Abstract
Endodontic surgery straddles the specialties of endodontics and dento alveolar surgery. With the advent of the operating microscope, newer endodontic filling materials and stem cell therapy, humungous strides have been taken in this area, thus enabling transmutation of peri apical surgery into an avant-garde treatment modality, this chapter is a modest attempt to expound the various aspects of the subject from the surgeons frame of reference. Hence greater import is laid on incisions, flaps,surgical techniques, rather than restorative materials and retro cavity preparation.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Definition
Endodontic surgery is a dental procedure to treat apical periodontitis in cases that did not heal after nonsurgical retreatment or, in certain instances, primary root canal therapy [1]. It is the branch of dentistry that deals with the diagnosis and treatment of lesions of endodontic origin, which cannot be treated by or do not respond to conventional root canal therapy.
2 Historical Frame of Reference
Guerini documented the first endodontic surgery as incision and drainage of an acute endodontic abscess, approximately 1500 years ago [2]. Infected root sections were removed, and the healthy tooth portion was retained in attempting to cure infected teeth for about 200 years. Histological bone regeneration was demonstrated in treated cases of infected periapical lesions in 1930 [3]. For a long time, pulpless teeth were implicated in a plethora of systemic disorders like nephritis and arthritis by the exponents of the focal theory of infection [4].
The terms apicoectomy, periapical surgery, periapical endodontics, root end surgery, apical microsurgery, and surgical endodontics have been used in the literature. Apicoectomy, which means cutting the root apex, limits the understanding of the procedure, which includes removal of the irritants in the root canal system and the periapical pathology as well. Today, endodontic surgery is one of the most puissant branches of dentistry and falls in the twilight zone among surgery, dentistry, and endodontics. Recent advances in techniques and materials have resulted in a paradigm shift toward a more judicious strategy for treating periapical pathologies. The new benchmark for success is tissue regeneration. Nonsurgical retreatment for endodontic failures and surgical endodontics has been radically revolutionized by the introduction of the “microscope”.
A periapical lesion is defined as any radiolucent image exceeding 1 mm in the periapical vicinity of the tooth. Lesions with a mean diameter > 5 mm are classified as large lesions, and those less than or equal to 5 mm are classified as small lesions [5]. In lesions greater than 10 mm, tooth extraction may or may not be done after considering factors like tooth mobility, pain, and the periodontal condition [6]. A periapical lesion may be noticed clinically or radiographically at a dimension of 5 mm [7].
3 Indications for Endodontic Surgery [8]
-
1.
Failures of nonsurgical treatment (treatment should have been done at least twice)
-
2.
Failure of nonsurgical treatment and retreatment is not feasible or impractical due to calcified canals, silver point filling, apical perforation, severely curved roots, and the presence of post and core or if the tooth is fractured at its apical one third
-
3.
To obtain a biopsy from the periapical region
-
4.
To retrieve broken instruments
3.1 Updated Indications (The European Society of Endodontology) (2006) [9]
-
1.
Radiological findings of apical periodontitis and/or symptoms associated with an obstructed canal (the obstruction proved not to be removable, displacement of the obstruction did not seem to be feasible, or the risk of damage was too great)
-
2.
Extruded material with clinical or radiological findings of apical periodontitis and/or symptoms continuing over a prolonged period
-
3.
Persisting or emerging disease following root canal treatment when root canal retreatment is inappropriate
-
4.
Perforation of the root or the floor of the pulp chamber and when it is impossible to treat from within the pulp cavity
4 Relative Contraindications
-
1.
Compromised medical status of the patient
-
2.
Anatomical considerations
-
3.
Surgeon’s skill and clinical expertise
-
4.
Vertically fractured tooth
-
5.
Unrestorable tooth
-
6.
Tooth with compromised periodontal support
-
7.
Nonfunctional tooth
-
8.
Tooth with short roots
Classification of Endodontic Surgery [8]
-
1.
Fistulative Surgery
-
(a)
Incision and drainage
-
(b)
Cortical trephination
-
(c)
Decompression procedures
-
(a)
-
2.
Periapical Surgery
-
(a)
Curettage
-
(b)
Root end resection
-
(c)
Root end preparation
-
(d)
Root end filling
-
(a)
-
3.
Corrective Surgery
-
(a)
Perforation repair
-
(i)
Mechanical (iatrogenic)
-
(ii)
Resorptive
-
(i)
-
(b)
Periodontal management
-
(i)
Root resection
-
(ii)
Tooth resection
-
(iii)
Intentional replantation
-
(i)
-
(a)
Principles of Endodontic Surgery
-
1.
Preoperative assessment and planning
-
2.
Achieving adequate anesthesia and hemostasis
-
3.
Appropriate surgical access through overlying soft and hard tissues
-
4.
Periapical curettage and root end preparation
-
5.
Would closure and care
-
6.
Postoperative management of the local surgical site
5 Preoperative Assessment and Planning
5.1 Anatomical Reflections
The nasal floor, maxillary sinus, inferior alveolar, and mental and greater palatine neurovascular bundles offer potential road blocks to the surgeon.
5.2 Important Considerations in the Maxilla and Maxillary Sinus
If the roots of the maxillary anteriors are very long and the lesion extends superiorly, proximity to the nasal floor should be borne in mind. Eberhardt et al. have commented that the mesiobuccal root apex of the maxillary second molar is closest to the sinus floor and the buccal root apex of the maxillary first premolar is the farthest [10]. The greater palatine neurovascular bundle presents a risk while working on the palatal roots of maxillary molars. If the vessel is severed, pressure must be applied by packs or bone wax and the eventuality of external carotid artery ligation should not be precluded. Vertical releasing incisions on the palate are to be eschewed, and if these are unavoidable, it is prudent to place the same between the maxillary canine and first premolar, where the artery has a narrow caliber. Palatal roots can be accessed buccally or across the sinus or palatally (direct approach). The contour and depth of the palatal vault greatly determine the surgeon’s accoutrement; greater the depth, greater the comfort.
Inadvertent loss of root tips into the maxillary sinus should be avoided and retrieved endoscopically if such a situation arises. Sinus communications, if they occur seldom, pose an impediment to healing neither are they implicated in sinustis [11]. The sinus membrane usually regenerates, and a thin bone forms at the apex [12]. Shallow vestibule, palatally or lingually inclined roots, compounds the surgeon’s difficulties.
5.3 Important Considerations in the Mandible
In the mandible, the facial artery, mental nerve, and inferior alveolar neurovascular bundle should be reckoned with. The facial artery can be safeguarded if incisions placed in the vicinity of the mandibular first molar are not extended beyond the vestibular depth. The route taken by the inferior alveolar neurovascular bundle is of particular significance. It winds buccal (second molar) to lingual (first molar) and then again buccal (the second premolar) before it exits the mental foramen [12]. In the vertical dimension, the mandibular second molar is closest to the canal as when compared to the mandibular first molar or second premolar. For all practical purposes, the mandibular second molar is not conducive to endodontic surgery and should be attempted bearing in mind these encumbrances. The mandibular anteriors offer a particular challenge while performing perpendicular root resection [12].
Excessive salivation, shallow vestibule, thick alveolus, and small rima oris are other determinants. A comprehensive assessment of all these variables is mandatory prior to embarking upon endodontic surgery (Table 16.1).
6 Investigations
Until recently, periapical radiographs were the workhorse of endodontic surgery. Their obvious shortcomings were due to compression of three-dimensional structures into a two-dimensional image and geometric distortion of anatomy. In the year 2000, Cone Beam Computer Tomography (CBCT) was introduced to dentistry. The limited CBCT offers higher resolution, and images are displayed in three planes: axial, coronal, and sagittal. Simultaneously, radiation dose is comparable to panoramic x-rays, and superimposition of neighboring structures is obviated [12]. The relationship between the teeth apices and anatomic structures, variations in root morphology, additional canals, and external root resorption are just a few of the diagnostic conundrums that can be assessed via CBCT.
Surgical workup also includes complete blood count, routine urine examination, viral markers, and a thorough medical history. Appropriate regulation of insulin, anticoagulants, and other drugs should be undertaken in liaison with the attending physician.
A well-informed patient is the best patient. The importance of informed consent cannot be overdrawn, and the patient is entitled to know about the prognosis, benefits, and surgical complications, anticipate damage to vital structures, and follow up. Communication regarding the above details is mandatory, and the patient should be made aware of alternate treatment modalities like extraction followed by implant placement.
7 Anesthesia and Hemostasis
7.1 Premedication
Nonsteroidal anti-inflammatory drugs (NSAIDs) in conflation with a long-acting local anesthetic can scale down postoperative pain. Ainsworth surmised that routine use of prophylactic antibiotics in periapical surgery is unwarranted [12]. A presurgical mouth rinse with chlorhexidine gluconate (0.12%) will reduce the salivary bacteria significantly, especially their growth on sutures and wound margins, but may obtrude with fibroblast reattachment to the root [12].
Most patients tolerate the surgical procedure under local anesthesia but for the apprehensive, conscious oral sedation with benzodiazepines or nitrous oxide/oxygen inhalation should be opted for. Diazepam 10 mg can be started on the night before the surgery, and another dose can be administered 1 hour before the procedure [13].
7.2 Local Anesthesia and Sedation
The merits and demerits of various local anesthetics have been elaborated in detail elsewhere in this book. Conventional nerve blocks are augmented by local infiltration. Of equal momentousness is the preference for the vasoconstrictor. Adrenaline in concentrations ranging from 1:50,000, 1: 100,000, and 1:200,000 have performed commendably.
8 Surgical Access
8.1 Armamentarium
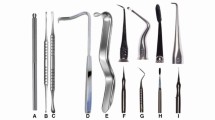
Incisions can be placed with no.11, no. 12, no 15, or no. 15-C blades (Fig. 16.1). Sharp, blunt dissection and elevation of the mucoperiosteal flap are accomplished by a Molts’ or Howarths’ periosteal elevator (Fig. 16.2). Endodontic tissue retractors like Austin, Seldon, or Minnesota should be judiciously selected in order to minimize trauma to the mucoperiosteal flap and neurovascular bundles (Fig. 16.3). Overlying bone is cut with No.4/6/8 round burs or 701/702 fissure burs (Fig. 16.4). These can be used to resect the root apex as well. Surgical handpiece with 45 ° angle head and rear air exhaust is advocated. Sharp surgical curettes like Lucas curette, angled periodontal curettes, and spoon excavators help to remove the inflamed soft tissue from the bony cavity (Fig. 16.5).
Root end resections can be done either with conventional burs or lasers (Er-YAG or Ho-YAG lasers) [14, 15]. The advantages of lasers include greater patient comfort, decreased vibrations, lesser surgical site contamination, and minimal trauma to the juxtaposed tissues.
Ultrasonic microsurgical tips are invaluable for root end preparation. Earlier, hand files and rotary burs were used. Teflon sleeves, pluggers, and Messing gun-type syringes can be used to place various root-end filling materials like MTA (Fig. 16.6). Review of literature validates the superiority of microsurgical techniques over conventional surgery (97% to 59%) [1]. The dental operating microscope, ultrasonic tips, and diamond coated micromirrors (Fig. 16.7) have found their niche in the surgeon’s armamentarium. Micromirrors (Fig. 16.8) can be used to inspect the buccal and lingual walls of the retrocavity. Microsurgical scalpels are useful for incising the intrasulcular areas and dissection of the interproximal papillae.
8.2 Surgical Management
The aims of periapical surgery are to visualize and debride the affected area and provide hermetic seal at the root end that aids in periodontal regeneration (Figs. 16.9, 16.10 and 16.11). Gutmann and Harrison [4] have categorized flaps as
-
1.
Full mucoperiosteal flaps
-
2.
Limited mucoperiosteal flaps
The main difference is that marginal interdental tissues are included in the full flaps, whereas the latter conserves them. Researchers opine that limited flaps prevent loss of papilla height, but careful adaptation of the reflected soft tissues rarely causes changes in gingival attachment level. It is vital to preserve the root attached tissues. In the absence of periodontal pathology, anatomic and functional status quo can be maintained via full mucoperiosteal flaps. Elevating palatal flaps is by and large a cumbersome affair. If the clinical situation demands a palatal approach, the envelope and triangular flaps can be considered. Contrary to popular teaching, vertical incisions can be placed on the palate, rather than stretching and renting a flap, which may impede healing. This approach is best reserved for palatal roots for posteriors. Anterior palatal cysts can be accessed labially or palatally, according to the surgeon’s discretion. To aid in surgical access, it is prudent to pass a long suture through the palatal flap and have the assistant retract the tissue. Table 16.2 details the advantages and disadvantages of various types of flaps that can be used for endodontic surgery.
8.3 Basic Principles of Flap Design
-
1.
Straight or parallel incisions are preferred over severely angled ones, in order to preserve the supraperiosteal blood supply of the attached gingiva and submucosa. Fewer vessels and collagen fibers are transected with parallel incisions, resulting in less hemorrhage and flap shrinkage
-
2.
Root eminences of canines and maxillary premolars are covered by thin bone with a poor blood supply and should be spared. Incisions should be placed between adjacent teeth on interdental bone
-
3.
Flaps should lie on solid healthy bone. At least 5 mm of bone should be present between the defect and incision line
-
4.
Do not incise frena and muscle attachments as it compromises the healing
-
5.
Do not incise through the dental papilla
-
6.
The entire mucoperiosteum should be included in the flap; this is imperative for uneventful healing
-
7.
Retractors should rest on solid bone
-
8.
The horizontal incision should extend at least one tooth beyond the pathologic area of interest
The position of the tooth in the arch, the dimensions of the periapical pathology, gingival recession, and the presence of artificial crowns also determine the choice of the incision that is placed.
8.4 Flap Elevation
The horizontal element of a full mucoperiosteal flap commences in the gingival sulcus and severs the gingival attachment fibers to the crestal bone. The interdental papilla should be incised at the midcol level. While incising a limited flap, the horizontal component must conform to the contour of the marginal gingiva and should be 2 mm apical to the depth of the gingival sulcus. The vertical incision should begin in the alveolar mucosa and proceed toward the crown till it abuts the horizontal incision. To achieve the above objectives fresh, sharp blades should be opted for.
Hemostasis and healing are enhanced if the entire mucoperiosteal flap is elevated as a single unit, due to adherence of the flap with its microvasculature. The broad end of the elevator can be maneuvered beneath the vertical incision, a few millimeters from the junction of the horizontal and vertical incision in the attached gingiva. This preserves the supracrestal root-attached fibers. This is followed by coronal dissection, and forces are directed toward the periosteum and bone. This technique is termed undermining elevation and should be continued throughout the length of the horizontal incision and apically to the alveolar mucosa [4]. An approximate distance of 1 cm from the apex should be exposed for adequate access. The bleeding tags seen on the bone contain periosteum that aids in healing and reattachment of the flap.
8.5 Flap Retraction
The soft tissues must be gently retracted to preclude the possibility of inadvertent crushing. This may lead to flap hypoxia, swelling, ecchymosis, and/or delayed healing. The retractor of a correct size should be selected and placed on cortical bone in such a way that the tissue is prevented from engaging with rotary instruments.
The periosteal surface of the flap should be irrigated with sterile, cool, and physiologic saline to keep it hydrated. The superficial surface is more resistant to dehydration due to the stratified squamous epithelium [16, 17].
8.6 Hard Tissue Management
Once the flap is raised, the surgeon encounters either intact cortical bone over the lesion or the lesion sans cortical bone. In the former scenario, it is imperative to localize the lesion and remove bone in the adjacent periapical area. Well-angled radiographs can aid in this aspect. Sounding the bone with the sharp end of the periosteal elevator can also be useful, as there is a change in resonance when one approaches the diseased area. It is also prudent to identify the root by calculating twice the crown length and then shave the thin bone at the apex. If digital technology is used, the distance from the alveolar crest to the root apex can be measured using the ruler function [3]. The root is smooth, hard, and yellow in color, surrounded by a periodontal ligament, and does not bleed on probing. Methylene blue dye staining can aid in locating the periodontal ligament.
Bone is vulnerable to thermal damage at any temperature above the normal body temperature. This is the crucial aspect of periapical surgery and influences the choice of burs, coolants, and handpieces. Bone when subject to temperatures between 40 °C and 50 ° C undergoes a spectrum of irreversible changes. These include reduction in microcirculation, tissue necrosis, fatty cell infiltration, and decrease in alkaline phosphatase.
While selecting bone cutting burs, sharp ones with wide spaces between the flutes are preferred. Round burs meeting these criteria promote excellent healing. Chilled saline effectively reduces the heat produced and flushes out the debris, thus enhancing efficiency. Excess pressure applied to the bone is detrimental to the tissues and handpiece. Gentle shaving or brushing motion must be used in short, multiple phases.
Hirsch et al. have used a piezoelectric device to create a bony aperture while performing apicoectomies in maxillary anteriors. The buccal bone was removed, preserved in Hank’s Balanced Salt Solution (HBSS), and later replaced in the bony crypt after the procedure, thus acting as an autologous bone graft. This is feasible in cases where there is minimal bone loss or in the presence of intact bone over the lesion [18].
The bony aperture should be wide enough to permit visual and surgical access into the lesion, enabling the insertion of bone curettes and excavators. In traditional root surgery, the size of the aperture is approximately 8–10 mm and 3-4 mm in microsurgery. The rate of healing is faster when the size of osteotomy is smaller. Granulomas and granulation tissues exhibit a propensity to bleed profusely, hindering the surgery. To circumvent this, local anesthetic with a vasoconstrictor can be injected within. Using a curette of appropriate dimension, the surgeon works from the periphery toward the center. The instrument is inserted between the tissue and the lateral edge of the cavity with its concave face toward the bone. This is continued all around the circumference of the cavity and slowly progresses toward the depth of the crypt in a scraping manner. After freeing all the tissue, it is gently grasped with a pair of tissue forceps and immersed in 10% buffered formalin solution. The specimen should not be left to dry. Lin et al. opine that complete curettage is not mandatory, if the irritant is eliminated [19]. Though a majority of periapical lesions have been diagnosed histopathologically as granulomas or cysts, there have been documented reports of perfectly innocuous looking periapical lesions diagnosed as odontogenic keratocysts, central giant cell granulomas, or squamous cell carcinoma [20, 21].
8.7 Root end Preparation
8.7.1 Root end Resection (Fig. 16.12)
Regeneration of alveolar bone, periodontal ligament, and cementum in the periapical area can be encouraged by removing the diseased root end tissues and placing a root end seal to stop the recontamination of the periapical region. Resection of apical 3 mm of the root apex will eliminate 78% of apical ramifications and 93% of lateral canals, which could contain material that would contribute to the periradicular disease [12]. The isthmus area should be included in the resection in roots with multiple canals. Anatomical obstructions, broken instruments, and perforations can be removed, orthograde sealing can be assessed, and trapped lingual tissue can be curetted out. In the case of apical fenestration, the apex can be reduced below the surrounding cortex to enable bone formation over the apex. The resection should enable the surgeon to prepare a root end cavity and place a restoration within.
A smooth, flat resected surface is considered ideal. This should be assessed for cracks, anatomical variations, and orthograde obturating material by means of an operating microscope at high power magnification and methylene blue staining [2]. The resection is made in order to surround the filling by normal dentin. Conventionally, a 30–45° bevel was placed, but the advent of the microscope has enabled a resection perpendicular to the long axis of the tooth. This substantially decreases the number of exposed dentinal tubules. Cohen opines that this aids in root end cavity preparation beyond the coronal extent of the root surface and apical stresses are well distributed, thus reducing apical fractures [11].
After the perpendicular root resection, the root must be conditioned to remove the smear layer produced. This exposes the collagen matrix of dentin and promotes growth. 5% aqueous citric acid has been used for this purpose. EDTA and tetracycline have also been studied, but have not found clinical popularity.
8.7.2 Root end Cavity Preparation
A 3 mm-deep Class I cavity is prepared along the long axis of the tooth, in order to place the filling material [22]. Ultrasonic tips have been specifically designed for this purpose. The tips produce less smear layer, need less beveling, and can be inserted through a smaller aperture. However, the ultrasonic vibrations can predispose to root fractures. This can be minimized when it is used at the lowest setting and with water coolant. Ultrasonic tips coated with stainless steel, diamond, or zirconium nitride are superior to uncoated tips. If the tips have a curvature of 70° or more, they are prone to fracture [23]. The root end filling is placed into the prepared cavity. When bonded materials like Retroplast are used, no root end preparation is needed; the filling is placed like a dome onto the resected root. This is termed bonded cap approach.
It is imperative to have a dry bloodless field prior to placement of the root end filling regardless of the setting properties of individual filling materials. To achieve this, adrenaline-saturated pellets, bone wax, Gelfoam, surgical, calcium sulfate, thrombin, collagen, or ferric sulfate may be used. Electrocautery may also be used, but this may delay bone healing.
8.7.3 Root end Filling (Fig. 16.13)
Amalgam used to be the quintessential retrograde filling material as it is cheap, easy to use, and radio opaque. However, it may stain the tissues and is sensitive to moisture [3]. Research has yielded a plethora of retrofilling materials, which has enhanced the outcome of endodontic surgery (Table 16.3).
After placing the root end filling, a radiograph must be obtained to assess its quality. If the radiograph reveals an incomplete root resection or an inadequate retrofill, the surgeon must rectify the above-said deficiencies. These are the most common surgical pitfalls that contribute to endodontic surgical failures.
The surgical site is gently cleaned and irrigated with sterile saline to remove debris of hemostatic agents and filling materials. Bone grafts or guided regeneration barriers can be placed into the crypt if indicated, but sterile technique should be adhered to, at all times to obviate infection. Calcium phosphate bioceramics, bioactive glass composite, and bioactive self-setting cements have been evaluated in vitro as well as in vivo [24, 25]. The flap is repositioned and delicately compressed with a moist gauze to vent out the excess blood, and tissue fluids 4–0 Silk or 6–0 monofilament suture materials are used. Tissue adhesives like cyanoacrylate and fibrin may be used as alternatives in the future. Suturing commences at the corners, approximately 2–3 mm from the wound margins. Interrupted sutures and sling sutures work well for closure of full mucoperiosteal flaps. Continuous locked suturing can also be done in marginal flaps as it reduces the time taken for suturing.
Following wound closure, moist gauze is placed on the flap for 5 min to stabilize clot formation and hemostasis. Compressing the flap with sterile ice packs in the immediate postoperative period minimizes the thickness of the fibrin clot and enhances wound healing. Intermittent cold compresses for 20 min on the day of surgery aids in patient comfort. Analgesics are prescribed, and verbal and written instructions are given to the patient and primary care provider. The wound must be cleaned gently with cotton. Chlorhexidine mouthwash is beneficial and can be continued till sutures are removed, i.e., on the fifth day postoperatively. Sutures can be removed after 3 days in microsurgical procedures.
9 Biology of Wound Healing [12]
The dynamics of healing in endodontic surgery involve various mechanisms germane to the nature of the individual tissues. The soft tissue incision heals by primary intention, whereas the bone defect and resected root surface heal via secondary intention. The endpoint of surgery should be regeneration, rather than repair where the normal tissue architecture and function are restored instead of a fibrous scar tissue.
The soft tissue healing progresses through three phases, i.e., inflammatory, proliferative, and maturation. The inflammatory phase begins with clot formation. The local microvasculature contracts, the platelets release serotonin, and a protein-rich exudate enters the wound site. Intravascular aggregation of platelets forms a platelet plug, and the extrinsic and intrinsic pathways are activated. This results in a randomly arranged thick fibrin clot. Within 6 hours of clot formation, polymorphonuclear leukocytes enter the wound and decontaminate the area by phagocytosis of bacteria. Their activity tapers off by 96 hours; monocytes and macrophages continue the phagocytic activity. A reduction in macrophages hampers the next phase of wound healing, especially in the older population where there is a step down of estrogen regulation of macrophages. The proliferative phase is dominated by fibroplasia and angiogenesis. The granulomatous nature of the wound transforms into granulation tissue by the activity of cytokines like platelet derived growth factor (PDGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF-1). By the third day, fibroblasts lay down Type III collagen, which matures to Type I. Myofibroblasts orient themselves parallel to the wound surface and contract, thus drawing the wound edges together. Concurrently, capillary networks form within the wound stimulated by proangiogenic factors like vascular endothelial growth factor (VEGF), FGF, transforming growth factor α, β (TGF-α, β), and interleukin-1 (IL-1). An epithelial seal is formed on the surface of the fibrin clot by the first day. In the next 5–7 days, the wound matures by the formation of larger collagen bundles.
In the osseous crypt, there is a hematoma and proliferation of granulation tissue, callus formation, and woven bone deposition, which is converted to lamellar bone. These events are regulated by TGF-β, PDGF, FGF, IGF, and bone morphogenic protein (BMP). At the root end, cementum forms over the resected surface. Cells responsible for cementogenesis are believed to originate from the ectomesenchymal cells in the tooth germ. By 28 days, the root end is covered by cementum.
10 Postoperative Complications
-
1.
Flap necrosis and breakdown, due to poor design and careless handling
-
2.
Transient paresthesia of mental and inferior alveolar nerves
-
3.
Exposure of the maxillary sinus, which heals in a majority of cases
-
4.
Perforation or Fracture of tooth roots
-
5.
Gingival recession and Scar tissue formation
-
6.
Staining of gingival tissues due to retrofilling materials like amalgam
11 Outcome of Endodontic Surgery
Clinical and radiographic assessments are made to determine the outcome of periapical surgery. The Periapical Index (PAI) has been used for radiographic assessment in both surgical and nonsurgical series [26]. Ingle opines that the terms ‘healed’, ‘healing’, ‘disease,’ and ‘asymptomatic’ can be used to describe the outcome.
-
1.
Healed cases show complete clinical and radiographic normalcy with no signs and symptoms or residual radiolucency
-
2.
Healing cases show a decrease in the size of the radiolucency and clinical normally within 4 years of surgery
-
3.
Diseased cases show radiolucency, new, increased, unchanged, or decreased after 4 years, regardless of radiographic appearance
-
4.
Asymptomatically functional teeth show clinical normalcy with or without persistent radiolucency, decreased or unchanged
12 Aids to Endodontic Surgery
Loupes, endoscopes, and the operating microscope have enhanced visualization of the operating field and thereby the quality of surgery.
12.1 Endoscopes
It has a rod lens system, camera head, and control unit with a monitor and light source placed on a mobile rack. The depth of perception is comparable with the naked eye. Tactile perception is excellent; diseased tissue behind and between roots can be visualized. Irrigation fluids can be used without clouding the visual field. Fabbro and Taschiere have reported success rates of 91.1 and 90.7% using the endoscope [27]. They offer rapid and easy adjustment of the viewing angle, direct viewing sans micromirrors, which is versatile and transportable.
12.2 Dental Operating Microscope
The resolution of the human eye is 0.2 mm. The power can be increased by moving closer to the surgical field, but can pose strain to the eye. Hence, magnifying lenses and illumination are used to bypass this problem.
The operating microscope offers up to 30 times magnification, coaxial light supply, and shadow free illumination. 200 mm objective lenses and 180° inclinable binoculars are optimum configurations. Light can be sourced from either xenon or quartz halogen bulbs. Digital camera, video camera, and co-observation tube are included in the setup. Endodontic microsurgery amalgamates magnification, illumination, and microinstrumentation, leading to predictable treatment outcomes. Deeper root end cavities can be prepared, which follow the contour of the root, hence minimizing lateral perforation [28]. The high cost of the equipment and need for specialized training deter many from adopting this tool on a routine basis, but the high success rates should be sufficient encouragement for clinicians and patients to adopt this new tool.
13 Future Perspectives
The role of mesenchymal stem cells in regeneration of periapical tissues has been explored with great enthusiasm. Dental pulp stem cells (DPSCs) and those from exfoliated deciduous teeth are multipotent in capacity and can be used along with scaffolds and signaling molecules [29]. These entities, when combined in the ideal proportion, aim to recreate the embryonic milieu, hence augmenting the biological concepts of wound healing. The future may usher in radical changes in our approach to endodontic surgery in terms of materials and techniques with respect to regenerative medicine.
References
Frank CS, Sweta BS, Meetu RK, Bekir K, Syngcuk K. Outcome of endodontic surgery: a meta analysis of the literature – part I: comparison of traditional root end surgery and endodontic microsurgery. JOE. 2010 Nov;36(11):1757–65.
Guerini VA. History of dentistry. Philadelphia: Lea and Febiger; 1909. p. 117.
Franco PB, Karlis V. In: Kademani D, Tiwana PS, editors. Apicoectomy in atlas of oral and maxillofacial surgery. St. Louis, MO: Elsevier; 2016.
Gutmann JL, Harrison JW. Surgical endodontics. St. Louis, MO: Ishiyaku euro America; 1994.
Quality assurance guidelines. Chicago: American Association of Endodontists; 1987, p. 1–27.
Simsek-Kaya G, Saruhan N, Yapia-Yavuz G, Ertas U. A decision analysis for periapical surgery: retrospective study. J Clin Exp Dent. 2018 Sep;10(9):e914–20.
El-Swiah JM, Walker RT. Reasons for apicoectomies: a retrospective study. Endod Dent Trauma. 1996;12:185–91.
Glickman GN, Hartwell GR. Endodontic surgery in ingle’s endodotnics. 6th ed. Hamilton, ON: BC Decker Inc; 2008.
European Society of Endodontology. Quality guidelines for endodontic treatment: consensus report of European society of endodontology. Int Endod J. 2006;39:921–30.
Eberhardt JA, Torabinejad M, Christiansen EL. A computed tomographic study of the distances between the maxillary sinus floor and apices of the maxillary posterior teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1992;73:345.
Benninger MS, Sebak BA, Levine HL. Mucosal regeneration of the maxillary sinus after surgery. Otolaryngol Head Neck Surgery. 1989;101:33.
Johnson BR, Fayad MI. Hargreaves KM, Berman LH, editors. Periradicular surgery in Cohen’s pathways of the pulp. 9th ed. St. Louis, MO: Elsevier.
Jastak JT, Yagiela JA. Regional anesthesia of the oral cavity. St. Louis, MO: Mosby; 1981.
Nedderman TA, Hartwell GR, Portell FR. A comparison of root surfaces following apical root resection with various burs: scanning electron microscope evaluation. J Endod. 1988;14:423.
Komori T, Yokoyama K, Matsumoto Y. YAG laser root resection of extracted human teeth. J Clin Laser Med Surg. 1997;15:9.
Harrison JW, Jurosky KA. Wound healing in the tissues of the periodontium following periradicular surgery. I. The incisional wound. J Endod. 1991;17:425.
Harrison JW, Jurosky KA. Wound healing in the tissues of the periodontium following perpendicular surgery. II. The dissectinal wound. J Endod. 1991;17:544.
Hirsch V, Meetu RK, Kim S. Apicoectomy of maxillary anterior teeth through a piezoelectric bony window osteotomy: two case reports introducing a new technique to preserve cortical bone. Restor Dent Endod. 2016 Nov;41(4):310–5.
Lin LM, Gaengler P, Langeland K. Periradicular curettage. Int Endod J. 1996;29:220.
Garlock JA, Pringle GA, Hicks ML. The odontogenic keratocyst: a potential endodontic misdiagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:452.
Jeevanand D, Ratika S, Rachappa M. Giant radicular cyst of the maxilla. BMJ Case Rep. 2014; https://doi.org/10.1136/bcr-2014-203678.
de Lange J, Putters T, Baas EM, van Ingen JM. Ultrasonic root end preparation in apical surgery: a prospective randomized study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:84.
Walmsley AD, Lumley PJ, Johnson WT, Walton RE. Breakage of ultrasonic root-end preparation tips. J Endod. 1996;22:287.
Komath M, Varma HK, Annie J, Vinod K, Simon D, Ramanathan M, Bhuvaneshwar GS. Designing bioactive scaffolds for dental tissue engineering in regenerative medicine: laborotary to clinic. Berlin: Springer; 2017.
Simon D, Manuel S, Varma HK. Novel nanoporous bioceramic spheres for drug delivery application: a preliminary in vitro investigation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Mar;115(3):e7–14.
Friedman S. Treatment outcome: the potential for healing and retained function in Ingle’s endodontics. 6th ed. Hamilton: BC Decker Inc; 2008.
Del Fabbro M, Taschieri S. Endodontic therapy using magnification devices: a systematic review. J Dent. 2010 Apr;38(4):269–75.
von Arx T. Failed root canals: the case for apicoectomy (periradicular surgery). J Oral Maxillofac Surg. 2005;63:832–7.
Shiehzadeh V, Aghmasheh F, Shiehzadeh F, Joulae M, Kosarieh E, Shiehzadeh F. Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: new method and three case reports. Indian J Dent Res. 2014 Mar–Apr;25(2):248–53.
Additional Reading (Algorithm for Periapical Surgery)
Lieblich SE. Periapical surgery: clinical decision making. Oral Maxillofac Surg Clin N Am. 2002;14:181.
Leiblich S. Current concepts of periapical surgery. Oral Maxillofacial Surg Clin N Am. 2015;27(3):383–2.
Acknowledgments
Dr. Khaleel Ahamed Thaha, Assistant Professor, Department of Conservative Dentistry and Endodontics, Government Dental College, Kozhikode, Kerala.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Simon, D. (2021). Endodontic Surgery. In: Bonanthaya, K., Panneerselvam, E., Manuel, S., Kumar, V.V., Rai, A. (eds) Oral and Maxillofacial Surgery for the Clinician. Springer, Singapore. https://doi.org/10.1007/978-981-15-1346-6_16
Download citation
DOI: https://doi.org/10.1007/978-981-15-1346-6_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1345-9
Online ISBN: 978-981-15-1346-6
eBook Packages: MedicineMedicine (R0)