Abstract
What is the clear definition of left colectomy? Unfortunately, the answer is not as clear as it is with a right colectomy. The resection can involve resection of the colonic segment anywhere between the left transverse colon and the upper rectum. For tumors involving the left transverse colon or splenic flexure, left hemicolectomy (LC) is the preferred operation. A LC is considered to be a resection of the mid-transverse colon to the descending/sigmoid junction. In complete mesocolic excision (CME) and central vascular ligation (CVL) for left transverse colon or splenic flexure colon cancer, ligation of the inferior mesenteric vein (IMV), left branch of the middle colic artery (lt-MCA), and left colic artery (LCA) at the root must be considered [1]. For resection of mid- or distal descending colon tumors, the oncological resection requires division of the inferior mesenteric artery at its origin. If the resection carried out involved the distal transverse colon up to the sigmoid-descending junction, it should be considered as a left segmentary colectomy [2].
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Introduction
What is the clear definition of left colectomy? Unfortunately, the answer is not as clear as it is with a right colectomy. The resection can involve resection of the colonic segment anywhere between the left transverse colon and the upper rectum. For tumors involving the left transverse colon or splenic flexure, left hemicolectomy (LC) is the preferred operation. A LC is considered to be a resection of the mid-transverse colon to the descending/sigmoid junction. In complete mesocolic excision (CME) and central vascular ligation (CVL) for left transverse colon or splenic flexure colon cancer, ligation of the inferior mesenteric vein (IMV), left branch of the middle colic artery (lt-MCA), and left colic artery (LCA) at the root must be considered [1]. For resection of mid- or distal descending colon tumors, the oncological resection requires division of the inferior mesenteric artery at its origin. If the resection carried out involved the distal transverse colon up to the sigmoid-descending junction, it should be considered as a left segmentary colectomy [2].
The question of whether extended right colectomy (ERC) or LC should be more strongly indicated for the tumors involving the left transverse colon or splenic flexure remains open. The rate of R0 resection as well as long-term oncological outcomes are not different between ERC and RC [2,3,4]. Nevertheless, the concept has been proposed that synchronous liver metastases are associated with the risk of distal positive lymph nodes, and ERC should be considered for metastatic patients suitable for curative treatment to ensure R0 resection of both tumor sites [5].
The attempts at an anastomosis of LC may be difficult because of inadequate length and tension. Under these circumstances, total or subtotal colectomy is a reasonable alternative. The inverted right colonic transposition (the so-called Deloyers procedure) and trans-mesenteric colorectal anastomosis represent another alternative [6].
Minimal invasive approaches to colon and rectal resection have resulted in earlier tolerance of diet, accelerated return of bowel function, lower analgesia requirement, and shorter length of hospital stay. Large multicentre randomized trials have shown comparable disease-free and overall survival between open and laparoscopic approaches for colon cancer [7,8,9]. However, all these studies exclude patients with transverse colon and splenic flexure lesions, probably because of technical difficulties specific to this location or the rarity of this condition. With the improvements in surgical techniques and instruments, increasing numbers of studies demonstrated that the laparoscopic LC is a feasible, safe, and effective procedure, as well as acceptable short-term and oncologic long-term outcomes [2, 10, 11].
Indications
The most common indication for LC is colon cancer (e.g., distal transverse colon cancer, splenic flexure colon cancer, proximal to mid descending colon cancer). Other indications include benign conditions such as diverticulitis, trauma, segmental Crohn’s colitis, ischemic colitis, polyps unresected through a colonoscopy, and colonic volvulus. Diverticular disease, typically with sigmoid colon resection, may require a LC if the descending colon is unsuitable for an anastomosis due to active diverticulitis or muscular hypertrophy.
Contraindications
Contraindication often depends on the surgeon’s level of expertise with less straightforward patients and diseases. Certainly, hemodynamically instability or cardiopulmonary disease that is severe enough to make peritoneal insufflation and Trendelenburg positioning dangerous represent a physiologic derangement that precludes the safe application of laparoscopy. Another relative contraindication includes large bowel obstruction. Depending on the degree of proximal intestinal dilatation present, the more limited volume of unencumbered working space, coupled with higher risk of bowel perforation during manipulation, may warrant an open approach. The application of self-expanding stents in obstructed colon as a bridge to laparoscopic surgery could be an alternative option. Severe adhesion due to previous surgeries pose a technical challenge to minimally invasive surgeons which may render patients not suitable for laparoscopic colectomy. Phlegmonous tissue which is usually encountered in severe, complicated Crohn’s disease or in diverticulitis may not also be resectable via laparoscopy due to the tissue friability, bleeding, and distortion anatomy that necessitates open exposure. Severe peritoneal carcinomatosis secondary to left-sided colon may also preclude laparoscopic LC. There are no common criteria to apply laparoscopic technique for combined resection for T4 colon cancer. Tumor invasion into other organs is not an absolute contraindication if en bloc resection could be achieved. However, laparotomic conversion is necessary if oncologically curative resection is not achieved laparoscopically. Significant intraoperative hemorrhage, in the presence of visceral lesion, incorrect dissection, all conditions that may affect the outcome, are contraindications of laparoscopy and the conversion is necessary.
Preoperative Assessment
All patients undergoing colonic surgery should have the same preoperative workup including anesthetic workup regardless of the surgical approaches. All patients should have a complete history and physical examination. Adjunct testing such as blood test, additional imaging (CT scan, barium enema), or cardiopulmonary testing is performed when indicated. The only special consideration for laparoscopic surgery is ensuring that the surgeon can identify the site of pathology at the time of operative intervention. The loss of tactile sensation in laparoscopic surgery stresses the importance of localizing techniques, especially for small lesions. These can be evaluated preoperatively by colonoscopy and tattooing of the lesion can be performed during the colonoscopy 1–2 days prior to the surgery especially for early colonic cancer which ensures the oncological safe margins. It is helpful to have flexible colonoscopy in the operating room as it may be needed intraoperatively to identify lesions if the location of the lesion remains doubtful. All elective colonic resections should follow ERAS preoperative protocol. The protocols include perioperative opioid-sparing analgesia, avoidance of nasogastric tubes and peritoneal drains, aggressive management of postoperative nausea and vomiting, and early oral feedings and ambulation. Mechanical oral bowel preparation is not needed for elective laparoscopic LC. An urinary catheter is placed at the beginning of the procedure and is removed on the first morning after the operation. Prophylaxis antibiotic and deep vein thrombosis (DVT) prophylaxis should be included.
OT Setup (Fig. 1a, b)
Authors routinely placed patients in lithotomy position with both arms tucked and the thighs positioned using stirrups at no more than a 10° angle to the torso. Lithotomy allows simultaneous access to the abdomen and perineum for colorectal anastomosis when using the circular stapler, as well as for intraoperative colonoscopy. It also provides additional space for a second assistant and an additional position for the operating surgeon when mobilizing the splenic flexure. The patient should be fixed securely on the table because the patient’s position could be changed during the operation. A beanbag is used to secure the patient to the table, along with reinforcement by adhesive tapes wrapping the patient’s chest to the table. The patient’s position can be adjusted intraoperatively at the stage of left flexure or rectal mobilization. The procedure is usually performed with one assistant. Surgeon will stand on the patient’s right while the camera assistant on surgeon’s right. During the approach of the middle colic artery, the surgeon may stand in between the patient’s legs. The monopolar device with hook, spatula, or scissor or energy-based devices are adapted for the plane dissection, depending on the surgeon’s preference. The adaptation of energy-based devices in laparoscopic colon cancer surgery could reduce chyle leakage, minimize bleeding on dissection planes, and facilitate complete plane dissection.
Surgical Techniques
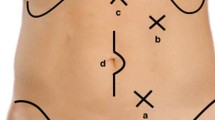
Ports Placements (Fig. 2)
Four trocars are placed. By open technique, Camera port is inserted infra or supra umbilically using a balloon trocar. Pneumoperitoneum 12–15 mmHg is created and the abdomen is inspected for findings. A 12 mm port is placed in the right lower quadrant, for the operator’s right hand. A 5 mm port is placed in the right hypochondrium for the operator’s left hand. A second 5 mm port is placed over the left side abdomen.
Operatives Details
-
1.
Vascular Pedicle Isolation and Ligation (Fig. 3a–f)
Patient is tilted head down 15° (Trendelenberg) and right side down for gravitational drag of the small bowel to the right and omentum and transverse colon slightly to cephalad. Omentum is swept cephalad direction and the transverse mesocolon is lifted up to expose the cleavage plane of the pancreas. Incision of transverse mesocolon at the level below lt-MCA either by monopolar diathermy or energy device to enter the lesser sac (Fig. 3a). The Treitz ligament is dissected with maximum care not to damage the jejunum (Fig. 3b). The peritoneal layer medial to the inferior Mesenteric Vein (IMV) is incised paralleled to the vessel. The IMV is easily visualized, or in case of more obese patients, search for, right below the inferior margin of the pancreas. The IMV is dissected free and then divided at the level close to the inferior border of the pancreas (Fig. 3c).
In order to perform central ligation of lt-MCA with a lateral-to-medial approach to the left-sided transverse mesocolon (TM), the posterior layer of TM is dissected along the pancreas and spread cephalad. The middle colic artery is identified from the superior mesenteric artery. Then the lt-MCA is dissected free and divided at the root (Fig. 3d).
The medial-to-lateral (MTL) approach to the left mesocolon easily brings the inferior mesenteric artery (IMA) into view. The root of sigmoid mesentery is retracted up to create tension on the peritoneum which is then incised using monopolar diathermy from caudal to cephalad position starting from the sacral promontory. Pneumo-dissection might help to open up further the embryological plane. The mesentery of sigmoid can be retracted away from the retroperitoneum by performing a blunt and bloodless dissection using monopolar or advanced energy devices. Proper Medial-To-Lateral (MTL) dissection will not expose the left ureter, left gonadal vessel, and psoas muscle, which are left undisturbed retroperitoneally. The retracting instrument can be inserted into the plane between the mesentery and the retroperitoneum, lifting the mesentery toward the anterior abdominal wall without grasping and tearing tissue. Dissection carried on cephalad till the root of IMA with careful identification and preservation of hypogastric nerves, which control urinary and sexual function. With D3 lymph node dissection at the IMA root (Fig. 3e), the left colic artery (LCA) is ligated near the origin from IMA (Fig. 3f).
-
2.
Splenic Flexure Mobilization (Fig. 4a, b)
The splenic flexure of colon is mobilized using medial-to-lateral approach. After the procedures of central vascular ligation, the lessor sac has been entered through the TM window and the mesentery root of the left colon is incised. By insertion of retracting instrument and tenting of mesentery of both transverse and descending colon, the pancreatico-colonic ligament is divided using either monopolar diathermy or advanced energy devices (Fig. 4a). Lifting the IMV arch allows furthering MTL dissection by opening a window between the Toldt fascia anteriorly and the Gerotal fascia posteriorly (Fig. 4b). The border between the two fascias, which indicates the embryonic plane of coalescence of posterior mesocolon and retroperitoneum is whitish, a clear sign of correct dissection plane (Fig. 4b). A tough elevation of mesocolon anteriorly toward the abdominal wall facilitates the dissection as far as the pericolic gutter, and downward to the level of sacral promontory.
-
3.
Mobilization of Colon (Fig. 5a–c)
If the medial approach was done adequately, colon (descending and sigmoid) can be easily mobilized from the Toldt’s fascia. Gently retract the descending colon medially, this thin Toldt’s fascia is scored and divided using monopolar diathermy and advanced energy devices (Fig. 5a). The splenic flexure proper can then be dissected down by dividing the spleno-colic ligament (Fig. 5b). The greater omentum is separated from the gastric curvature. The gastrocolic ligament is also divided (Fig. 5c). The sigmoid and descending colon is fully mobilized until it is a midline structure. For a tension-free anastomosis, sometimes mobilization of hepatic flexure may be indicated.
-
4.
Construction of Anastomosis and Specimen Extraction (Fig. 6a–e)
Authors prefer extracorporeal hand-sewn end-to-end colo-colonic anastomosis, which offers the advantages of tension-free anastomosis, and less risk of jejunum compression which results in postoperatively intestinal obstruction. The specimen is extracted through the umbilical port, which extended to about 3–6 cm. To avoid contamination, a wound protector is used. Care to be taken when extracting the colon with the lesion as too much of traction can disrupt the colonic wall and marginal artery which will jeopardize anastomosis. Excessive traction may also cause contamination, and in the worst scenario tumor cell seeding in colonic malignancies. After division of the mesocolon, routine Indocyanine Green (ICG) is used to assure good vasculature of the remaining colon before every transaction. After restoration of bowel continuity, the colon is placed back into the abdomen and insufflation is reestablished. Closure of the mesenteric gap is recommended to minimize the risk of internal herniation.
Alternatively, intracorporeal colocolic functional end-to-end anastomosis, which is technically a side-to-side approach, can be performed if adequate bowel is preserved in some of the cases. The superiority of side-to-side anastomosis compared with hand-sewn is having better blood flow and wider diameter thus reducing intraluminal pressure and proximal ischemia. Advantage of performing intracorporeal anastomosis is avoidance of bowel twisting in the wrong orientation and avoidance of excessive traction on bowel during anastomosis. A totally laparoscopic approach represents the better treatment particularly for obese patients, as it avoids the exteriorization of heavy and short mesenteries through much thicker abdominal walls and the risk of microlacerations which may affect the success of the anastomosis. The intracorporeal transections of the transverse and descending colon are accomplished using 60 mm/3.5 mm blue-load articulating linear endoscopic staplers. The specimen, completely separated from all attachments, is then kept aside in the abdominal cavity. The transverse and the left colon are lined up side to side (isoperistaltic manner), and a stapled side-to-side colocolic anastomosis (SSSA) is conducted with one fire of a 60 mm blue endostaper load (Fig. 6a). The enterotomy is closed using a 3–0 PDS double layer running suture (Fig. 6b). Antiperistaltic SSSA is also feasible; however, it may run higher risk of tensioned anastomosis. Antiperistaltic SSSA required more intestinal mobilization than isoperistaltic SSSA [12].
Extended right hemicolectomy (ERC) or subtotal colectomy has significant technical advantages over left colectomy, especially under the circumstances of obstructing tumors of the left colon, synchronous cancers in other segments, clinically evident diverticular disease, or inadequate remaining bowel length for anastomosis. Technically, it utilizes a highly mobile segment of the bowel, the ileum, to transpose it toward the left colon and perform the intracorporeal ileocolonic anastomosis without tension.
Trans-mesenteric colo-colonic or colorectal anastomosis are feasible laparoscopically and allow tension-free anastomosis in patients with a short proximal colonic segment after extended LH. The proximal colon is mobilized as completely as possible. The gasto-colic ligament is divided and the second position of duodenum is exposed. An ileal mesenteric window is creased in the avascular area between the superior mesenteric and ileocolic pedicles (Fig. 6c). Then the proximal transverse colon is pulled through the mesenteric window to create a tension-free anastomosis (Fig. 6d). In most cases, division of the middle colic vessels is necessary for full mobilization; therefore, it is important to preserve the marginal vessels to avoid the risk of ischemia after middle colic vessel ligation.
If trans-mesenteric anastomosis is still not feasible, the inverted right colonic transposition procedure is an alternative salvage. After full mobilization and middle colic vessel ligation, the right colon is rotated 1800 counterclockwise around the ileocolic vessel axis such that the cecum is cephalad while the hepatic flexure is caudal (Fig. 6e). The right colon can easily be anastomosed tension-free to the colonic or rectal stump. All patients undergoing the Deloyers procedure have routine appendectomy.
(a) Incision of transverse mesocolon at the level below lt-MCA to enter the lesser sac. (b) The Treitz ligament is dissected with maximum care not to damage the jejunum. (c) The IMV is divided at the level close to the inferior border of the pancreas. (d) The lt-MCA is divided at the root. (e) D3 lymph node dissection at the IMA root. (f) LCA is ligated near the origin from IMA
(a) Isoperistaltic SSSA is conducted with one fire of a 60 mm blue endostaper load (b) The enterotomy is closed using a 3–0 PDS double layer running suture (c) An ileal mesenteric window is creased in the avascular area between the superior mesenteric and ileocolic pedicles (d) The proximal transverse colon is pulled through the mesenteric window (e) Deloyers procedure: the right colon is rotated 1800 counterclockwise around the ileocolic vessel axis such that the cecum is cephalad while the hepatic flexure is caudal
Postoperative Care
Authors follow postoperative ERAC protocol management. Postoperatively, the patients are placed on an enhanced recovery pathway. The orogastric tube is removed in the operating room prior to awakening from anesthesia. Following the operation, the patient is given oral fluid diet and progressed to an oral diet from the first postoperative day as long as patients tolerate well. The urine catheter is removed on the next day and if drain is inserted, the drain is removed prior to discharge. Postoperative analgesia as per pain team. The average length of hospital stay is 3–5 days.
References
Watanabe J, Ota M, Suwa Y, et al. Evaluation of lymph flow patterns in splenic flexure colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int J Color Dis. 2017;32(2):201–7.
Martínez-Pérez A, Brunetti F, Vitali GC, et al. Surgical treatment of colon cancer of the splenic flexure: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2017;27(5):318–27.
Secco GB, Ravera G, Gasparo A, et al. Segmental resection, lymph nodes dissection and survival in patients with left colon cancer. Hepato-Gastroenterology. 2007;54(74):422–6.
Gravante G, Elshaer M, Parker R, et al. Extended right hemicolectomy and left hemicolectomy for colorectal cancers between the distal transverse and proximal descending colon. Ann R Coll Surg Engl. 2016;98(5):303–7.
Manceau G, Mori A, Bardier A, et al. Lymph node metastases in splenic flexure colon cancer: is subtotal colectomy warranted? J Surg Oncol. 2018;118(6):1027–33.
Chen YC, Fingerhut A, Shen MY, et al. Colorectal anastomosis after laparoscopic extended left colectomy: techniques and outcome. Color Dis. 2020;22(9):1189–94.
Clinical Outcome of Surgical Therapy Study Group, Nelson H, Sargent DJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–9.
Veldkamp R, Kuhry E, Hop WC, et al. Laparascopic surgery versus open surgery for colon cancer: short-term outcome of a randomised trial. Lancet Oncol. 2005;6(7):477–84.
Jayne DG, Thrpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97(11):1638–45.
Grieco M, Cassini D, Spoletini D, et al. Laparoscopic resection of splenic flexure colon cancers: a retrospective multi-center study with 117 cases. Updat Surg. 2019;71(2):349–57.
Okuda J, Yamamoto M, Tanaka K, et al. Laparoscopic resection of transverse colon cancer at splenic flexure: technical aspects and results. Updat Surg. 2016;68(1):71–5.
Matsuda A, Miyashita M, Matsumoto S, et al. Isoperistaltic versus antiperistaltic stapled side-to-side anastomosis for colon cancer surgery: a randomized controlled trial. J Surg Res. 2015;196(1):107–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Shen, MY., Leow, Y.C., Chen, W.TL. (2023). Laparoscopic Left Hemicolectomy. In: Lomanto, D., Chen, W.TL., Fuentes, M.B. (eds) Mastering Endo-Laparoscopic and Thoracoscopic Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-19-3755-2_70
Download citation
DOI: https://doi.org/10.1007/978-981-19-3755-2_70
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3754-5
Online ISBN: 978-981-19-3755-2
eBook Packages: MedicineMedicine (R0)