Abstract
As the usage of electrocautery, ultrasonic scalpels, and lasers have become commonplace, operative staff and patients alike are at increased risk of exposure to dangerous surgical smoke emanating from these devices. Terms like “smoke,” “plume,” and less commonly “aerosol” are used to refer to by-products of laser tissue ablation and electrocautery, whereas “plume,” “aerosol,” and “vapor” are associated with ultrasonic dissection. “Smoke,” although not formally accurate in all cases, is a widely accepted term used to describe surgically generated gaseous by-product [1].
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Introduction
As the usage of electrocautery, ultrasonic scalpels, and lasers have become commonplace, operative staff and patients alike are at increased risk of exposure to dangerous surgical smoke emanating from these devices. Terms like “smoke,” “plume,” and less commonly “aerosol” are used to refer to by-products of laser tissue ablation and electrocautery, whereas “plume,” “aerosol,” and “vapor” are associated with ultrasonic dissection. “Smoke,” although not formally accurate in all cases, is a widely accepted term used to describe surgically generated gaseous by-product [1].
Surgical smoke contains particulates like carbon monoxide, polyaromatic carbons, benzene, hydrogen cyanide, formaldehyde, viable and nonviable cellular material, viruses, and bacteria [2]. These particulates pose a risk to surgeons, operating theater personnel, and patients because they harbor these chemicals and biological components, and have shown to carry mutagenic and carcinogenic potential.
Factors affecting the amount and content of smoke produced does include type of procedure, surgeon’s technique, pathology of target tissue (e.g., presence of bacteria or virus), type of energy device, power levels used, and the amount of cutting, coagulation, or ablation performed [3]. The smoke produced by each energy device has its own unique properties, comprising of aerodynamic particle size, chemical makeup, and biological constituents. For instance, electrocautery produces the smallest aerodynamic particle size, followed by laser tissue ablation creating larger ones while harmonic scalpels produce the largest particle size. The smaller the size, the further the distance these particles travel, and they pose a higher chemical concern. Larger particles, on the other hand, raise more concerns from a biological aspect [1].
Studies have compared the deposition of particulate matter in ten different tissues, and have shown that the liver produced the highest number of particles, skeletal muscle and renal tissues produced medium mass of particulate matter, while other tissues produced significantly less particulate mass [4].
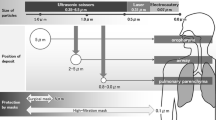
Particles that are greater than 5μm can deposit on walls of the nose, pharynx, trachea, and bronchus whereas particles smaller than 2 μm are deposited in the bronchioles and alveoli. Considering that 77% of plume is in the inspirable range, it is concerning that smoke can cause acute and chronic inflammatory changes, including alveolar congestion, interstitial pneumonia, bronchiolitis, and emphysematous changes in the respiratory tract [5].
Multiple carcinogens have been identified in surgical smoke, with butadiene and benzene showing 17- and 10-fold higher concentrations than second-hand smoking. Several laboratory and animal studies have demonstrated smoke from laser and electrocautery surgery causing acute and delayed carcinogenic effects on humans. Although there is no direct evidence at present to show that surgical smoke is carcinogenic to humans, there are persistent concerns [5].
Besides chemical components, mutagenicity and cytotoxicity also pose great concerns to users of lasers, electrocautery, and powered surgical instruments. Tomita et al. quantified the mutagenic effect created by thermal destruction of just 1g of tissue to be equivalent to three to six cigarettes [6]. Additionally, studies have shown smoke produced from breast tissue has the mutagenicity of a TA98 strain of Salmonella, and another study demonstrated that it induced cytotoxicity in human small airway epithelial cells and mouse macrophages [7].
Surgical smoke, produced with or without a heating process, contains bio-aerosols with viable and nonviable cellular material that consequentially poses a risk of infection such as HIV, hepatitis B virus, and human papillomavirus (HPV) [8]. Although the possibility of disease transmission via surgical smoke exists, actual documented cases of pathogen transmission are rare. Only one such case has essentially been proven, whereby a surgeon contracted laryngeal papillomatosis after treating anogenital condyloma with a laser. HPV types 6 and 11, the same types in anogenital papillomatosis, were found in this individual’s larynx, a very uncommon area of infection, which would suggest direct contact as a route of transmission [9].
Patients are also at risk from surgical smoke, particularly during laparoscopic procedures whereby smoke gets trapped in the peritoneal cavity. Potential complications include carbon monoxide toxicity, port-site metastases via chimney effect, and toxicity to peritoneal compartment and its contents [1]. The chimney effect, first described in 1995, stipulates that cancer cells are aerosolized during laparoscopic surgery and can leak from around the cannula during the procedure. The localized inflammation from the trauma caused by cannula and trocar insertion increases the potential for cancer cells to implant. It was also suggested that pneumoperitoneum creates a pressure gradient with resulting outflow of gas and floating tumor cells through port wounds, creating a chimney effect that does not occur in a standard wound [10]. Smoke also limits surgical field visibility, which poses direct harm to patients.
Mitigating the Risks
Once we recognize that surgical smoke is essentially an occupational hazard, it is important to minimize its production and have proper evacuation systems or protocols in place. It is also vital to raise awareness among surgeons and operating theater personnel regarding the dangers of surgical smoke.
Surgeons can minimize the production of surgical smoke by avoiding unnecessary tissue ablation and using shorter, precise bursts. Assistants may also aid in capturing smoke with a suction wand. A recently unpublished study had shown that a suction wand can effectively capture 95–99% of smoke if the tube’s orifice is within 2 inches of the smoke source [11].
Small particles less than 1.1 μm constitute 77% of particulate matter found in surgical smoke [12]. Because of this, most conventional surgical masks do not have sufficient filtering or snug-fitting attributes to provide respiratory protection. A study by Gao et al. had shown that wearing at least N95 respirator and N100 filtering face piece respirator could offer more protection to wearers [13].
Evacuation Systems
The National Institute for Occupational Safety and Health (NIOSH) of the United States recommends a combination of general room and local exhaust ventilation (LEV) to remove airborne contaminants generated by surgical devices. They advocate suction devices with a capture velocity of 100–150 feet per minute [13]. Three such suction devices utilizing LEV include smoke evacuation wands, electrosurgical unit (ESU) pencils (Fig. 1), and cell foam technology.
ESU pencils are attached to tubing, which in turn connects to smoke evacuation filters. The latest device based on cell foam technology operates by having an open cell foam core sandwiched between layers of nonporous plastic to keep smoke within the device and prevent loss of suction power. The LEV machines used are in turn connected to ultra-low particulate (penetration) air (ULPA) filters that include activated charcoal which absorbs and deodorizes chemicals and odors present in smoke [13]. Filters should also be used in the exhaust port of the collection device to prevent contents of smoke from leaking [14].
Alternatively, high-efficiency particulate air (HEPA) filters that are placed on top entry ports of suction canisters can trap particulates effectively. Combination of HEPA filters with activated carbon called “high efficiency gas absorption” (HEGA) filters successfully prevent surgeons from volatile organic compounds and chemical vapors. Additionally, using activated carbon fiber filter during laparoscopic operations can dramatically reduce carcinogens by more than 85% [15].
Special Considerations
The COVID-19 pandemic had drastic ramifications towards society and many had to adapt to the “new normal” and change work practices. The same applies to surgeons as there were raised concerns of the risk of coronavirus transmission in the operating room. Specifically, the elevated risk during intubation and extubation from the anesthetic standpoint, as well as the risk of release of potential infectious particulates in laparoscopic smoke.
Past research had shown that laparoscopy can lead to aerosolization of blood-borne viruses but there has been no evidence to support that this effect is seen with COVID-19, nor that it is isolated to laparoscopic procedures. However, to err on the side of caution, it is prudent to treat the coronavirus as exhibiting similar aerosolization properties. The UK and Ireland Intercollegiate Board have advised to consider laparoscopy only in selected cases whereby the clinical benefit of the patient outweighs the risk of viral transmission [16].
Assigning designated operating theaters for confirmed and suspect cases of COVID-19 can aid to streamline patient movements, limit the number of staff and equipment needed, as well as limiting contamination to specific areas. Negative pressure ventilation can curb contamination of surgical smoke via doors and vents. There had been recommendations to stop positive pressure ventilation during the procedure and for at least 20 min after the patient has left the theater; however, the risks associated with positive pressure ventilation have not been quantified [17].
Smoke extraction is crucial and can be achieved with a general ventilation system, local extraction at the site of surgery, and use of personal filtration masks, as discussed before. The smoke evacuator can be of two types one without the triple filter and the other one with a triple filtering tube (Figs. 2 and 3a, b). At present, the most effective smoke evacuation system is the triple filter, which includes a prefilter that traps large particles, an ULPA filter, and a special charcoal that captures toxic chemicals.
There are however nonfiltration devices available in the market that can evacuate smoke as well. The Ultravision™ system removes smoke particulates during electrosurgical procedures, as an aid to maintain clear visual field. This system is not restricted by particle size, and it has been demonstrated to remove more than 99% of all smoke particulates [16].
Methods recommended for laparoscopic surgery include the use of balloon ports to reduce the risk of inadvertent displacement of trocars thus reducing the risk of loss of pneumoperitoneum to the operating theater environment. These trocars also have valves preventing gas leakage whenever an instrument is passed through into the peritoneal cavity. Pneumoperitoneum should be maintained throughout the procedure at the lowest possible pressure and decompressed slowly at the end if an incision is required for specimen extraction [16].
Conclusion
Surgical smoke contains harmful particulates and although more research is required to determine its direct effect on health, we must be wary of its long-term effects. There are many mitigation strategies that can be applied, ranging from filtration masks to sophisticated smoke evacuation systems. The most important step however is to first and foremost educate healthcare workers that surgical smoke is an occupational hazard, and should be treated seriously as such.
References
Barrett W, Garber S. Surgical smoke: a review of the literature. Surg Endosc. 2003;17(6):979–87. https://doi.org/10.1007/s00464-002-8584-5.
Steege AL, Boiano JM, Sweeney MH. Secondhand smoke in the operating room? Precautionary practices lacking for surgical smoke. Am J Ind Med. 2016;59(11):1020–31. https://doi.org/10.1002/ajim.22614.
Gatti JE, Bryant CJ, Noone RB, Murphy JB. The mutagenicity of electrocautery smoke. Plastic Reconstr Surg. 1992;89(5):785–6. https://doi.org/10.1097/00006534-199205000-00002.
Karjalainen M, Kontunen A, Saari S, et al. The characterisation of surgical smoke from various tissues and its implications for occupational safety. PLoS One. 2018;13(4):e0195274. https://doi.org/10.1371/journal.pone.0195274.
Liu Y, Song Y, Hu X, Yan L, Zhu X. Awareness of surgical smoke hazards and enhancement of surgical smoke prevention among the gynaecologists. J Cancer. 2019;10(2):2788–99. https://doi.org/10.7150/jca.31464.
Yoshifumi T, Shigenobu M, Kazuto N, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89(2):145–9. https://doi.org/10.1016/0165-1218(81)90120-8.
Sisler JD, Shaffer J, Soo J-C, et al. In vitro toxicological evaluation of surgical smoke from human tissue. J Occup Med Toxicol. 2018;13(1):12. https://doi.org/10.1186/s12995-018-0193-x.
Alp E, Bijl D, Bleichrodt R, Hansson B, Voss A. Surgical smoke and infection control. J Hosp Infect. 2006;62(1):1–5. https://doi.org/10.1016/j.jhin.2005.01.014.
Lobraico RV, Schifano MJ, Brader KR. A retrospective study on the hazards of the carbon dioxide laser plume. J Laser Applic. 1998;1(1):6–8. https://doi.org/10.2351/1.4745215.
Hubens G, Pauwels M, Hubens A, Vermeulen P, Marck EV, Eyskens E. The influence of a pneumoperitoneum on the peritoneal implantation of free intraperitoneal colon cancer cells. Surg Endosc. 1996;10(8):809–12. https://doi.org/10.1007/s004649900166.
Schultz L. An analysis of surgical smoke plume components, capture and evacuation. AORN J. 2014;99(2):289–98. https://doi.org/10.1016/j.aorn.2013.07.020.
Benson SM, Novak DA, Ogg MJ. Proper use of surgical N95 respirators and surgical masks in the OR. AORN J. 2013;97(4):457–70. https://doi.org/10.1016/j.aorn.2013.01.015.
Fan JK-M, Chan FS-Y, Chu K-M. Surgical smoke. Asian J Surg. 2009;32(4):253–7. https://doi.org/10.1016/s1015-9584(09)60403-6.
Gao S, Koehler RH, Yermakov M, Grinshpun SA. Performance of facepiece respirators and surgical masks against surgical smoke: simulated workplace protection factor study. Ann Occup Hyg. 2016;60:608–18. https://doi.org/10.1093/annhyg/mew006.
Choi SH, Choi DH, Kang DH, et al. Activated carbon fibre filters could reduce the risk of surgical smoke exposure during laparoscopic surgery: application of volatile organic compounds. Surg Endosc. 2018;32(10):4290–8. https://doi.org/10.1007/s00464-018-6222-0.
Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. 2020;107(11):1406–13. https://doi.org/10.1002/bjs.11679.
Team RCSEC. Intercollegiate General Surgery Guidance on COVID-19 UPDATE. The Royal College of Surgeons of Edinburgh. Published March 30, 2020. https://www.rsced.ac.uk/news-public-affairs/news/2020/march/intercollegiate-general-surgery-guidance-on-covid-19-update. Accessed 11 July 2020.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Malik, S., Khairi, F., Wijerathne, S. (2023). Surgical Smoke: Risks and Mitigation Strategies. In: Lomanto, D., Chen, W.TL., Fuentes, M.B. (eds) Mastering Endo-Laparoscopic and Thoracoscopic Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-19-3755-2_11
Download citation
DOI: https://doi.org/10.1007/978-981-19-3755-2_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3754-5
Online ISBN: 978-981-19-3755-2
eBook Packages: MedicineMedicine (R0)