Abstract
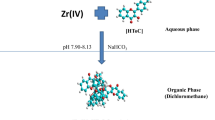
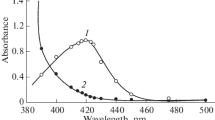
Micro determination of Zirconium (IV) has been carried out using 3-Hydroxy-2-[2′-(5′-methylthienyl)]-4H-chromen-4-one (HMTC) as an analytical reagent. Zr (IV) forms a 1:4 (M:L) yellow coloured complex with HMTC extracted into dichloromethane from ammoniacal medium (pH 7.05–7.09). The complex system shows a maximum at 424–440 nm and follows Beer’s law in the range 0.0–0.9 µg Zr (IV) ml−1 with an optimum range of determination as 0.27–0.79 µg Zr (IV) ml−1as detected from Ringbom plot. Zr (IV)-HMTC complex has molar absorptivity of 8.22 × 104 Lmol−1 cm−1, specific absorptivity of 0.900 ml g−1 cm−1 and Sandell’s sensitivity value 0.0011 µg Zr (IV)cm−2; the linear regression equation being Y = 0.981X − 0.036 (Y = absorbance, X = µg Zr (IV) ml−1) with the correlation coefficient 0.9987. Detection limit of the procedure is 0.0174 µg ml−1. The repercussions obtained are highly consistent with the standard deviation of ±0.0039 absorbance unit and has been confirmed by student’s t-test with 0.5% limit. The proposed technique has been successfully applied in diverse synthetic and industrial samples.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Schroeder AH, Balassa JJ (1966) Abnormal trace metals in man: zirconium. J Chronic Diseases 19(5):573–586
Lee DBN, Roberts M, Bluchel CG, Odell RA (2010) Zirconium: biomedical and nephrological. Appl ASAIO J 56(6):550–556
Ingelfinger JR (2016) A new era for the treatment of hyperkalemia. J Med 372(3):275–277
Chawaria M, Sharma HK (2018) A non-extractive spectrophotometric determination of Zirconium with6-Chloro-3-hydroxy-2-(2’-hydroxyphenyl)-4-oxo-4 H-1-benzopyran using propan-1-ol-H2O mixture as solvent. Internat J Green Herbal Chem 7(2):230–236
Chawaria M, Kumar A (2017) Extractive spectrophotometric determination of zirconium with 6-chloro-3-hydroxy-2-phenyl-4H-chromen-4-one as an analytical reagent. J Chem Bio Phy Sci 7(3):600–604
Lasheen TA, Hussein GM, Khawassek YM, Cheira MF (2013) Spectrophotometric determination of zirconium (IV) and hafnium (IV) with pyrazolo (1, 5-a) quinazolin-6-onederivative reagent. Anal Chem Indian J 12(10):368–376
Sumathi G, Sreenivasulu Reddy RT Direct and derivative spectrophotometric determination of zirconium(IV) with 2-hydroxynaphthaldehyde-phydroxybenzoichydrazone. J Appl Chem 2(1):81–85
Jain A, Prakash O, Kakkar LR (2010) Spectrophotometric determination of zirconium with 5,7-dibromo-8-hydroxyquinoline in presence of thiocyanate. J Anal Chem 65(8):820–824
Algar J, Flynn JP (1934) A new method for the synthesis of flavones. Proc Royal Irish Acad 42B:1–8; Oyamada T(1934) A new general method for the synthesis of flavonol derivatives. J Chem Soc Jpn 55:1256–1261
Agnihotri N, Mehta JR (2006) A highly sensitive extractive spectrophotometric determination of zirconium(IV) using 2-(2'-furyl)-3-hydroxy-4-oxo-4H-1-benzopyran. J Indian Chem Soc 83(8):846–848
Ringbom A (1938) On the accuracy of colorimetric analytical methods I. Z Anal Chem 115:332–343
Job P (1928) Formation and stability of inorganic compexes in solution. Ann Chim 9:113
Vosburgh WC, Cooper GC (1941) The identification of complex ion in solution by spectrophotometric measurements. J Am Chem Soc 63:437
Yoe JH, Jones AL (1944) Colorimetric determination of iron with disodium-1,2-dihydroxybenzene-3,5-disulfonate.Ind. Eng Chem (Anal.Ed.) 16:111
Hanwell MD, Curtis DE, lonie DC, Vandermeersch T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization and analysis platform. J Cheminform 4(17)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Dhonchak, C., Kaur, N., Agnihotri, R., Berar, U., Agnihotri, N. (2021). Trace Determination of Zirconium (IV) as its 3-Hydroxy-2-[2′-(5′-Methylthienyl)]-4H-Chromen-4-One Complex and Structural Elucidation by Quantization Technique. In: Marriwala, N., Tripathi, C.C., Kumar, D., Jain, S. (eds) Mobile Radio Communications and 5G Networks. Lecture Notes in Networks and Systems, vol 140. Springer, Singapore. https://doi.org/10.1007/978-981-15-7130-5_25
Download citation
DOI: https://doi.org/10.1007/978-981-15-7130-5_25
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7129-9
Online ISBN: 978-981-15-7130-5
eBook Packages: EngineeringEngineering (R0)