Abstract
Coronavirus disease 2019 (COVID-19) caused by the new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in early December 2019, in Wuhan city, the capital city of Hubei Province. This virus spread easily and rapidly worldwide. A lot of serology testing for COVID-19 are now available, and very powerful tools to understand the immune response to SARS-CoV-2, vaccine efficacy, hard immunity among communities, and so on. However, there remains several uncertainties about serology testing, compared to the direct virus detection test, such as reverse transcription polymerase chain reaction (RT-PCR) and antigen test. In this chapter, we introduce the current understanding of serological testing for COVID-19 to clarify how to use and select during the COVID-19 pandemic.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 What is SARS-CoV-2 Serology Testing?
Diagnostic modalities for COVID-19 infection used in clinical settings include the nucleic acid amplification test (NAAT), antigen testing, and serology testing. Serology testing, also known as antibody testing, is usually conducted after complete recovery from COVID-19. While serological immunoassays are now widely available from many diagnostic manufacturers globally, there is significant debate regarding their clinical utility, as well as the appropriate clinical and analytical performance characteristics for routine applications during the current pandemic.
To date, there have been few guidelines or statements regarding serological testing. Serological testing has been used to confirm the prevalence of COVID-19 infection in certain cohorts, such as communities or healthcare professionals.
In addition, serological testing can be used for the following purposes:,Footnote 1 Footnote 2
-
to quantitatively evaluate the antibody response in COVID-19 patients;
-
to assist in identifying potential convalescent plasma donors;
-
to assist in identifying immunity and evaluating the antibody response to vaccines;
-
to assist in monitoring the progression of herd immunity.
As such, serological testing offers marked potential to understand COVID-19. However, just like for NAAT, each serological test exhibits different sensitivity and specificity among each testing method, and the antigen used for detection in each assay differs, which will consequently affect the sensitivity and specificity as well.
In this chapter, we briefly describe the type and selection of serological testing and a case study involving a cohort of essential workers in Kyoto city.
2 Antibody Testing for COVID-19: Types and Selection
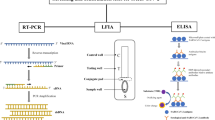
The diagnosis of COVID-19 is mainly based on viral nucleic acid detection, such as by the reverse transcription-polymerase chain reaction (RT-PCR). Although RT-PCR assays generally have high sensitivity (71–98%), false-negative results can occur due to inappropriate sample collection.Footnote 3 Furthermore, more than 80% of people infected with COVID-19, especially young people, are known to be asymptomatic or have mild disease. It is difficult to diagnose such people with COVID-19. Therefore, it is problematic to clarify the actual prevalence of COVID-19 using direct detection methods.
If pathogenic microorganisms, including SARS-CoV-2, infect humans, immune responses occur in the body. In this process, the production of specific antibodies (immunoglobulins) by the host's plasma B cells on encountering the pathogens comprises one of the most important immune responses. These responses can occur even if the person has no obvious symptoms of infection (for example, fever, cough, and shortness of breath). Antibody tests, also known as serology tests, detect the host's immune response to the infection rather than detecting the virus itself. Antibody tests are used to detect specific antibodies in human blood and are considered as important methods in epidemiological research and to monitor seropositivity. Moreover, a serology test can provide information on the immune status after vaccination, and could help determine appropriate donors of convalescent plasma.
For example, in the guidelines of the Centers for Disease Control and Prevention (CDC) in America, the antibody test has been designed and validated for surveillance and research purposes. Its purpose is to estimate the percentage of the population previously infected with SARS-CoV-2, which is necessary to guide the response to the pandemic and protect the public’s health.Footnote 4
2.1 CDC Guidelines and EUA of Antibody Testing by FDA
The CDC summarized antibody tests for COVID-19 in their “Interim Guidelines for COVID-19 Antibody Testing”, as followsFootnote 5:
-
Several serologic assays for SARS-CoV-2 have received Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA), which has independently reviewed their performance.
-
Currently, there is no known advantage of assays testing for IgG, IgM, IgG, or total antibody.
-
It is important to minimize false-positive test results by choosing an assay with high specificity and by testing populations and individuals with an elevated likelihood of previous exposure to SARS-CoV-2. Alternatively, an orthogonal testing algorithm (that is, employing two independent tests in sequence when the first test yields a positive result) can be used when the expected positive predictive value of a single test is low.
-
Antibodies most commonly become detectable 1–3 weeks after symptom onset, at which time evidence suggests that infectiousness markedly decreases and some degree of immunity from future infection has developed. However, additional data are needed before revising public health recommendations based on serologic test results, including decisions on discontinuing physical distancing and using personal protective equipment.
At the end of December 2020, many antibody tests for COVID-19 had been made available and approved by the FDA under EUA.Footnote 6 (Table 1) These tests show high sensitivity and specificity. However, the sensitivity of LFAs is relatively low compared with other laboratory assays, such as ELISA and other high-throughput assays. According to the “Interim Guidelines for COVID-19 Antibody Testing” by the CDC, antibody tests with sufficiently high specificity (for example, > 99.5%) are required to ensure a high positive predictive value (for example, 95%). Therefore, ELISA and other high-throughput assays are more appropriate than LFAs for seroprevalence surveillance.
2.2 Types of Antibody Testing
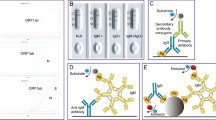
Antibody tests for COVID-19 are markedly diverse in terms of test methods, target proteins, and immunoglobulin classes they detect (Table 1). Actually, multiple different types of assays are available to detect different aspects of the immune response and functionality of antibodies. Test methods vary widely, including: the lateral flow assay (LFA), which is often used for point-of-care testing, enzyme-linked immunosorbent assay (ELISA), and other automated high-throughput assays, such as the electro-chemiluminescent immunoassay (ECLIA), chemiluminescent immunoassay (CLIA), and chemiluminescent microparticle immunoassay (CMIA).Footnote 7 These assays mainly use spike protein, nucleocapsid protein, or their combination as target protein. The spike protein of SARS-CoV-2, which plays an important role in fusion and entry into the host cell, consists of an S1 domain that contains the receptor-binding domain (RBD) and S2 domain. The nucleocapsid protein of SARS-CoV-2 has a role in binding and packing of the viral RNA genome into a helical nucleocapsid structure during viral replication. These tests are divided into two categories to detect either binding (binding antibody detection) or neutralizing (neutralizing antibody detection) antibodies. The characteristics of each assay are summarized in Table 2.Footnote 8
2.2.1 Binding Antibody Detection
Lateral flow assays (LFAs) are widely used in human health for point-of-care testing (POCT) because of their convenience and ease of use. LFAs can be performed by a healthcare professional or by the patient in some instances, and in a range of settings, such as a hospital laboratory, clinic, or home. Sample preparation is very simple, and it only takes about 10–30 min to obtain the results. Typical LFAs use a test strip and require a drop of whole blood, plasma, or serum to detect the presence of immunoglobulins (for example, IgA, IgG, or IgM) against SARS-CoV-2 antigen. Although some LFAs need a specific instrument for each assay to measure the results, they are generally judged visually.
The ELISA assay, currently one of the most commonly used assays for antibody testing, is a laboratory-based test with an average time-to-result of 2–5 h. ELISA typically uses a plate whose surface of each well is coated with a specific antigen to bind to and detect the corresponding host immunoglobulins (for example, IgA, IgG, or IgM) in whole blood, plasma, or serum samples. Then, the antigen–antibody complex is detected by a second antibody and a colorimetric or fluorescent substrate.
Other assays are usually performed using automated clinical laboratory equipment, and many samples are analyzed simultaneously. For example, the CLIA assay is similar to ELISA in that it takes advantage of high binding affinity between antigens and host antibodies but uses chemical probes that emit light through a chemical reaction to generate a positive signal. CLIA has a shorter average time-to-result (1–2 h).Footnote 9
CLIA and ELISA are both high-throughput laboratory-based assays with high concordance.Footnote 10
Surrogate virus neutralization tests (sVNT) are another type of binding antibody detection test. These assays are aimed to detect potential neutralizing antibodies that inhibit the interaction between RBD and angiotensin-converting enzyme 2 (ACE2, the cell receptor responsible for mediating infection by SARS-CoV-2).
These assays can be performed in BSL-2 laboratories.
2.2.2 Neutralizing Antibody Detection
The neutralizing antibody detection assay (neutralization assay) is a laboratory-based test that uses live virus and cell culture methods to determine if patient antibodies can prevent viral infection in vitro. The test procedures are laborious and must be performed in laboratories with designated biosafety certificates (for example, BSL-3) to culture SARS-CoV-2-infected cells and has a time-to-result of 3–5 days.Footnote 11
2.3 Dynamics of Seroconversion in COVID-19 Patients
The timelines for the SARS-CoV-2 viral load and dynamics of IgA, IgG, and IgM are shown in Fig. 1.Footnote 12 Antibodies are not detectable in the early phase of COVID-19 infection, but they can be detected after viral load increase.
Dynamics of seroconversion in COVID-19 patientsFootnote
Wölfel et al. (2020).
Figure 2 shows the seropositive rate from symptom onset among COVID-19 patients. The results of two total antibody assays (A_Ab and C_Ab), two IgG assays (B_IgG and C_IgG), and two IgM assays (B_IgM and C_IgM) were evaluated, using 269 serum samples from 27 hospitalized patients at Kyoto University Hospital, Kyoto, Japan. The positive rate of antibody testing gradually rises until 15 days from symptom onset. The positive rate of pan-immunoglobulin assays is high and similar to that of IgG assays, and these are higher than that of IgM assays. As described in previous studies,Footnote 14 the positive rate is relatively low in the early stage of COVID-19, because the host's immune response remains insufficiently established. Therefore, antibody testing may be less effective in this period. There are no clear data on how long the antibodies for SARS-CoV-2 are detectable. IgG could be detectable at least 49 days after symptom onset.Footnote 15 On the other hand, IgM might not be detectable beyond 30 days after onset.
2.4 Antibody Test Selection
To use antibody tests appropriately and effectively, it is important to comprehensively understand their performance characteristics and limitations. At present, antibody testing can be used most effectively for seroprevalence surveillance. The use of antibody tests to diagnose COVID-19 infection and evaluate vaccine immunity or convalescent serum is still controversial and requires further evaluation. When antibody tests are used in clinical settings, those with a high sensitivity of greater than 99.5% should be chosen, especially in areas with a low prevalence of COVID-19.
Moreover, the tests should be repeated if necessary. The results of a single antibody test are not sufficiently accurate in low-prevalence populations.Footnote 16 Thus, in these situations, a single test is rarely used to make a decision on whether a person has been infected previously or truly has specific antibodies to the virus. A second test that detects the presence of antibodies to a different viral protein would generally be needed to increase the accuracy of the overall test results. One concern regarding antibody testing should be noted: COVID-19 patients who are asymptomatic or with mild disease might have a lower immune response than patients with severe disease, and immunoglobulins could decline to undetectable levels throughout the course of infection. Therefore, well-designed research is needed, such as seroprevalence surveillance repeated every two or three months in the same study population.
3 Epidemiological Study of COVID-19
The novel coronavirus, SARS-CoV-2 infection (COVID-19), was first reported from China at the end of 2019. This virus has spread very rapidly, and the resulting pandemic remains an international public health emergency. Data on how many people have been infected comprise one of the most important epidemiological factors influencing public health policies. To date, many studies have been reported on national or local seroprevalence surveillance. During the present global pandemic of COVID-19, national surveillance data play a critical role in combatting and controlling the COVID-19 outbreak. In addition, local epidemiological data also play a key role, because local administrations should manage infection control in each outbreak area. Therefore, we perform seroprevalence surveillance in Kyoto city.
3.1 Previous Study on Seroprevalence of COVID-19
Seroprevalence surveillances of COVID-19 have been conducted all over the world, and several examples are presented as follows:
In the US, nationwide repeated cross-sectional seroprevalence research was conducted using a total of 177,919 serum samples from all 50 states between July and September 2020,Footnote 17 although about 7.2 million people had been reported to be infected at the end of September 2020. Commercially available high-throughput IgG assays, all of which have high specificity, were used, including the Abbott ARCHITECT assay, Ortho-Clinical Diagnostics VITROS assay, and Roche Elecsys assay. At that time, the overall prevalence of COVID-19 in the US was less than 10%, ranging from almost 0% (Alaska State) to over 20% (New York State). The outbreak of COVID-19 remains an existential threat in the US. Confirmed cases of COVID-19 have doubled since October 2020.Footnote 18 Therefore, a prevalence rate of 20% might be insufficient for herd immunity.
In the UK, seroprevalence was reported by Public Health England in October 2020.Footnote 19 This research was conducted using the Euroimmun ELISA assay and healthy blood donors. London was the most prevalent area (about 15%) in the UK, followed by the north west (10%), and east of England (8%).
In Germany, in early 2020, a seroprevalence of 0.91% was reported, using the Euroimmun ELISA assay.Footnote 20
In Japan, the Ministry of Health, Labour and Welfare reported a COVID-19 seroprevalence of 0.038% in Tokyo, 0.02% in Osaka, and 0.004% in Miyagi Prefecture in June 2020. The Abbott ARCHITECT assay and Roche Elecsys assay were used in this research.Footnote 21
3.2 Prevalence Surveillance in Kyoto City
We started seroprevalence surveillance in Kyoto city from July 2020. The initial research was conducted between July and August 2020. Antibody testing was performed using the YHLO CLIA IgG and IgM kit, a high-throughput CLIA assay showing high-level concordance with the Roche Elecsys assay. In our research, the sensitivity and specificity of this IgG assay were 91.9% (95% confidence interval: 87.7–95.1) and 100% (95%CI: 96.4–100), respectively. Among the 1737 serum samples from healthcare workers and essential workers analyzed, only eight (0.46%) were seropositive for SARS-CoV-2.
As shown in Fig. 3, the daily number of confirmed cases of COVID-19 has been increasing since the end of October 2020.Footnote 22 This local surveillance should be continued to evaluate and estimate the actual prevalence of COVID-19 in this region.
Only a year has passed since we first encountered COVID-19. Throughout that year, we have been trying to understand what “it” is and how “it” behaves. Due to concerted efforts, various testing modalities can now be used in clinical settings. At the present point, we have reached the stage where we need to determine how to appropriately apply serology testing in order to control the COVID-19 pandemic through our case study in Kyoto city.
Notes
- 1.
Bohn et al. (2020).
- 2.
Interim Guidelines for COVID-19 Antibody Testing.
- 3.
The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing.
- 4.
Interim Guidelines for COVID-19 Antibody Testing.
- 5.
Interim Guidelines for COVID-19 Antibody Testing.
- 6.
EUA Authorized Serology Test Performance.
- 7.
Ghaffari et al. (2020).
- 8.
Bastos et al. (2020).
- 9.
Ghaffari et al. (2020).
- 10.
Vashist (2020).
- 11.
Ghaffari et al. (2020).
- 12.
Ghaffari et al. (2020).
- 13.
Wölfel et al. (2020).
- 14.
Wölfel et al. (2020).
- 15.
Zhang et al. (2020).
- 16.
Interim Guidelines for COVID-19 Antibody Testing.
- 17.
Bajema et al. (2020).
- 18.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU).
- 19.
Sero-surveillance of COVID-19.
- 20.
Fischer et al. (2020).
- 21.
Result of Serosurveillance, in Japanese.
- 22.
COVID-19 in Kyoto Prefecture.
References
Bajema KL et al (2020) Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Internal Medicine, 24 Nov 2020
Bastos ML et al (2020) Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ, 1 July 2020
Bohn MK et al (2020) IFCC Interim Guidelines on Serological Testing of Antibodies against SARS-CoV-2. Clin Chem Lab Med, 7 Oct 2020
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html
EUA Authorized Serology Test Performance. www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
Fischer B et al (2020) SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Euro Surveill, 16 July 2020
Ghaffari A et al (2020) COVID-19 Serological Tests: How Well Do They Actually Perform?, 4 July 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7400479/
Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing. www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/diagnostics/idsa-covid-19-gl-dx-v2.0.0.pdf
Interim Guidelines for COVID-19 Antibody Testing. www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html
Kyoto Prefecture. COVID-19 in Kyoto Prefecture, in Japanese. https://kyoto.stopcovid19.jp/
Ministry of Health, Labour and Welfare. Result of Serosurveillance, in Japanese. www.mhlw.go.jp/content/10906000/000640184.pdf
Public Health England. Sero-surveillance of COVID-19. www.gov.uk/government/publications/national-covid-19-surveillance-reports/sero-surveillance-of-covid-19
Vashist SK (2020) In Vitro diagnostic assays for COVID-19: Recent advances and emerging trends. Diagnostics, April 5
Wölfel R et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature, December 11
Zhang J et al. (2020) Serological detection of 2019-nCoV respond to the epidemic: A useful complement to nucleic acid testing. medRxiv (preprint), March 10
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 RIETI
About this chapter
Cite this chapter
Yamamoto, M., Matsumura, Y., Nagao, M. (2022). Application of SARS-CoV-2 Serology Testing: A Case Study. In: Yano, M., Matsuda, F., Sakuntabhai, A., Hirota, S. (eds) Socio-Life Science and the COVID-19 Outbreak. Economics, Law, and Institutions in Asia Pacific. Springer, Singapore. https://doi.org/10.1007/978-981-16-5727-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-5727-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5726-9
Online ISBN: 978-981-16-5727-6
eBook Packages: Political Science and International StudiesPolitical Science and International Studies (R0)