Abstract
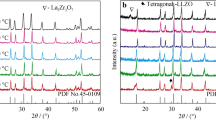
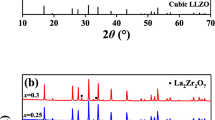
Synthesis of Al-Y-doped LLZO has been conducted by solid-state reaction. The synthesized powder was treated by different treatments which were sintered, non-sintered, and also the different CIP pressure. The sintering treatment was conducted at 1050 and 1200 °C. Meanwhile, the applied pressures were 20, 30, and 40 MPa. XRD analysis equipped with the Le Bail Refinement found that the Al-Y-doped LLZO was crystallized within cubic phase, with a percentage composition of 84.89%. Impedance analysis followed by ZView fitting and conductivity calculation found that the green pellet formed by 40 MPa CIP pressure provide the highest ionic conductivity, i.e., 1.06 × 10–5 S cm−1. The experiment result shows that the ionic conductivity increases by the increase of the applied pressure.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Jiang L, Wang Q, Li K, Ping P (2018) A self cooling and flame retardant electrolyte for safer lithium ion batteries. Sustain Energy Fuels 2018(2):1323–1331. https://doi.org/10.1039/c8se00111a
Xu K (2004) Non-aqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104:4303–4417
Awaka J, Kijima N, Hayakawa H, Akimoto J (2009) Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J Solid State Chem 182:2046–2052
Inaguma Y, Chen L, Itoh M, Nakamura T, Uchida T, Ikuta M, Wakihara M (1993) High ionic conductivity in lithium lanthanum titanate. Solid State Commun 86:689
Birke P, Scharner S, Huggins RA, Weppner W (1997) Electrolytic stability limit and rapid lithium insertion in the fast-ion-conducting Li0.29La0.57TiO3 perovskite-type compound. J Electrochem Soc 144(6):L167–L169
Kanno R, dan Murayama M (2001) Lithium ionic conductor thio-LISICON. J Electrochem Soc 148:A742–A746
Stramare S, Thangadurai V, Weppner W (2003) Lithium lanthanum titanates: a review. Chem Mater 15:3974–3990
Mizuno F, Hayashi A, Tadanaga K, Tatsumisago M (2005) New, highly ion-conductive crystals precipitated from Li2S-P2S5 glasses. Adv Mat 17:918–921
Hayashi A, Minami K, Ujiie S, Tatsumisago M (2010) Preparation and ionic conductivity of Li7P3S11-z glass-ceramic electrolytes. J Non-Crystal Solids 356:2670–2673
Mo Y, Ong SP, Ceder G (2012) First principles study of the Li10GeP2S12 lithium super ionic conductor material. Chem Mat 24:15–17
Rangasamy E, Wolfenstine J, Sakamoto J (2012) The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics 206:28–32
Rangasamy E, Wolfenstine J, Allen J, Sakamoto J (2013) The effect of 24c-site (A) cation substitution on the tetragonal-cubic phase transition in Li7-xLa3-xAxZr2O12 garnet-based ceramic electrolyte. J Power Sources 230:261–266
El-shinawi H, Paterson GW, MacLaren D, Cussen EJ, Corr SA (2016) Low temperature densification of Al-doped Li7La3Zr2O12: a reliable and controllable synthesis of fast-ion conducting garnets. J Mat Chem. https://doi.org/10.1039/C6TA06961D
Bitzer M, Gestel TV, Uhlenbruck S, Buchkremer H-P (2016) Sol-gel synthesis of thin solid Li7La3Zr2O12 electrolyte films for Li-ion batteries. Thin Solid Films 615:128–134
Li C, Liu Y, He J, Brinkman KS (2017) Ga-substituted Li7La3Zr2O12: an investigation based on grain coarsening in garnet-type lithium ion conductors, 695:3744–3752
Zhang Y, Deng J, Hu D, Chen F, Shen Q, Zhang L, dan Dong S (2019) Synergistic regulation of garnet-type Ta-doped Li7La3Zr2O12 solid electrolyte by Li+ concentration and Li+ transport channel size. Electrochimica Acta 296:823–829
Xu B, Duan H, Xia W, Guo Y, Kang H, Li H, Liu H (2016) Multistep sintering to synthesize fast lithium garnets. J Power Sources 302:291–297
Allen JL, Wolfenstein J, Rangasamy E, Sakamoto J (2012) Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J Power Sources 206:315–319
Kumar PJ, Nishimura K, Senna M, Duevel A, Heitjans P, Kawaguchi T, Sakamoto N, Wakiya N, Suzuki H (2016) A novel low-temperature solid-state route for nanostructured cubic garnet Li7La3Zr2O12 and its application to Li-ion battery. R Soc Chem Adv. https://doi.org/10.1039/C6RA09695F
Rettenwander D, Welzl A, Cheng L, Fleig J, Musso M, Suard E, Doeff MM, Redhammer GJ, dan Amthauer G (2015) Synthesis, crystal chemistry, and electrochemical properties of Li7−2xLa3Zr2−xMoxO12 (x = 0.1−0.4): stabilization of the cubic garnet polymorph via substitution of Zr4+ by Mo6+. Inorg Chem 54:10440−10449
Rangasamy E, Wolfenstein J, Sakamoto J (2011) The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition of Li7La3Zr2O12. Solid State Ionics. https://doi.org/10.1016/j.ssi.2011.10.022
Kokal I, Somer M, Notten PHL, Hintzen HT (2011) Sol-gel synthesis and lithium ion conductivity of Li7La3Zr2O12 with garnet-related type structure. Solid State Ionics 185:42–46
Toby BH (2006) R factors in Rietveld analysis: How good is good enough? Powder Diff 21(1)
Werner P, Sieber H, Hillebrand R, Hesse D (1996) Time-dependent interfacial reaction mechanism in a spinel-forming solid state reaction studied by TEM. Mat Res Soc Symp Proc 446:191–196
Galusek D, Znášik P, Majling J (1999) The influence of cold isostatic pressing on compaction and properties of Mg-PSZ ceramics. J. Mat. Sci. Lett. 18:1347–1351
Hu Z, Liu H, Ruan H, Hu R, Su Y, Zhang L (2016) High Li-ion conductivity of Al-doped Li7La3Zr2O12 synthesized by solid-state reaction. Ceram Int. https://doi.org/10.1016/j.ceramint.2016.04.149
Xia W, Xu B, Duan H, Tang X, Guo Y, Kang H, Li H, Liu H (2017) Reaction mechanisms of lithium garnet pellets in ambient air: the effect of humidity and CO2. J Am Ceram Soc 1–8
Larraz G, Orera A, Sanjuan M (2013) Cubic phase of garnet-type Li7La3Zr2O12: the role of hydration. J Mat Chem A 1:11419–11428
Boulant A, Bardeau JF, Jouanneaux A, Emery J, Buzare JY, Bohnke O (2010) Reaction mechanisms of Li0.30La0.57TiO3 powder with ambient air: H+/Li+ exchange with water and Li2CO3 formation 39:3968–3975
Sharafi A, Yu S, Naguib M, Lee M, Ma C, Meyer HM, Nanda J, Chi M, Siegel DJ, Sakamoto J (2017) Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. J Mat Chem A. https://doi.org/10.1039/c7ta03162a
Acknowledgements
Authors thank The Ministry of Education and Culture, Directorate General of Higher Education, Republic Indonesia for funding this research under the scheme of PDUPT 2021.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Arifah, S.K., Rahmawati, F., Hidayat, Y. (2022). Cold Isostatic Pressing Treatment in the Preparation of Al and Y-Doped LLZO (Li6.15La3Zr1.75Al0.2Y0.25O12-δ) Solid Electrolyte. In: Kolhe, M., Muhammad, A., El Kharbachi, A., Yuwono, T.Y. (eds) Recent Advances in Renewable Energy Systems. Lecture Notes in Electrical Engineering, vol 876. Springer, Singapore. https://doi.org/10.1007/978-981-19-1581-9_26
Download citation
DOI: https://doi.org/10.1007/978-981-19-1581-9_26
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1580-2
Online ISBN: 978-981-19-1581-9
eBook Packages: EnergyEnergy (R0)