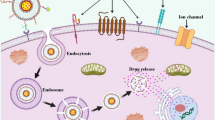
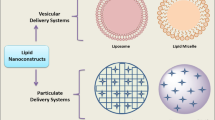
Abstract
With the booming development of bionanotechnology, a variety of multifunctional nanoparticle platforms have been explored to facilitate drug delivery in cancer treatment. Thanks to their good biocompatibility and versatile structures, the lipid-based nanoparticles can serve as ideal candidates to carry therapeutic reagents to cancer tissues. Additionally, the natural polymeric coating of liposomes could easily fuse with cellular membrane and minimize clearance by blood circulation. To date, their composition, properties, targeting strategies, reasonable design methods, and pharmacokinetics have been extensively studied to facilitate preclinical and clinical translations. In this chapter, we will introduce the structure and function of the lipid-based nanoparticle system, and a comprehensive review of the lipid-based nanoparticle drug delivery system will be presented.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Kreuter J (2007) Nanoparticles – a historical perspective. Int J Pharm 331(1):1–10
Gregoriadis G (1976) The carrier potential of liposomes in biology and medicine. N Engl J Med 295(13):704–710
Tran MA, Watts RJ, Robertson GP (2009) Use of liposomes as drug delivery vehicles for treatment of melanoma. Pigm Cell Melanoma Res 22(4):388–399
Torchilin VP (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4(2):145–160
Bangham AD, Standish MM, Watkins JC (1965) Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13(1):238–252
Sessa G, Weissmann G (1968) Phospholipid spherules (liposomes) as a model for biological membranes. J Lipid Res 9(3):310–318
Klausner RD, Kleinfeld AM, Hoover RL, Karnovsky MJ (1980) Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem 255(4):1286–1295
Gregoriadis G (1995) Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol 13(12):527–537
Allen TM (1998) Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs 56(5):747–756
Gubernator J (2011) Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv 8(5):565–580
Allen TM∗, Hansen CB, de Menezes DEL (1995) Pharmacokinetics of long-circulating liposomes. Adv Drug Deliv Rev 16(1–2):267–284
Lian T, Ho RJY (2011) Trends and developments in liposome drug delivery systems. J Pharm Sci 90(6):667–680
Mu LM, Ju RJ, Liu R et al (2017) Dual-functional drug liposomes in treatment of resistant cancers. Adv Drug Deliv Rev 115:46–56
Michelia MR, Bovab R, Maginib A, Emilianib C∗ (2012) Lipid-based nanocarriers for CNS-targeted drug delivery. Recent Pat CNS Drug Discov 7(1):71–86
Senior JH (1987) Fate and behavior of liposomes in vivo: a review of controlling factors. Crit Rev Ther Drug Carrier Syst 3(2):123–193
Mazzacuva F, Isacchi B, Bergonzi M et al (2011) Development and evaluation of conventional and PEGylated curcumin liposomes, absorption and tissue distribution studies in mice. Planta Med 77(12):87–120
Laverman P, Carstens MG, Boerman OC et al (2001) Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther 298(2):607–612
Lv Z, Yang Y, Wang J et al (2018) Optimization of the preparation conditions of borneol-modified ginkgolide liposomes by response surface methodology and study of their blood brain barrier permeability. Molecules 23(2):303–317
Yoshioka H, Goto H (1998) Inhibition adsorption of proteins on the liposome surface. US patent 5, 846, 458, 1998
Hope MJ, Mui B, Ansell S et al (1998) Cationic lipids, phosphatidylethanolamine and the intracellular delivery of polymeric, nucleic acid-based drugs. Membr Biochem 15(1):1–14
Ellens H, Bentz J, Szoka FC (1984) PH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry 23(7):1532–1538
Miyazaki M, Yuba E∗, Hayashi H et al (2018) Hyaluronic acid-based pH-sensitive polymer-modified liposomes for cell-specific intracellular drug delivery systems. Bioconjug Chem 29(1):44–55
Xia Y, Tian J, Chen X∗ (2016) Effect of surface properties on liposomal siRNA delivery. Biomaterials 79:56–68
Dakwar GR, Braeckmans K, Demeester J et al (2015) Disregarded effect of biological fluids in siRNA delivery: human ascites fluid severely restricts cellular uptake of nanoparticles. ACS Appl Mater Interfaces 7(43):24322–24329
Wang Y∗, Miao L, Satterlee A et al (2015) Delivery of oligonucleotides with lipid nanoparticles. Adv Drug Deliv Rev 87:68–80
Luo YL, Xu CF, Li HJ et al (2018) Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano 12(2):994–1005
Christensen D, Bøllehuus Hansen L, Leboux R et al (2019) A liposome-based adjuvant containing two delivery systems with the ability to induce mucosal immunoglobulin a following a parenteral immunization. ACS Nano 13(2):1116–1126
Bume G, Cevc G (1990) Liposomes for the sustained drug release in vivo. Biochim Biophys Acta 1029(1):91–97
Lasic DD, Martin FJ, Gabizon A et al (1991) Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta 1070(1):187–192
ElBayoumi TA, Torchilin VP (2010) Liposomes methods and protocols volume 1: pharmaceutical nanocarriers. In: Tamer AE, Vladimir PT (eds) Current trends in liposome research. Springer, London, pp 1–28
Pattni BS, Chupin VV, Torchilin VP∗ (2015) New developments in liposomal drug delivery. Chem Rev 115(19):10938–10966
Yingchoncharoen P, Kalinowski DS, Richardson DR (2016) Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharm Rev 68(3):701–787
Bangham AD (1982) Preparation of liposomes and methods for measuring their permeabilities. In: Hesketh TR, Kornberg HL, Metcalfe JC et al (eds) Technique in life science – technique in lipid and membrane biochemistry. Elsevier, Amsterdam, pp 1–25
Olson F, Hunt CA, Szoka FC et al (1979) Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta 557(1):9–23
Vemuri S, Rhodes CT (1995) Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharm Acta Helv 70(2):95–111
Hauser H (1982) Methods of preparation of lipid vesicles: assessment of their suitability for drug encapsulation. Trends Pharmacol Sci 3:274–277
Tyrrell DA, Heath TD, Colley CM et al (1976) New aspects of liposomes. Biochim Biophys Acta 457(3–4):259–302
Szoka F, Papahadjopoulos D (1978) Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A 75(9):4194–4198
Milsmann MHW, Schwendener RA, Weder HG (1978) The preparation of large single bilayer liposomes by a fast and controlled dialysis. Biochim Biophys Acta 512(1):147–155
Enoch HG, Strittmatter P (1979) Formation and properties of 1000-A-diameter, single-bilayer phospholipid vesicles. Proc Natl Acad Sci U S A 76(1):145–149
Gerritsen WJ, Verkley AJ, Zwaal RF et al (1978) Freeze-fracture appearance and disposition of band 3 protein from the human erythrocyte membrane in lipid vesicles. Eur J Biochem 85(1):255–261
Huang CH (1969) Phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry 8(1):344–352
Deamer D, Bangham AD (1976) Large volume liposomes by an ether vaporization method. Biochim Biophys Acta 443(3):629–634
Torchilin VP (2007) Micellar nanocarriers: pharmaceutical perspectives. Pharm Res 24(1):1–16
Edwards K, Johnsson M, Karlsson G et al (1997) Effect of polyethylene glycol-phospholipids on aggregate structure in preparations of small unilamellar liposomes. Biophys J 73(1):258–266
Lukyanov AN, Torchilin VP (2004) Micelles from lipid derivatives of water soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev 56(9):1273–1289
Rosen MJ, Kunjappu JT (2012) Surfactants and interfacial phenomena, 4th edn. Wiley, Hoboken
Lukyanov AN, Gao Z, Mazzola L et al (2002) Polyethylene glycol diacyl lipid micelles demonstrate increased accumulation in subcutaneous tumors in mice. Pharm Res 19(10):1424–1429
Gao Z, Lukyanov AN, Chakilam AR et al (2003) Diacyl lipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. J Drug Target 11(2):87–92
Wong HL, Bendayan R, Rauth AM et al (2007) Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev 59(6):491–504
Schwarz C, Mehnert W, Lucks JS et al (1994) Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Control Release 30(1):83–96
Wissing SA, Kayser O, Müller RH (2004) Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev 56(9):1257–1272
Müller RH, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm 50(1):161–177
Mehnert W, Mäder K (2001) Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–196
Gasco MR (1993) Method for producing solid lipid microspheres having a narrow size distribution. US Patent US5250236. 1991 Aug 2
Boltri L, Canal T, Esposito PA et al (1993) Lipid nanoparticles: evaluation of some critical formulation parameters. Proc Int Symp Control Release Bioact Mater 20:346–347
Sjöström B, Westesen K, Bergenståhl B (1993) Preparation of submicron drug particles in lecithin-stabilized o/w emulsions: II. Characterization of cholesteryl acetate particles. Int J Pharm 94(1–3):89–101
Westesen K, Siekmann B, Koch MHJ (1993) Investigations on the physical state of lipid nanoparticles by synchrotron radiation X-ray diffraction. Int J Pharm 93(1–3):189–199
Cavalli R, Caputo O, Gasco MR (1993) Solid lipospheres of doxorubicin and idarubicin. Int J Pharm 89(1):9–12
zurMühlen A, Schwarz C, Mehnert W (1998) Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur J Pharm Biopharm 45(2):149–155
Hadinoto K, Sundaresan A, Cheow WS (2013) Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm 85(3):427–443
Raemdonck K, Braeckmans K, Demeester J et al (2014) Merging the best of both worlds: hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem Soc Rev 43(1):444–472
Thevenot J, Troutier AL, David L et al (2007) Steric stabilization of lipid/polymer particle assemblies by poly(ethylene glycol)-lipids. Biomacromolecules 8(11):3651–3660
Fenart L, Casanova A, Dehouck B et al (1999) Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther 291(3):1017–1022
Mieszawska AJ, Gianella A, Cormode DP et al (2012) Engineering of lipid-coated PLGA nanoparticles with a tunable payload of diagnostically active nanocrystals for medical imaging. Chem Commun 48(47):5835–5837
Messerschmidt SK, Musyanovych A, Altvater M et al (2009) Targeted lipid-coated nanoparticles: delivery of tumor necrosis factor-functionalized particles to tumor cells. J Control Release 137(1):69–77
Hasan W, Chu K, Gullapalli A et al (2012) Delivery of multiples iRNAs using lipid coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett 12(1):287–292
Troutier AL, Delair T, Pichot C et al (2005) Physicochemical and interfacial investigation of lipid/polymer particle assemblies. Langmuir 21(4):1305–1313
Fang RH, Aryal S, Hu CMJ et al (2010) Quick synthesis of lipid-polymer hybrid nanoparticles with low polydispersity using a single-step sonication method. Langmuir 26(22):16958–16962
Valencia PM, Basto PA, Zhang L et al (2010) Single-step assembly of homogenous lipid-polymeric and lipid-quantum dot nanoparticles enabled by microfluidic rapid mixing. ACS Nano 4(3):1671–1679
Zhang L, Chan JM, Gu FX et al (2008) Self-assembled lipid–polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano 2(8):1696–1702
Chan JM, Zhang L, Yuet KP et al (2009) PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 30(8):1627–1634
Bershteyn A, Chaparro J, Yau R et al (2008) Polymer-supported lipid shells, onions, and flowers. Soft Matter 4(9):1787–1791
Cheow WS, Hadinoto K (2011) Factors affecting drug encapsulation and stability of lipid-polymer hybrid nanoparticles. Colloids Surf B Biointerfaces 85(2):214–220
Liu Y, Pan J, Feng SS (2010) Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: effects of surfactants on particles size, characteristics and in vitro performance. Int J Pharm 395(1–2):243–250
Chu CH, Wang YC, Huang HY et al (2011) Ultrafine PEG coated poly(lactic-co-glycolic acid) nanoparticles formulated by hydrophobic surfactant-assisted one-pot synthesis for biomedical applications. Nanotechnology 22(18):185601
Wacker M (2013) Nanocarriers for intravenous injection–the long hard road to the market. Int J Pharm 457(1):50–62
Pastorino F, Brignole C, Marimpietri D et al (2003) Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res 63(21):7400–7409
Kaur S, Banerjee R (2012) Lipid-coated PLGA nanoparticles as robust siRNA delivery vehicles. Nanomedicine 7(6):803
Tayebi L, Vashaee D, Parikh AN (2012) Stability of uni- and multillamellar spherical vesicles. Chem Phys Chem 13(1):314–322
Pignatello R, Musumeci T, Graziano AC et al (2016) A study on liposomal encapsulation of a lipophilic prodrug of LHRH. Pharm Dev Technol 21(6):664–671
Maestrelli F, González-Rodríguez ML, Rabasco AM et al (2006) Effect of preparation technique on the properties of liposomes encapsulating ketoprofen-cyclodextrin complexes aimed for transdermal delivery. Int J Pharm 312(1–2):53–60
Choi HS, Liu W, Misra P et al (2007) Renal clearance of quantum dots. Nat Biotechnol 25(10):1165–1170
Owens DE III, Peppas NA (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307(1):93–102
Awasthi VD, Garcia D, Goins BA et al (2003) Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm 253(1–2):121–132
Wisse E, Jacobs F, Topal B et al (2008) The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther 15(17):1193–1199
Daemen T, Velinova M, Regts J et al (1997) Different intrahepatic distribution of phosphatidylglycerol and phosphatidylserine liposomes in the rat. Hepatology 26(2):416–423
Shaw DJ, Costello B (1993) Introduction to colloid and surface chemistry: Butterworth-Heinemann. Elsevier, Oxford
Hunter RJ (1981) Zeta potential in colloid science: principles and applications, 1st edn. Academic, London
Brandhonneur N, Chevanne F, Vié V et al (2009) Specific and non-specific phagocytosis of ligand-grafted PLGA microspheres by macrophages. Eur J Pharm Sci 36(4–5):474–485
He C, Hu Y, Yin L et al (2010) Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31(13):3657–3666
Davis ME, Chen ZG, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–782
Liu Y, Li K, Pan J et al (2010) Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials 31(2):330–338
Ge J, Li K, Ding D et al (2012) Lipid-PEG-Folate encapsulated nanoparticles with aggregation induced emission characteristics: cellular uptake mechanism and two-photon fluorescence imaging. Small 8(23):3655–3663
Li K, Jiang Y, Ding D et al (2011) Folic acid-functionalized two-photon absorbing nanoparticles for MCF-7 cancer cell imaging. Chem Commun 47(26):7323–7325
Gabizon A, Papahadjopoulos D (1988) Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A 85(18):6949–6953
Williams KJ, Phillips MC, Rodrigueza WV (1998) Structural and metabolic consequences of liposome-lipoprotein interactions. Adv Drug Deliv Rev 32(1–2):31–43
Szebeni J, Wassef NM, Spielberg H et al (1994) Complement activation in rats by liposomes and liposome-encapsulated hemoglobin: evidence for anti-lipid antibodies and alternative pathway activation. Biochem Biophys Res Commun 205(1):255–263
Tall AR, Tabas I, Williams KJ (1986) Lipoprotein-liposome interactions. Methods Enzymol 128(4):647–657
Sabín J, Prieto G, Ruso JM et al (2009) Interactions between DMPC liposomes and the serum blood proteins HSA and IgG. J Phys Chem B 113(6):1655–1661
Goren D, Horowitz AT, Zalipsky S et al (1996) Targeting of stealth liposomes to erB-2 (Her/2) receptor: in vitro and in vivo studies. Br J Cancer 74(11):1749–1756
Riviere K, Huang Z, Jerger K et al (2011) Antitumor effect of folate-targeted liposomal doxorubicin in KB tumor-bearing mice after intravenous administration. J Drug Target 19(1):14–24
Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer-chemotherapy – mechanism of tumor itropic accumulation of proteins and the antitumor agent Smancs. Cancer Res 46:6387–6392
Ganta S, Devalapally H, Shahiwala A et al (2008) A review of stimuli responsive nanocarriers for drug and gene delivery. J Control Release 126(3):187–204
van Vlerken LE, Duan Z, Seiden MV et al (2007) Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res 67(10):4843–4850
Noble CO, Kirpotin DB, Hayes ME et al (2004) Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets 8(4):335–353
El-Hammadi MM, Arias JL (2019) An update on liposomes in drug delivery: a patent review (2014–2018). Expert Opin Ther Pat 29(11):891–907
Doi Y, Shimizu T, Ishima Y et al (2019) Long-term storage of PEGylated liposomal oxaliplatin with improved stability and long circulation times in vivo. Int J Pharm 564:237–243
Hamishehkar H, Bahadori MB, Vandghanooni S et al (2018) Preparation, characterization and anti-proliferative effects of sclareol loaded solid lipid nanoparticles on A549 human lung epithelial cancer cells. J Drug Deliv Sci Tec 45:272–280
Nausicaa C, Benedetta F, Casimiro G et al (2018) Solid lipid nanoparticles carrying temozolomide for melanoma treatment. Preliminary in vitro and in vivo studies. Int J Mol Sci 19(2):255
Bae YH (2009) Drug targeting and tumor heterogeneity. J Control Release 133(1):2–3
Cho K, Wang X, Nie S et al (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14(5):1310–1316
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor-targeted of nanocarriers for anti-cancer drug delivery. J Control Release 148(2):35–146
Bareford LM, Swaan PW (2007) Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev 59(8):748–758
Clague MJ, Urbé S, Aniento F et al (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 269(1):21–24
Lee RJ, Wang S, Low PS (1996) Measurement of endosome pH following folate receptor-mediated endocytosis. Biochim Biophys Acta 1312(3):237–242
Nishi T, Forgac M (2002) The vacuolar (H+)-ATPases–nature’s most versatile proton pumps. Nat Rev Mol Cell Biol 3(2):94–103
Rudenko G, Henry L, Henderson K et al (2002) Structure of the LDL receptor extracellular domain at endosomal pH. Science 298(5602):2353–2358
Kamen BA, Smith AK (2004) A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev 56(8):1085–1097
Lakadamyali M, Rust MJ, Zhuang X (2006) Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124(5):997–1009
Weijer R, Broekgaarden M, Kos M et al (2015) Enhancing photodynamic therapy of refractory solid cancers: combining second-generation photosensitizers with multi-targeted liposomal delivery. J Photochem Photobiol Chem 23:103–131
Jain RK, Stylianopoulos T (2010) Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 7(11):653–664
Park JW, Hong K, Kirpotin DB et al (2002) Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res 8(4):1172–1181
Orcutt KD, Rhoden JJ, Ruiz-Yi B et al (2012) Effect of small molecule binding affinity on tumor uptake in vivo. Mol Cancer Ther 11(6):1365–1372
Juweid M, Neumann R, Paik C et al (1992) Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res 52(19):5144–5153
Yang G, Yang T, Zhang W et al (2014) In vitro and in vivo antitumor effects of folate-targeted ursolic acid stealth liposome. J Agric Food Chem 62(10):2207–2215
Min HK, Kim CS, Han J et al (2019) Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf B 173:539–548
Hattori Y, Shimizu S, Ozaki K et al (2019) Effect of cationic lipid type in folate-PEG-modified cationic liposomes on folate receptor-mediated siRNA transfection in tumor cells. Pharmaceutics 11(4):181
Silindir-Gunay M, Karpuz M, Ozturk N et al (2019) Radiolabeled, folate-conjugated liposomes as tumor imaging agents: formulation and in vitro evaluation. J Drug Deliv Sci Technol 50:321–328
Handali S, Moghimipour E, Kouchak M et al (2019) New folate receptor targeted nano liposomes for delivery of 5-fluorouracil to cancer cells: strong implication for enhanced potency and safety. Life Sci 227:39–50
Kumar P, Huo P, Liu B (2019) Formulation strategies for folate-targeted liposomes and their biomedical applications. Pharmaceutics 11(8):381
Trinder D, Zak O, Aisen P (1996) Transferrin receptor-independent uptake of differic transferrin by human hepatoma cells with antisense inhibition of receptor expression. Hepatology 23(6):1512–1520
Kalinowski D, Richardson DR (2005) The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev 57(4):547–583
Prost AC, Ménégaux F, Langlois P et al (1998) Differential transferrin receptor density in human colorectal cancer: a potential probe for diagnosis and therapy. Int J Oncol 13(4):871–875
Shinohara H, Fan D, Ozawa S et al (2000) Site-specific expression of transferrin receptor by human colon cancer cells directly correlates with eradication by antitransferrin recombinant immunotoxin. Int J Oncol 17(4):643–651
Gomme PT, McCann KB, Bertolini J (2005) Transferrin: structure, function and potential therapeutic actions. Drug Discov Today 10(4):267–273
Daniels TR, Delgado T, Helguera G et al (2006) The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol 121(2):159–176
Chang J, Jallouli Y, Kroubi M et al (2009) Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int J Pharm 379(2):285–292
Ulbrich K, Hekmatara T, Herbert E et al (2009) Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharm Biopharm 71(2):251–256
Tang J, Wang Q, Yu Q et al (2019) A stabilized retro-inverso peptide ligand of transferrin receptor for enhanced liposome-based hepatocellular carcinoma-targeted drug delivery. Acta Biomater 83:379–389
Fu J, Li W, Xin X et al (2019) Transferrin modified nano-liposome co-delivery strategies for enhancing the cancer therapy. J Pharm Sci
Riaz MK, Zhang X, Wong KH et al (2019) Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int J Nanomedicine 14:2879–2902
Jhaveri A, Luther E, Torchilin V (2019) The effect of transferrin-targeted, resveratrol-loaded liposomes on neurosphere cultures of glioblastoma: implications for targeting tumour-initiating cells. J Drug Targeting 27(5–6):601–613
Wang Y, Yang Y, Yu Y et al (2020) Transferrin modified dioscin loaded PEGylated liposomes: characterization and in vitro antitumor effect. J Nanosci Nanotechnol 20(3):1321–1331
Li S, Zhao H, Mao X et al (2019) Transferrin receptor targeted cellular delivery of doxorubicin via a reduction-responsive peptide-drug conjugate. Pharm Res 36(12):168
dos Santos Rodrigues B, Kanekiyo T, Singh J (2019) ApoE-2 brain-targeted gene therapy through transferrin and penetratin tagged liposomal nanoparticles. Pharm Res 36(11):161
Witton CJ, Reeves JR, Going JJ et al (2003) Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol 200(3):290–297
Abd El-Rehim DM, Pinder SE, Paish CE et al (2004) Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer 91(8):1532–1542
Bossuyt V, Fadare O, Martel M et al (2005) Remarkably high frequency of EGFR expression in breast carcinomas with squamous differentiation. Int J Surg Pathol 13(4):319–327
Mamot C, Drummond DC, Noble CO et al (2005) Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res 65(24):11631–11638
Zu Kim Y, hee Park Y, Choi HJ et al (2018) Compositions and methods related to anti-EGFR antibody drug conjugates: U.S. Patent 10,118,965[P]. 2018-11-6
Guo P, Yang J, Liu D et al (2019) Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis. Sci Adv 5(3):eaav5010
Abumanhal-Masarweh H, da Silva D, Poley M et al (2019) Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J Control Release 307:331–341
Takenaka T, Nakai S, Katayama M et al (2019) Effects of gefitinib treatment on cellular uptake of extracellular vesicles in EGFR-mutant non-small cell lung cancer cells. Int J Pharm 572:118762
Li JJ, Feng GY, Chen CC et al (2019) Biological evaluation of an EGFR targeting liposomal drug in a peritoneal tumor-bearing mouse model. J Nucl Med 60(supplement 1):1041
Deshpande PP, Biswas S, Torchilin VP (2013) Current trends in the use of liposomes for tumor-targeted. Nanomedicine (Lond) 8(9):1509–1528
Kang H, O’Donoghue MB, Liu H et al (2010) A liposome-based nanostructure for aptamer directed delivery. Chem Commun 46(2):249–251
Xing H, Tang L, Yang X et al (2013) Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J Mater Chem B Mater Biol Med 1(39):5288–5297
Moosavian SA, Sahebkar A (2019) Aptamer-functionalized liposomes for targeted cancer therapy. Cancer Lett 448:144–154
Hong S, Ding P, Luo Y et al (2019) Aptamer-integrated α-Gal liposomes as bispecific agents to trigger immune response for killing tumor cells. J Biomed Mater Res Part A 107(6):1176–1183
Frohnmeyer E, Tuschel N, Sitz T et al (2019) Aptamer lateral flow assays for rapid and sensitive detection of cholera toxin. Analyst 144(5):1840–1849
Yang X, Zhao J, Duan S et al (2019) Enhanced cytotoxic T lymphocytes recruitment targeting tumor vasculatures by endoglin aptamer and IP-10 plasmid presenting liposome-based nanocarriers. Theranostics 9(14):4066–4083
Guo X, Dong C, Liu Q et al (2019) The sustained and targeted treatment of hemangiomas by propranolol-loaded CD133 aptamers conjugated liposomes-in-microspheres. Biomed Pharmacother 114:108823
Cheng Y, Ou Z, Li Q et al (2019) Cabazitaxel liposomes with aptamer modification enhance tumor-targeting efficacy in nude mice. Mol Med Rep 19(1):490–498
Lammers T, Hennink WE, Storm G (2008) Tumour-targeted nanomedicines: principles and practice. Br J Cancer 99(3):392–397
Denekamp J, Hobson B (1982) Endothelial-cell proliferation in experimental tumours. Br J Cancer 46(5):711–720
Denekamp J (1984) Vasculature as a target for tumour therapy. Prog Appl Microcirc 4:28–38
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10
Yao Y, Wang T, Liu Y et al (2019) Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma. Artif Cells Nanomed Biotechnol 47(1):1374–1383
Byrne JD, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60(15):1615–1626
Hood JD, Bednarski M, Frausto R et al (2002) Tumor regression by targeted gene delivery to the neovasculature. Science 296(5577):2404–2407
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10(1):9–22
Tang Z, Feng W, Yang Y et al (2019) Gemcitabine-loaded RGD modified liposome for ovarian cancer: preparation, characterization and pharmacodynamic studies. Drug Des Dev Ther 13:3281–3290
Li XT, Tang W, Xie HJ et al (2019) The efficacy of RGD modified liposomes loaded with vinorelbine plus tetrandrine in treating resistant brain glioma. J Liposome Res 29(1):21–34
Vihinen P, Ala-aho R, Kähäri VM (2005) Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets 5(3):203–220
Genís L, Gálvez BG, Gonzalo P et al (2006) MT1-MMP: universal or particular player in angiogenesis? Cancer Metastasis Rev 25(1):77–86
Lyu Y, Xiao Q, Yin L et al (2019) Potent delivery of an MMP inhibitor to the tumor microenvironment with thermosensitive liposomes for the suppression of metastasis and angiogenesis. Signal Transduction Targeted Ther 4(1):1–9
Andresen T L, Jensen S S, Henriksen JR et al (2019) Cationic liposomes: WIPO Patent 2019012107[P]
Seraj S, Lee J, Ahn HJ (2019) Systemic delivery of Eg5 shRNA-expressing plasmids using PEGylated DC-Chol/DOPE cationic liposome: long-term silencing and anticancer effects in vivo. Biochem Pharmacol 166:192–202
Ichihara H, Motomura M, Matsumoto Y (2019) Therapeutic effects and anti-metastasis effects of cationic liposomes against pancreatic cancer metastasis in vitro and in vivo. Biochem Biophys Res Commun 511(3):504–509
Zhang M, Lemay SG (2019) Interaction of anionic bulk nanobubbles with cationic liposomes: evidence for reentrant condensation. Langmuir 35(11):4146–4151
Wui SR, Kim KS, Ryu JI et al (2019) Efficient induction of cell-mediated immunity to varicella-zoster virus glycoprotein E co-lyophilized with a cationic liposome-based adjuvant in mice. Vaccine 37(15):2131–2141
Drummond DC, Meyer O, Hong K et al (1999) Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev 51(4):691–743
Allen TM, Hansen CB, Menezes DLD (1995) Pharmacokinetics of long-circulating liposomes. Adv Drug Deliv Rev 16(2):267–284
Senior J, Crawley JCW, Gregoriadis G (1985) Tissue distribution of liposomes exhibiting long half-lives in the circulation after intravenous injection. Biochim Biophys Acta 839:1–8
Ahl PL, Bhatia SK, Meers P et al (1997) Enhancement of the in vivo circulation lifetime of L-a-distearoylphosphatidylcholine liposomes: importance of liposomal aggregation versus complement opsonization. Biochim Biophys Acta 1329(2):370–382
Gregoriadis G, Senior J (1980) The phospholipid component of small unilamellar liposomes controls the rate of clearance of entrapped solutes from the circulation. FEBS Lett 119(1):43–46
Abra RM, Hunt CA (1981) Liposome disposition in vivo. III. Dose and vesicle-size effects. Biochim Biophys Acta 666(3):493–503
Hwang KJ (1987) Liposome pharmacokinetics. In: Ostro MJ (ed) Liposomes: from biophysics to therapeutics. Marcel Dekker Inc, New York, pp 109–156
Allen TM, Hansen C (1991) Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta 1068(2):133–141
Huang SK, Mayhew E, Gilani S et al (1992) Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon carcinoma. Cancer Res 52(24):6774–6781
Woodle MC, Lasic DD (1992) Sterically stabilized liposomes. Biochim Biophys Acta 1113(2):171–199
Papahadjopoulos D, Allen TM, Gabizon A et al (1991) Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A 88(24):11460–11464
Gabizon A, Papahadjopoulos D (1988) Liposome formulations with prolonged circulation time in blood and enhanced uptake in tumors. Proc Natl Acad Sci U S A 85(18):6949–6953
Gabizon A, Price DC, Huberty J et al (1990) Effect of liposome composition and other factors on the targeting of liposomes to experimental tumors: biodistribution and imaging studies. Cancer Res 50(19):6371–6378
Fielding RM, Mukwaya G, Sandhaus RA (1998) Long circulating liposomes: old drugs, new therapeutics. J Control Release 44(1):1–9
Papahadjopoulos D, Jacobson K, Nir S et al (1973) Phase transitions in phospholipid vesicles: fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta 311(3):330–348
Bally MB, Nayar R, Masin D et al (1990) Liposomes with entrapped doxorubicin exhibit extended blood residence times. Biochim Biophys Acta 1023(1):133–139
van Etten EWM, van Vianen W, Tijhuis RHG et al (1995) Sterically stabilized amphotericin B-liposomes: toxicity and biodistribution in mice. J Control Release 37(1):123–129
Nosova AS, Koloskova OO, Nikonova AA et al (2019) Diversity of PEGylation methods of liposomes and their influence on RNA delivery. Med Chem Comm 10(3):369–377
Li B, Cai M, Lin L et al (2019) MRI-visible and pH-sensitive micelles loaded with doxorubicin for hepatoma treatment. Biomater Sci-UK 7(4):1529–1542
Kanamala M, Palmer BD, Ghandehari H et al (2018) PEG-benzaldehyde-hydrazone-lipid based PEG-sheddable pH-sensitive liposomes: abilities for endosomal escape and long circulation. Pharm Res-DORDR 35(8):154
Shen Z, Ye H, Kröger M et al (2018) Aggregation of polyethylene glycol polymers suppresses receptor-mediated endocytosis of PEGylated liposomes. Nanoscale 10(9):4545–4560
Wei H, Zhao Y, Guo Y et al (2018) PEGylated self-assembled nano-bacitracin A: probing the antibacterial mechanism and real-time tracing of target delivery in vivo. ACS Appl Mater Interfaces 10(13):10688–10705
Ma K, Fu D, Liu Y et al (2018) Cancer cell targeting, controlled drug release and intracellular fate of biomimetic membrane-encapsulated drug-loaded nano-graphene oxide nanohybrids. J Mater Chem B 6(31):5080–5090
Kang X, Chen H, Li S et al (2018) Magnesium lithospermate B loaded PEGylated solid lipid nanoparticles for improved oral bioavailability. Colloids Surf B Biointerfaces 161:597–605
Shimizu T, Abu Lila AS, Fujita R et al (2018) A hydroxyl PEG version of PEGylated liposomes and its impact on anti-PEG IgM induction and on the accelerated clearance of PEGylated liposomes. Eur J Pharm Biopharm 127:142–149
Kuang Y, Zhang K, Cao Y et al (2017) Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl Mater Interfaces 9(14):12217–12226
Porter CJH, Trevaskis NL, Charman WN (2007) Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 6(3):231–248
Williams H D, Ford L, Igonin A et al (2019) Unlocking the full potential of lipid-based formulations using lipophilic salt/ionic liquid forms. Adv Drug Deliv Rev
Anselmo AC, Mitragotri S (2016) Nanoparticles in the clinic. Bioeng Transl Med 1(1):10–29
Beloqui A, Solinís MÁ, Rodríguez-Gascón A et al (2016) Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine 12(1):143–161
Desai N (2012) Challenges in development of nanoparticle-based therapeutics. AAPS J 14(2):282–295
Susan H (2015) Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front Pharmacol 6:219
Wagner V, Dullaart A, Bock AK et al (2006) The emerging nanomedicine landscape. Nat Biotechnol 24(10):1211–1217
Van DVFM (2014) The emerging landscape for nanomedicine in atherosclerosis. Endocrine BioScientifica 35
Schütz CA, Juillerat-Jeanneret L, Mueller H et al (2013) Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine 8(3):449–467
Mishra DK, Ruchita S, Mishra PK (2018) Lipid based nanocarriers: a translational perspective. Nanomedicine 14(7):2023–2050
Bobo D, Robinson KJ, Islam J et al (2016) Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 33(10):2373–2387
Eifler AC, Thaxton CS (2011) Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. Methods Mol Biol 726(726):325
Vivot A, Jacot J, Zeitoun JD et al (2017) Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000–2015. Ann Oncol 28(5):1111–1116
James ND, Coker RJ, Tomlinson D et al (1994) Liposomal doxorubicin (Doxil): an effective new treatment for Kaposi’s sarcoma in aids. Clin Oncol 6(5):294–296
Gabizon A et al (1994) Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 54(4):987–992
Karthik V, Yi L, Dennis N et al (2014) Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. Br J Clin Pharmacol 77(6):998–1010
Gabizon A, Shmeeda H, Barenholz Y (2003) Pharmacokinetics of pegylated liposomal doxorubicin. Clin Pharmacokinet 42(5):419
Hattori Y, Shi L, Ding W et al (2009) Novel irinotecan-loaded liposome using phytic acid with high therapeutic efficacy for colon tumors. J Control Release 136(1):30–37
Yingchoncharoen P, Kalinowski DS, Richardson DR (2016) Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev 68(3):701–787
Babu A, Templeton AK, Munshi A et al (2014) Nanodrug delivery systems: a promising technology for detection, diagnosis, and treatment of cancer. AapsPharmscitech 15(3):709–721
Etheridge ML, Campbell SA, Erdman AG et al (2013) The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine 9(1):1–14
Ventola CL (2017) Progress in nanomedicine: approved and investigational nanodrugs. Pharm Ther 42(12):742
Min Y, Caster JM, Eblan MJ et al (2015) Clinical translation of nanomedicine. Chem Rev 115(19):11147–11190
Lytton-Jean AKR, Kauffman KJ, Kaczmarek JC et al (2015) Cancer nanotherapeutics in clinical trials. Cancer Treat Res 166:293–322
Bolwell BJ, Cassileth PA, Gale RP (1988) High dose cytarabine: a review. Leukemia 2(5):253
Lengfelder E, Haferlach C, Saussele S et al (2009) High dose ara-C in the treatment of newly diagnosed acute promyelocytic leukemia: long-term results of the German AMLCG. Leukemia 23(12):2248–2258
Wiernik PH, Banks PL, Case CD et al (1992) Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood 79(2):313–319
Vogler WR, Velezgarcia E, Weiner RS et al (1992) A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: a southeastern cancer study group study. J Clin Oncol 10(7):1103–1111
Muhammad A, Champeimont J, Mayr UB et al (2012) Bacterial ghosts as carriers of protein subunit and DNA-encoded antigens for vaccine applications. Expert Rev Vaccines 11(1):97–116
Kanasty R, Dorkin JR, Vegas A et al (2013) Delivery materials for sirna therapeutics. Nat Mater 12(11):967–977
Jin GR et al (2016) Multifunctional organic nanoparticles with aggregation induced emission (AIE) characteristics for targeted photodynamic therapy and RNA interference therapy. Chem Commun 52(13):2752–2755
Senzer N, Nemunaitis J, Nemunaitis D et al (2013) Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther 21(5):1096–1103
Lane DP (1995) On the regulation of the p53 tumour suppressor, and its role in the cellular response to DNA damage. Philos Trans R Soc Lond Biol 347(1319):83–87
Yokoyama M (2005) Drug targeting with nano-sized carrier systems. J Artif Organs 8(2):77–84
Bottini M, Sacchetti C, Pietroiusti A et al (2014) Targeted nanodrugs for cancer therapy: prospects and challenges. J Nanosci Nanotechnol 14(1):98–114
Yang HX, Qin XL et al (2019) An in vivo miRNA delivery system for restoring infarcted myocardium. ACS Nano
Nahta R, Hung MC, Esteva FJ (2004) The her-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64(7):2343–2346
Mikhail AS, Negussie AH, Pritchard WF et al (2017) Lyso-thermosensitive liposomal doxorubicin for treatment of bladder cancer. Int J Hyperth 33(7):1–28
Yang J (2012) Stimuli-responsive drug delivery systems. Adv Drug Deliv Rev 64(11):965–966
Yin Q, Shen J, Zhang Z et al (2013) Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv Drug Deliv Rev 65(13–14):1699–1715
Gu M, Wang X, Toh TB et al (2017) Applications of stimuli-responsive nanoscale drug delivery systems in translational research. Drug Discov Today 23(5):1043–1052
Hare JI, Lammers T, Ashford MB et al (2016) Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev 108:25–38
Moore R (2014) Challenges to nanomedicine. Springer, New York
Eliasof S, Lazarus D, Peters CG et al (2013) Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc Natl Acad Sci U S A 110(37):15127–15132
Malinoski FJ (2014) The nanomedicines alliance: an industry perspective on nanomedicines. Nanomedicine 10(8):1819–1820
Bregoli L, Movia D, Gavigan-Imedio JD et al (2016) Nanomedicine applied to translational oncology: a future perspective on cancer treatment. Nanomed Nanotechnol Biol Med 12(1):81–103
Sharma A, Madhunapantula SRV, Robertson GP (2012) Toxicological considerations when creating nanoparticle based drugs and drug delivery systems? Expert Opin Drug Metab Toxicol 8(1):47–69
Adiseshaiah PP, Crist RM, Hook SS et al (2016) Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol 13(12):750–765
Nie S (2010) Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 5(4):523–528
Maity AR, Stepensky D (2016) Pharmacokinetics and pharmacodynamics of nano-drug delivery systems. In: Intracellular delivery III. Springer, Cham, pp 341–362
Sahakyan N, Haddad A, Richardson S et al (2017) Personalized nanoparticles for cancer therapy: a call for greater precision. Anti Cancer Agents Med Chem 17(8):1033–1039
Onoue S, Yamada S, Chan K (2014) Nanodrugs: pharmacokinetics and safety. Int J Nanomedicine 9:1025
Acknowledgments
We are grateful to the funding support from the National Natural Science Foundation of China (31870991) and Thousand Young Talents Program.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Li, Y., Zhang, C., Min, T., Ping, Y., Li, K. (2020). Lipid-Based Tumor-targeted Systems. In: Huang, R., Wang, Y. (eds) New Nanomaterials and Techniques for Tumor-targeted Systems. Springer, Singapore. https://doi.org/10.1007/978-981-15-5159-8_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-5159-8_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5158-1
Online ISBN: 978-981-15-5159-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)