Abstract
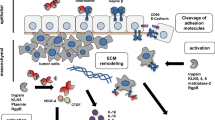
Meprins are glycosylated, multi-domain zinc endopeptidases belonging to the astacin family of metalloproteases. They have been found to have a diversified role in several physiological and pathological conditions including digestion, tissue remodeling, wound healing, neurodegenerative diseases, and cancer. The two isoforms, meprin α and β, are coded by separate genes on two different chromosomes. Expression of meprin α has been related with colorectal cancer and is used as a prognostic marker for the differentiation of chronic pancreatitis from malignant one. Meprin β is expressed in liver metastasis, neuroendocrine tumors, and endometrial cancer. The chapter aims at understanding the structure and function of theses proteases acting as molecular switches in tumor development, progression, and metastasis which can help in the designing of inhibitors acting as potential leads in future drug therapy.
Similar content being viewed by others
References
Arolas JL, Broder C, Jefferson T, Guevara T, Sterchi EE, Bode W, Stöcker W, Becker-Pauly C, Gomis-Rüth FX (2012) Structural basis for the sheddase function of human meprin β metalloproteinase at the plasma membrane. Proc Natl Acad Sci 109:16131–16136
Banerjee S, Oneda B, Yap LM, Jewell DP, Matters GL, Fitzpatrick LR, Seibold F, Sterchi EE, Ahmad T, Lottaz D, Bond JS (2009) MEP1A allele for meprin a metalloprotease is a susceptibility gene for inflammatory bowel disease. Mucosal Immunol 2:220–231
Barnes K, Ingram J, Kenny AJ (1989) Proteins of the kidney microvillar membrane. Structural and immunochemical properties of rat endopeptidase-2 and its immunohistochemical localization in tissues of rat and mouse. Biochem J 264:335–346
Becker C, Kruse MN, Slotty KA, Kohler D, Harris JR, Rosmann S, Sterchi EE, Stocker W (2003) Differences in the activation mechanism between the α and β subunits of human meprin. Biol Chem 384:825–831
Bertenshaw GP, Norcum MT, Bond JS (2003) Structure of homo- and hetero-oligomeric meprin metalloproteases: dimers, tetramers, and high molecular mass multimers. J Biol Chem 278:2522–2532
Beynon RJ, Shannon JD, Bond JS (1981) Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J 199:591–598
Bond JS, Beynon RB (1995) The astacin family of metallo endopeptidase. Protein Sci 4:1247–1126
Bond JS, Beynon RJ, Reckelhoff JF, David CS (1984) Mep-1 gene controlling a kidney metalloendopeptidase is linked to the major histocompatibility complex in mice. Proc Natl Acad Sci 81:5542–5545
Broder C, Becker-Pauly C (2013) The metalloproteases meprin α and meprin β: unique enzymes in inflammation, neurodegeneration, cancer and fibrosis. Biochem J 450:253–264
Bylander J, Li Q, Ramesh G, Zhang B, Reeves WB, Bond JS (2008) Targeted disruption of the meprin metalloproteinase β gene protects against renal ischemia-reperfusion injury in mice. Am J Phys Renal Phys 294:F480–F490
Carr JC, Sherman SK, Wang D, Dahdaleh FS, Bellizzi AM, O’Dorisio MS, O’Dorisio TM, Howe JR (2013) Overexpression of membrane proteins in primary and metastatic gastrointestinal neuroendocrine tumors. Ann Surg Oncol 20:739–746
Chaudhuri A, Biswas S, Chakraborty S (2019a) Exploring protein-protein intermolecular recognition between meprin-α and endogenous protease regulator cystatinC coupled with pharmacophore elucidation. J Biomol Struct Dyn 37:440–453
Chaudhuri A, Hudait N, Chakraborty SS (2019b) Pharmacophore modeling coupled with molecular dynamic simulation approach to identify new leads for meprin-β metalloprotease. Comput Biol Chem 80:292–306
Claudia B, Christoph B (2013) The metalloproteases meprin alpha and meprin beta: unique enzymes in inflammation, neurodegeneration, cancer and fibrosis. Biochem J 450:253–264
Dietrich JM, Jiang W, Bond JS (1996) A novel Meprin β’ mRNA in mouse embryonal and human colon carcinoma cells. J Biol Chem 271:2271–2278
Gorbea CM, Marchand P, Jiang W, Copeland NG, Gilbert DJ, Jenkins NA, Bond JS (1993) Cloning, expression, and chromosomal localization of the mouse meprin β subunit. J Biol Chem 268:21035–21043
Hedrich J, Lottaz D, Meyer K, Yiallouros I, Jahnen-Dechent W, Stöcker W, Becker-Pauly C (2010) Fetuin-a and cystatin C are endogenous inhibitors of human meprin metalloproteases. Biochemistry 49:8599–8607
Jiang W, Sadler PM, Jenkins NA, Gilbert DJ, Copeland NG, Bond JS (1993) Tissue-specific expression and chromosomal localization of the α subunit of mouse meprin a. J Biol Chem 268:10380–10385
Karmilin K, Schmitz C, Kuske M, Körschgen H, Olf M, Meyer K, Hildebrand A, Felten M, Fridrich S, Yiallouros I, Becker-Pauly C, Weiskirchen R, Jahnen-Dechent FJ, Stöcker W (2019) Mammalian plasma fetuin-B is a selective inhibitor of ovastacin and meprin metalloproteinases. Sci Rep 9:546
Lottaz D, Maurer CA, Hahn D, Buchler MW, Sterchi EE (1999) Nonpolarized secretion of human meprin α in colorectal cancer generates an increased proteolytic potential in the stroma. Cancer Res 59:1127–1133
Matters GL, Bond JS (1999) Expression and regulation of the meprin beta gene in human cancer cells. Mol Carcinog 25:169–178
Matters GL, Manni A, Bond JS (2005) Inhibitors of polyamine biosynthesis decrease the expression of the metalloproteases meprin alpha and MMP-7 in hormone-independent human breast cancer cells. Clin Exp Metastasis 22:331–339
Niyitegeka JM, Bastidas AC, Newman RH, Taylor SS, Ongeri EM (2015) Isoform-specific interactions between meprin metalloproteases and the catalytic subunit of protein kinase a: significance in acute and chronic kidney injury. Am J Phys Renal Phys 308:F56–F68
Ongeri EM, Anyanwu O, Reeves WB, Bond JS (2011) Villin and actin in the mouse kidney brush-border membrane bind to and are degraded by meprins, an interaction that contributes to injury in ischemia-reperfusion. Am J Phys Renal Phys 301:F871–F882
Ouyang HY, Xu J, Luo J, Zou RH, Chen K, Le Y, Zhang YF, Wei W, Guo RP, Shi M (2016) MEP1A contributes to tumor progression and predicts poor clinical outcome in human hepatocellular carcinoma. Hepatology 63:1227–1239
Ramsbeck D, Hamann A, Schlenzig D, Schilling S, Buchholz M (2017) First insight into structure-activity relationships of selective meprin β inhibitors. Bioorg Med Chem Lett 27:2428–2431
Ramsbeck D, Hamann A, Richter G, Schlenzig D, Geissler S, Nykiel V, Cynis H, Schilling S, Buchholz M (2018) Structure-guided design, synthesis, and characterization of next-generation meprin β inhibitors. J Med Chem 61:4578–4592
Red Eagle AR, Hanson RL, Jiang W, Han X, Matters GL, Imperatore G, Knowler WC, Bond JS (2005) Meprin beta metalloprotease gene polymorphisms associated with diabetic nephropathy in the Pima Indians. Hum Genet 118:12–22
Ricardo SD, Bond JS, Johnson GD, Kaspar J, Diamond JR (1996) Expression of subunits of the metalloendopeptidase meprin in renal cortex in experimental hydronephrosis. Am J Phys 270:F669–F676
Schäffler H, Li W, Helm O, Krüger S, Böger C, Peters F, Röcken C, Sebens S, Lucius R, Becker-Pauly C, Arnold P (2019) The cancer-associated meprin β variant G32R provides an additional activation site and promotes cancer cell invasion. J Cell Sci 132:jcs220665
Schönemeier B, Metzger J, Klein J, Husi H, Bremer B, Armbrecht N, Dakna M, Schanstra JP, Rosendahl J, Wiegand J, Jäger M, Mullen W, Breuil B, Plentz RR, Lichtinghagen R, Brand K, Kühnel F, Mischak H, Manns MP, Lankisch TO (2016) Urinary peptide analysis differentiates pancreatic cancer from chronic pancreatitis. Pancreas 45:1018–1026
Schütte A, Ermund A, Becker-Pauly C, Johansson ME, Rodriguez-Pineiro AM, Bäckhed F, Müller S, Lottaz D, Bond JS, Hansson GC (2014) Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc Natl Acad Sci 111:12396–12401
Sterchi EE, Green JR, Lentze MJ (1982) Non-pancreatic hydrolysis of N-benzoyl-l-tyrosyl-p-aminobenzoic acid (PABA-peptide) in the human small intestine. Clin Sci 62:557–560
Sterchi EE, Green JR, Lentze MJ (1983) Nonpancreatic hydrolysis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid (PABA peptide) in the rat small intestine. J Pediatr Gastroenterol Nutr 2:539–547
Sterchi EE, Stöcker W, Bond JS (2008) Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Asp Med 29:309–328
Sunnerhagen M, Pursglove S, Fladvad M (2002) The new MATH: homology suggests shared binding surfaces in meprin tetramers and TRAF trimers. FEBS Lett 530:1–3
Tan K, Jäger C, Schlenzig D, Schilling S, Buchholz M, Ramsbeck D (2018) Tertiary-amine-based inhibitors of the astacin protease Meprin α. Chem Med Chem 13:1619–1624
Wang X, Chen J, Wang J, Yu F, Zhao S, Zhang Y, Tang H, Peng Z (2016) Metalloproteases meprin-ɑ (MEP1A) is a prognostic biomarker and promotes proliferation and invasion of colorectal cancer. BMC Cancer 16:383
Yura RE, Bradley SG, Ramesh G, Reeves WB, Bond JS (2009) Meprin a metalloproteases enhance renal damage and bladder inflammation after LPS challenge. Am J Phys Renal Phys 296:F135–F144
Zapata JM, Martinez-Garcia V, Lefebvre S (2007) Phylogeny of the TRAF/MATH domain. Adv Exp Med Biol 597:1–24
Acknowledgments
This work has been supported by a grant from the Department of Biotechnology, Govt. of India (BT/PR12326/BID/7/502/2014) to SSC and AC.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Chakraborty, S.S., Chaudhuri, A., Dholey, Y., Bera, A.K. (2021). Meprins: Ancient Enzymes Newly Discovered in Cancer Progression. In: Chakraborti, S., Ray, B.K., Roychowdhury, S. (eds) Handbook of Oxidative Stress in Cancer: Mechanistic Aspects. Springer, Singapore. https://doi.org/10.1007/978-981-15-4501-6_145-1
Download citation
DOI: https://doi.org/10.1007/978-981-15-4501-6_145-1
Received:
Accepted:
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4501-6
Online ISBN: 978-981-15-4501-6
eBook Packages: Springer Reference Biomedicine and Life SciencesReference Module Biomedical and Life Sciences
Publish with us
Chapter history
-
Latest
Meprins: Ancient Enzymes Newly Discovered in Cancer Progression- Published:
- 07 January 2022
DOI: https://doi.org/10.1007/978-981-15-4501-6_145-2
-
Original
Meprins: Ancient Enzymes Newly Discovered in Cancer Progression- Published:
- 28 October 2021
DOI: https://doi.org/10.1007/978-981-15-4501-6_145-1