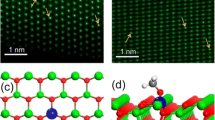
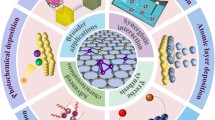
Abstract
The quantity of information available on subject such as synthesis and catalysis is vast. However, it is significant to integrate both subjects and present a comprehensive article. The present chapter is a small contribution to acclaim the role of synthesis in the development of catalysis industry and research. Three main categories of catalysts that evolved over the years are bulk, dispersed or supported and single atom catalysts (SACs)—which is an ultimate possible form of the dispersed catalysts. With the advancement in research on synthetic techniques, the dream of dispersion of single atoms on support could be realized by managing the associated high surface energy, which is a chief obstacle causing agglomeration and migration of metal ions. SACs are promising catalysts with exceptionally high activity, selectivity and turn over number. The catalyst industry is heavily dependent on bulk and dispersed catalysts, while SAC is at research stage. This chapter aims to give a brief account of all three classes and synthetic methods used to prepare industrial catalysts. It also gives an overview of novel/modified methodologies used to prepare nanoparticles of mixed metal bulk oxides and supported catalysts at large scale. Atomic layer deposition, wet chemical co-precipitation route, photo-deposition and atom trapping are four different approaches adopted to synthesize SACs are also discussed. Finally, it emphasizes synthesis–activity correlations by using examples from our laboratory on thermal reactions ranging from low-temperature CO oxidation, CO + N2O to high-temperature sulfuric acid decomposition catalytic reactions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Schmal M (2016) Heterogeneous catalysis and its industrial applications. Springer International Publishing
Thomas JM, Thomas WJ (2014) Principles and practice of heterogeneous catalysis, 2nd edn. Wiley-VCH, Weinheim, Germany
Ross J (2018) Contemporary catalysis, fundamentals and current applications, 1st edn. Elsevier
Védrine JC (2017) Heterogeneous catalysis on metal oxides. Catalysts 7:341
Mabilon G, Durand D, Prigent M (1991) Kinetics of the physico-chemical and catalytic activity evolution of a Pt-Rh catalyst in automotive exhaust gas. In: Crucq A (ed), Catalysis and automotive pollution control II. Elsevier, Amsterdam, pp 569
Shinjoh H, Muraki H, Fujitani Y (1991) Effect of severe thermal aging on noble metal catalysts. In: Crucq A (ed) Catalysis and automotive pollution control II. Elsevier, Amsterdam, pp 617
Leigh GJ, Haber-Bosch (2004) Other industrial processes. In: Smith BE, Richards RL, Newton WE (eds) Catalysts for nitrogen fixation. Nitrogen fixation: origins, applications, and research progress, vol 1. Springer, Dordrecht
Staffell I, Scamman D, Abad AV, Balcombe P, Dodds PE, Ekins P, Shah N, Ward KR (2019) The role of hydrogen and fuel cells in the global energy system. Energy Environ Sci 12:463
Kozuch S, Martin JML (2012) Turning over. Definitions in catalytic cycles. ACS Catal 2:2787
Patel S, Kundu S, Halder P, Marzbali MH, Chiang K, Surapaneni A, Shah K (2020) Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int J Hydrogen Energy 45:29978
Golodets GI (1983) Heterogeneous catalytic reactions involving molecular oxygen, studies in surface science and catalysis, vol 15. Elsevier, Amsterdam
Hagen J (2015) Industrial catalysis: a practical approach, 3rd edn. Wiley-VCH Verlag GmbH & Co, KGaA
Satterfield CN (1993) Heterogeneous catalysis in industrial processes. McGraw-Hill, Singapore
Ross JRH (2011) Heterogeneous catalysis 1st edn. In: Fundamentals and applications, Elsevier
Somorjai GA (1994) Introduction to surface chemistry and catalysis, John Wiley & Sons, Inc., New York, pp 444
Bond GC (1972) Principles of catalysis, 2nd edn. The chemical society, Burlington House, London
Pai MR, Banerjee AM, Rawool SA, Singhal A, Nayak C, Ehrman SH, Tripathi AK, Bharadwaj SR (2016) A comprehensive study on sunlight driven photocatalytic hydrogen generation using low cost nanocrystalline Cu-Ti oxides. Sol Energy Mater Sol Cells 154:104
Widmann D, Behman RJ (2018) Dynamic surface composition in a Mars-van Krevelen type reaction: CO oxidation on Au/TiO2. J Catalysis 357:263
Mars P, van Krevelen DW (1954) Oxidation carried out by means of vandium oxide catalysts. Chem Eng Sci Suppl 3:41
Dadyburjor DB, Jewur SS, Ruckenstein E (1979) Selective oxidation of hydrocarbons on composite oxides. Catal Rev Sci Eng 19:293
Schmidt F (2001) New catalyst preparation technologies—observed from an industrial viewpoint. Appl Catal A Gen 221:15
Regalbuto J (2007) Catalyst preparation, science and engineering, CRC Press, Boca Raton, FL
Saab R, Polychronopoulou K, Zheng L, Kumar S, Schiffer A (2020) Synthesis and performance evaluation of hydrocracking catalysts: A review. J Ind Eng Chem 89:83
Bartholomew CH, Farrauto RJ (2006) Fundamentals of industrial catalytic processes. John Wiley & Sons Inc., Hoboken, NJ
Deutschmann O, Knözinger H, Kochloefl K, Turek T (2011) Ullmann’s encyclopedia of industrial chemistry, heterogeneous catalysis and solid catalysts, 7th edn. Wiley-VCH Verlag GmbH, Weinheim
Pai MR, Wani BN, Belapurkar AD, Gupta NM (2004) Role of substitution in catalytic activity of La–Th–V–O mixed oxides for the reaction of methanol. J Mol Catal A: Chemical 223:275
Pai MR, Wani BN, Gupta NM (2002) Synthesis, characterization and thermal behavior of Th-Mn-VO oxides. J Mater Sci Lett 21:1187
Pai MR, Wani BN, Gupta NM (2005) Effect of La substitution on thermal stability of ThV2O7. Thermochim Acta 425(1-2):109
Pai MR, Wani BN, Gupta NM (2006) The role of substitution-induced micro-structural defects on the redox behavior and catalytic activity of LaxTh1− x (VO3− δ) 4 mixed oxide catalysts. J Phys Chem Solids 67:1502
Achary SN, Pai MR, Mishra R, Tyagi AK (2008) Crystal structure of thorium metavanadate (Th (VO3)4). J Alloys Compd 453:332
Sokolovski VD (1990) Principles of oxidative catalysis on solid oxides. Catal Rev-Sci Eng 32:1
Grasselli RK, Burrington JD (1981) Selective oxidation and ammoxidation of propylene by heterogeneous catalysis. Adv Catal 30:133
Courty P, Ajot H, Delmon B (1973) Acad CR Sci Ser C 276:1147
Sazonov VA, Popovskii VV, Boreskov GK (1968) Kinetica i Kataliz 9:307
Pankratyev YD, Tichy J (1975) Binding energy of oxygen with a Vanadium-Molybdenum oxide on silica catalyst. React Kin Catal Lett 2:319
Dunn JP, Koppula PR, Stenger HG, Wachs IE (1998) Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts. Appl Catal B Environ 19:103
Bingre R, Louis B, Nguyen P (2018) An overview on zeolite shaping technology and solutions to overcome diffusion limitations. Catalysts 8(4):163
Li WG, Hu YJ, Jiang H, Li CZ (2019) Aerosol spray pyrolysis synthesis of porous anatase TiO2 microspheres with tailored photocatalytic activity. Acta Metall Sin (Engl Lett) 32(3):286
Siegel RW, Ramaswami S, Hahn H, Zongquan L, Ting L, Grousky R (1988) Synthesis, characterization, and properties of nanophase TiO2. J Mater Res 3:1367
El-Shall MS, Slack W, Vann W, Kane D, Hanely D (1994) Synthesis of nanoscale metal oxide particles using laser vaporization/condensation in a diffusion cloud chamber. J Phys Chem 98(12):3067
Edelstein AS, (1994) Nanoparticles from a supersaturated vapor. In: Hadjipanayis GC, Siegel RW (eds) Nanophase Materials. Springer Netherlands, pp 73–80
Kodas TT (1989) Aerosol spray pyrolysis synthesis techniques. Adv Mater 6:180
Messing GL, Zhang SC, Jayanthi GV (1993) Ceramic powder synthesis by spray pyrolysis. J Am Ceram Soc 76(11):2707
Chow GM, Gonsalves KE (1996) (eds) Nanotechnology, molecularly designed materials. American chemical society, Washington, DC, pp 64–78
Skandan G, Chen Y-J, Glumac N, Kear BH (1999) Synthesis of oxide nanoparticles in low pressure flames. Nanostructured Materials 11(2):149–158
Khaleel A, Richards RM (2001) Ceramics. In: Klabunde KJ (ed) Nanoscale materials in chemistry. Wiley- Interscience, New York, pp 85–120
Mehrotra RC, Singh A (1997) Recent trends in metal alkoxide chemistry. In: Karlin KD (ed) Progress in inorganic chemistry. John Wiley&Sons Inc., pp 239
Herrig H, Hempelmann R (1996) A colloidal approach to nanometre-sized mixed oxide ceramic powders. Mater Lett 27:287
Thimmaiah S, Rajamathi M, Singh N, Bera P, Meldrum F, Chandershekhar N, Seshadsi R (2001) A solvothermal route to capped nanoparticles of g-Fe2O3 and CoFe2O4. J Mater Chem 11:3215
Laine RM, Waldner K, Bickmore C, Treadwell DR (1999) Ultrafine powders by flame spray pyrolysis. US Patent, 5, 958, 361
Pramanik P, Roy JC, Sen A, Pati RK (2001) Novel chemical methods for preparation of nanosized oxide ceramics. In: International symposium on metastable, mechanically alloyed and nanocrystaline materials; Proceedings of ISMANAM-2000, Oxford, UK, Material Science Forum, vols 360–362, 623–630
Shin H, Lee S, Jung HS, Kim J-B (2013) Effect of ball size and powder loading in the milling efficiency of a laboratory-scale wet ball mill. Ceram Int 39(8):8963
Anderson JA, García MF (2005) Catalysis science series- volume 5: supported metals in catalysis. Imperial College press
Geus JW, van Dillen AJ (2008) Preparation of supported catalysts by deposition–precipitation. In: Ertl G, Knozinger H, Schüth F, Weitkamp J (eds) Handbook of heterogeneous Catalysis, vol 1. Wiley-VCH Verlag GmbH& Co. KGaA, Weinheim, Germany, pp 428
Geus JW, van Veen JR (1999) Catalysis, an integrated approach to homogeneous, heterogeneous and industrial catalysis. In: Moulijn JA, van Leeuwen PWNM, van Santen RA (eds) Elsevier, Amsterdam, pp 467
Van Dillen AJ, Terorde RJAM, Lensveld DJ, Geus JW, de Jong KP (2003) Synthesis of supported catalysts by impregnation and drying using aqueous chelated metal complexes. J Catal 216:257
Nijhuis TA, Beers AEW, Vergunst T, Hoek I, Kapteijn F, Moulijn JA (2001) Preparation of monolithic catalysts. Catal Rev Sci Eng 43:345
Vergunst T, Linders MJG, Kapteijn F, Moulijn JA (2001) Carbon-based monolithic structures. Catal Rev Sci Eng 43:291
Geus JW (1983) Production and thermal pretreatment supported catalysts. Stud Surf Sci Catal 16:1
van der Grift CJG, Boon AQM, van Veldhuizen AJW, Trommar HGJ, Geus JW, Quinson JF, Brun M (1990) Preparation and characterization of porous silica spheres containing a copper (oxide) catalyst. Appl Catal 65:225
Mul G, Hirschon AS (2001) Effect of preparation procedures on the activity of supported palladium/lanthanum methanol decomposition catalysts. Catal Today 65:59
Liu X, Khinast JG, Glasser BJ (2008) A parametric investigation of impregnation and drying of supported catalysts. Chem Eng Sci 63:4517
Schwarz JA, Contescu C, Contescu A (1995) Methods for preparation of catalytic materials. Chem Rev 95(3):477
Geenen PV, Boss HJ, Pott GT (1982) A study of the vapor-phase epoxidation of propylene and ethylene on silver and silver-gold alloy catalysts. J Catal 77:499
Munnik P, de Jongh PE, de Jong KP (2015) Recent developments in the synthesis of supported catalysts. Chem Rev 115:6687
Geus JW, van Dillen AJ (1999) Preparation of supported catalysts by deposition‐precipitation. In: Ertl G, Knözinger H, Weitkamp J (eds) Preparation of solid catalysts. Wiley‐VCH Verlag GmbH, pp 460–487
Mehrabadi BAT, Eskandari S, Khan U, White RD, Regalbuto JR (2017) A review of preparation methods for supported metal catalysts. Adv Catal 61:1
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0°C. Chem Lett 16(2):405
Somorjai GA, Park JY (2008) Molecular surface chemistry by metal single crystals and nanoparticles from vacuum to high pressure. Chem Soc Rev 37:2155
Crespo-Quesada M, Yarulin A, Jin M, Xia Y, Kiwi-Minsker L (2011) Structure sensitivity of alkynol hydrogenation on shape- and size-controlled palladium nanocrystals: Which sites are most active and selective? J Am Chem Soc 133:12787
Yang X-F, Wang A, Qiao B, LI J, Liu J, Zhang T (2013) Single-atom catalysts: A new Frontier in heterogeneous catalysis. Acc Chem Res 46:1740
Wang G, Su J, Gong Y, Zhou M, Li J (2010) Chemistry on single atoms: spontaneous hydrogen production from reactions of transition‐metal atoms with methanol at cryogenic temperatures. Angew Chem Int Ed 49(7):1302
Qiao B, Wang A, Yang X, Allard LF, Jiang Z, Cui Y, Liu J, Li J, Zhang T (2011) Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem 3:634
Lu J, Aydin C, Browning ND, Gates BC (2012) Imaging isolated gold atom catalytic sites in zeolite NaY. Angew Chem Int Ed 51(24):5842-5846, https://doi.org/10.1002/anie.201107391
Heiz U, Sanchez A, Abbet S, Schneider WD (1999) Catalytic oxidation of carbon monoxide on monodispersed platinum clusters: each atom counts. J Am Chem Soc 121:3214
Kaden WE, Wu T, Kunkel WA, Anderson SL (2009) Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science. Science 326:826-829, 10.1126/science.1180297
Abbet S, Sanchez A, Heiz U, Schneider WD, Ferrari AM, Pacchioni G, Rosch N (2000) Acetylene cyclotrimerization on supported size-selected Pdn clusters (1 < = n < = 30): one atom is enough!. J Am Chem Soc 122:3453
Wang Q, Zhang D, Chen Y, Fu W-F, Lv X-J (2019) Single-atom catalysts for photocatalytic reactions. ACS Sustain Chem Eng 7:6430
Asim N, Ahmadi S, Alghoul MA, Hammadi FY, Saeedfar K, Sopian K (2014) Research and development aspects on chemical preparation techniques of photoanodes for dye sensitized solar cells. Int J Photoenergy 2014:518156
Sun S, Zhang G, Gauquelin N, Chen N, Zhou J, Yang S, Chen W, Meng X, Geng D, Banis MN, Li R, Ye S, Knights S, Botton GA, Sham T-K, Sun X (2013) Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci Rep 3:1775
Moses-DeBusk M, Yoon M, Allard LF, Mullins DR, Wu Z, Yang X, Veith G, Stocks GM, Narula CK (2013) CO oxidation on supported single Pt atoms: Experimental and ab Initio density functional studies of CO interaction with Pt Atom on θ-Al2O3(010) surface. J Am Chem Soc 135:12634
Liu P, Zhao Y, Qin R, Mo S, Chen G, Gu L, Chevrier DM, Zhang P, Guo Q, Zang D, Wu B, Fu G, Zheng N (2016) Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352(6287):797
Carrillo C, Johns TR, Xiong H, DeLaRiva A, Challa SR, Goeke RS, Artyushkova K, Li W, Kim CH, Datye AK (2014) Trapping of mobile Pt species by PdO nanoparticles under oxidizing conditions. J Phys Chem Lett 5(12):2089
Jones J, Xiong H, DeLaRiva AT, Peterson EJ, Pham H, Challa SR, Qi G, Oh S, Wiebenga MH, Hernández XIP, Wang Y, Datye AK (2016) Thermally stable singe-atom platinum-on-ceria catalysts via atom tapping. Science 353(6295):150
Weckhuysen BM, Keller DE (2003) Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal Today 78:25
Pai MR, Wani BN, Sreedhar B, Singh S, Gupta NM (2006) Catalytic and redox properties of nano-sized La0.8Sr0.2Mn1−xFexO3−δ mixed oxides synthesized by different routes. J Mol Catal A: Chem 246:128
Pai MR, Wani BN, Bharadwaj SR (2006) Synthesis, characterization and redox behavior of nano-size La0.8Sr0.2Mn0.8Fe0.2O3-δ. J Ind Chem Soc 83:336-341
Nishihata Y, Mizuki J, Akao T, Tanaka H, Uenishi M, Kimura M, Okamoto T, Hamada N (2002) Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 418:164
Kartha KK, Pai MR, Banerjee AM, Pai RV, Meena SS, Bharadwaj SR (2011) Modified surface and bulk properties of Fe-substituted lanthanum titanates enhances catalytic activity for CO+ N2O reaction. J Mol Catal A 335:158
Pai MR, Banerjee AM, Kartha K, Pai RV, Kamble VS, Bharadwaj SR (2010) Mechanism of CO + N2O reaction via transient CO32− species over crystalline Fe-Substituted lanthanum titanates. J Phys Chem B 114:6943
Kodama T, Gokon N (2007) Thermochemical cycles for high temperature solar hydrogen production. Chem Rev 107:4048
Onuki K, Kubo S, Terada A, Sakaba N, Hino R (2009) Thermochemical water-splitting cycle using iodine and sulfur. Energy Environ Sci 2:491
Barbarossa V, Brutti S, Diamanti M, Sau S, De Maria G (2006) Catalytic thermal decomposition of sulphuric acid in sulphur–iodine cycle for hydrogen production. Int J Hydrogen Energy 31:883
Ginosar DM, Petkovic LM, Glenn AW, Burch KC (2007) Stability of supported platinum sulfuric acid decomposition catalysts for use in thermochemical water splitting cycles. Int J Hydrogen Energy 32(4):482
Petkovic LM, Ginosar DM, Rollins HW, Burch KC, Pinhero PJ, Farrell HH (2008) Pt/TiO2 (rutile) catalysts for sulfuric acid decomposition in sulfur-based thermochemical water-splitting cycles Appl Catal A 338:27
Nagaraja BM, Jung KD, Ahn BS, Abimanyu H, Yoo KS (2009) Catalytic decomposition of SO3 over Pt/BaSO4 materials in sulfur-iodine cycle for hydrogen production. Ind Eng Chem Res 48:1451
Rashkeev SN, Ginosar DM, Petkovic LM, Farrell HH (2009) Catalytic activity of supported metal particles for sulfuric acid decomposition reaction. Catal Today 139:291
Dokiya M, Kameyama T, Fukuda K, Kotera Y (1977) The study of thermochemical hydrogen preparation—III: an oxygen-evolving step through the thermal splitting of sulfuric acid. Bull Chem Soc Jpn 50:2657
Banerjee AM, Pai MR, Meena SS, Tripathi AK, Bharadwaj SR (2011) Catalytic activities of cobalt, nickel and copper ferrospinels for sulfuric acid decomposition: the high temperature step in the sulfur based thermochemical water splitting cycles. Int J Hydrogen Energy 36:4768
Banerjee AM, Pai MR, Tewari R, Naina Raje, Tripathi AK, Bharadwaj SR, Das D (2015) A comprehensive study on Pt/Al2O3 granular catalyst used for sulfuric acid decomposition step in sulfur–iodine thermochemical cycle: Changes in catalyst structure, morphology and metal-support interaction. Appl Catal B: Environ 162:327-337
Banerjee AM, Pai MR, Bhattacharya K, Tripathi AK, Kamble VS, Bharadwaj SR, Kulshreshtha SK (2008) Catalytic decomposition of sulfuric acid on mixed Cr/Fe oxide samples and its application in sulfur–iodine cycle for hydrogen production. Int J Hydrogen Energy 33(1):319
Banerjee AM, Shirole AR, Pai MR, Tripathi AK, Bharadwaj SR, Das D, Sinha PK (2012) Catalytic activities of Fe2O3 and chromium doped Fe2O3 for sulfuric acid decomposition reaction in an integrated boiler, preheater, and catalytic decomposer. Appl Catal B Environ 127:36
Nadar A, Banerjee AM, Pai MR, Meena SS, Pai RV, Tewari R, Yusuf SM, Tripathi AK, Bharadwaj SR (2017) Nanostructured Fe2O3 dispersed on SiO2 as catalyst for high temperature sulfuric acid decomposition—Structural and morphological modifications on catalytic use and relevance of Fe2O3-SiO2 interactions. Appl Catal B Environ 217:154
Nadar A, Banerjee AM, Pai MR, Pai RV, Meena SS, Tewari R, Tripathi AK (2018) Catalytic properties of dispersed iron oxides Fe2O3/MO2 (M= Zr, Ce, Ti and Si) for sulfuric acid decomposition reaction: Role of support. Int J Hydrogen Energy 43:37
Nadar A, Banerjee AM, Pai MR, Meena SS, Patra AK, Sastry PU, Singh R, Singh MK, Tripathi AK (2021) Immobilization of crystalline Fe2O3 nanoparticles over SiO2 for creating an active and stable catalyst: A demand for high temperature sulfuric acid decomposition. Appl Catal B Environ 283:119610
Jin X, Dang L, Lohrman J, Subramaniam B, Ren S, Chaudhari RV (2013) Lattice-matched bimetallic CuPd-graphene nanocatalysts for facile conversion of biomass-derived polyols to chemicals. ACS Nano 7(2):1309
Acknowledgements
Authors sincerely thank Dr. A. K. Tyagi, Outstanding Scientist, Director, Chemistry Group and Head, Chemistry Division, BARC for his keen interest, support and encouragement. MRP sincerely thank her mentor and PhD guide, Dr. N. M. Gupta, former Head, Applied Chemistry Division, BARC for introducing to the exciting field of heterogeneous catalysis.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pai, M.R., Rawool, S.A., Singh, R.V., Banerjee, A.M., Tripathi, A.K. (2022). Role of Synthesis in Evolution of Catalyst: Bulk, Dispersed to Single Atom. In: Tyagi, A.K., Ningthoujam, R.S. (eds) Handbook on Synthesis Strategies for Advanced Materials. Indian Institute of Metals Series. Springer, Singapore. https://doi.org/10.1007/978-981-16-1803-1_17
Download citation
DOI: https://doi.org/10.1007/978-981-16-1803-1_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1802-4
Online ISBN: 978-981-16-1803-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)