoxyethoxy)ethoxy side groups. Macromolecules 24(1), 23–34 (1991). doi:10.1021/ma00001a005
Andrianov, A.K., Marin, A.: Degradation of polyaminophosphazenes: effects of hydrolytic environment and polymer processing. Biomacromolecules 7(5), 1581–1586 (2006). doi:10.1021/bm050959k
Andrianov, A.K., Marin, A., Peterson, P.: Water-soluble biodegradable polyphosphazenes containing N-ethylpyrrolidone groups. Macromolecules 38(19), 7972–7976 (2005). doi:10.1021/ma0509309
Crommen, J., Vandorpe, J., Schacht, E.: Degradable polyphosphazenes for biomedical applications. J. Controlled Release 24(1–3), 167–180 (1993). doi:10.1016/0168-3659(93)90176-6
Cui, Y.J., Zhao, M., Tang, X.Z., Luo, Y.P.: Novel micro-crosslinked poly(organophosphazenes) with improved mechanical properties and controllable degradation rate as potential biodegradable matrix. Biomaterials 25(3), 451–457 (2004). doi:10.1016/s0142-9612(03)00532-5
Qiu, L.Y., Zhu, K.J.: Novel biodegradable polyphosphazenes containing glycine ethyl ester and benzyl ester of amino acethydroxamic acid as cosubstituents: Syntheses, characterization, and degradation properties. J. Appl. Polym. Sci. 77(13), 2987–2995 (2000). doi:10.1002/1097-4628(20000923)77:13<2987:aid-app24>3.0.co;2-f
Singh, A., Krogman, N.R., Sethuraman, S., Nair, L.S., Sturgeon, J.L., Brown, P.W., Laurencin, C.T., Allcock, H.R.: Effect of side group chemistry on the properties of biodegradable L-alanine cosubstituted polyphosphazenes. Biomacromolecules 7(3), 914–918 (2006). doi:10.1021/bm050752r
Tian, Z.C., Zhang, Y.F., Liu, X., Chen, C., Guiltinan, M.J., Allcock, H.R.: Biodegradable polyphosphazenes containing antibiotics: synthesis, characterization, and hydrolytic release behavior. Polymer Chemistry 4(6), 1826–1835 (2013). doi:10.1039/c2py21064a
Yuan, W.Z., Song, Q., Zhu, L., Huang, X.B., Zheng, S.X., Tang, X.Z.: Asymmetric penta-armed poly(epsilon-caprolactone)s with short-chain phosphazene core: synthesis, characterization, and in vitro degradation. Polym. Int. 54(9), 1262–1267 (2005). doi:10.1002/pi.1840
Singla, A.K., Garg, A., Aggarwal, D.: Paclitaxel and its formulations. Int. J. Pharm. 235(1–2), 179–192 (2002). doi:10.1016/s0378-5173(01)00986-3
Maeda, H., Wu, J., Sawa, T., Matsumura, Y., Hori, K.: Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Controlled Release 65(1–2), 271–284 (2000). doi:10.1016/s0168-3659(99)00248-5
Modok, S., Mellor, H.R., Callaghan, R.: Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr. Opin. Pharmacol. 6(4), 350–354 (2006). doi:10.0116/j.coph.2006.01.009
Kwak, H.H., Shim, W.S., Choi, M.K., Son, M.K., Kim, Y.J., Yang, H.C., Kim, T.H., Lee, G.I., Kim, B.M., Kang, S.H., Shim, C.K.: Development of a sustained-release recombinant human growth hormone formulation. J. Controlled Release 137(2), 160–165 (2009). doi:10.1016/j.jconrel.2009.03.014
Kim, H.K., Chung, H.J., Park, T.G.: Biodegradable polymeric microspheres with “open/closed” pores for sustained release of human growth hormone. J. Controlled Release 112(2), 167–174 (2006). doi:10.1016/j.jconrel.2006.02.004
Kim, H.K., Park, T.G.: Microencapsulation of human growth hormone within biodegradable polyester microspheres: Protein aggregation stability and incomplete release mechanism. Biotechnol. Bioeng. 65(6), 659–667 (1999). doi:10.1002/(sici)1097-0290(19991220)65:6<659:aid-bit6>3.0.co;2-9
Kim, Y.M., Park, M.R., Song, S.C.: Injectable polyplex hydrogel for localized and long-term delivery of siRNA. ACS Nano 6(7), 5757–5766 (2012). doi:10.1021/nn300842a
Park, K.H., Song, S.C.: Morphology of spheroidal hepatocytes within injectable, biodegradable, and thermosensitive poly(organophosphazene) hydrogel as cell delivery vehicle. J. Biosci. Bioeng. 101(3), 238–242 (2006). doi:10.1263/jbb.101.238
Potta, T., Chun, C., Song, S.C.: Chemically crosslinkable thermosensitive polyphosphazene gels as injectable materials for biomedical applications. Biomaterials 30(31), 6178–6192 (2009). doi:10.1016/j.biomaterials.2009.08.015
Potta, T., Chun, C., Song, S.C.: Injectable, dual cross-linkable polyphosphazene blend hydrogels. Biomaterials 31(32), 8107–8120 (2010). doi:10.1016/j.biomaterials.2010.07.029
Potta, T., Chun, C., Song, S.C.: Dual cross-linking systems of functionally photo-cross-linkable and thermoresponsive polyphosphazene hydrogels for biomedical applications. Biomacromolecules 11(7), 1741–1753 (2010). doi:10.1021/bm100197y
Potta, T., Chun, C., Song, S.C.: Controlling the degradation rate of thermoresponsive photo-cross-linked poly(organophosphazene) hydrogels with compositions of depsipeptide and PEG chain lengths. Polym. Degrad. Stab. 96(7), 1261–1270 (2011). doi:10.1016/j.polymdegradstab.2011.04.010
Potta, T., Chun, C., Song, S.C.: Rapid photocrosslinkable thermoresponsive injectable polyphosphazene hydrogels. Macromol. Rapid Commun. 31(24), 2133–2139 (2010). doi:10.1002/marc.201000350
Potta, T., Chun, C., Song, S.C.: Design of polyphosphazene hydrogels with improved structural properties by use of star-shaped multithiol crosslinkers. Macromol. Biosci. 11(5), 689–699 (2011). doi:10.1002/mabi.201000438
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Liu, X. (2015). Biodegradable Thermogelling Poly(Organophosphazenes) and Their Potential Biomedical Applications. In: Loh, X. (eds) In-Situ Gelling Polymers. Series in BioEngineering. Springer, Singapore. https://doi.org/10.1007/978-981-287-152-7_3
Download citation
DOI: https://doi.org/10.1007/978-981-287-152-7_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-151-0
Online ISBN: 978-981-287-152-7
eBook Packages: EngineeringEngineering (R0)