Abstract
The ductus arteriosus (DA) must be closed after birth for the establishment of adult circulation. Extremely preterm infants, however, frequently have patent ductus arteriosus (PDA), which associates with significant morbidity and mortality. At birth, the separation of the fetus from its maternal environment constitutes a dramatic change for the infant. Initiation of respiration changes serum composition and oxygen tension resulting in the closure of the DA. We found that serum osmolality was significantly decreased early after birth in rats, and that the hypo-osmolality sensor transient receptor potential melastatin (TRPM) 3 had increased expression in the rat DA compared to the aorta. Our data suggest that a decrease in serum osmolality promotes DA closure via TRPM3 and that an increase in osmolality dilates the DA. For extremely preterm infants, recent recommendations include immediate amino acid supplementation. We examined plasma amino acid composition and found an association between low glutamate concentration and PDA. Our data suggest that glutamate induces DA contraction through the AMPA receptor GluR-mediated noradrenaline production. Based on these data, maintaining proper levels of serum osmolality and amino acid composition may improve the therapeutic outcome of PDA in extremely preterm infants.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In the fetus, the ductus arteriosus (DA) diverts ventricular output away from the high-resistance pulmonary vascular bed into the systemic circulation. Although its patency is essential for fetal circulation, once pulmonary circulation is established, the DA must be closed.
Despite considerable progress in medical management for preterm infants, 25% of PDA in extremely preterm infants is still resistant to pharmacological therapies, i.e., cyclooxygenase inhibitors, which have been the only pharmacological therapeutic strategy since the mid-1970s [1,2,3]. Therefore, novel strategies for PDA in extremely preterm infants are required.
At birth, dynamic changes occur in serum components. A previous study demonstrated that serum osmolality decreased transiently after birth, but recovered over the next few days in full-term human neonates [4]. However, the significance of this transient decrease in osmolality has never been addressed. Amino acid composition is also changed after birth. Amino acid administration beginning immediately after birth has recently been suggested [5]. However, the role of amino acids on DA regulation has not been reported. Therefore, we investigated the roles of serum osmolality and amino acid composition in DA closure.
2 Novel Mechanisms Regulating DA Closure
2.1 Decreased Serum Osmolality Promotes DA Contraction
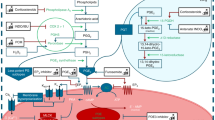
Under physiological conditions, body-fluid osmolality is tightly regulated to near 300 mOsm/kg through the intake or excretion of water and salt [6]. Hypotonicity is sensed, partly, by the transient receptor potential (TRP) channels, such as TRP vanilloid 4 (TRPV4) [7] or TRP melastatin 3 (TRPM3) [8, 9], which acts as a Ca2+ channel. Because changes in intracellular Ca2+ are associated with DA contraction [10,11,12], we focused on TRP channels.
We found that rats exhibited a similar transient hypo-osmolality after birth as in humans. Hypotonic stimulation induced constriction of DA rings and increased Ca2+ transient in DA smooth muscle cells. TRPM3 was highly expressed in the rat DA, and TRPM3-targeted siRNA abolished the Ca2+ response to hypo-osmolality. The TRPM3 stimulator, pregnenolone sulfate, induced rat DA constriction ex vivo and in vivo. Contrary to these findings, intra-peritoneal hypertonic fluid administration impaired rat DA closure.
In humans, neonatal serum hypo-osmolality was observed in relatively mature preterm infants (≥28 weeks). In extremely preterm infants (<28 weeks), however, this hypo-osmolality was not observed. Instead, a rapid increase in osmolality occurred after birth in extremely preterm infants. The degree of osmolar increase was greater in patients with PDA.
These data suggest that a transient decrease in serum osmolality may promote DA closure during the first few days of life (Fig. 37.1) [13].
2.2 Glutamic Acid Contributes to DA Closure
First, we analyzed amino acid composition in human neonates. Aminogram results in human neonates at day 2 revealed that the plasma glutamate concentration was significantly lower in extremely preterm infants (<28 weeks gestation) with PDA than in those without PDA and relatively mature preterm infants (28–29 weeks gestation).
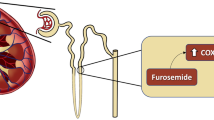
It has been shown that glutamate stimulates noradrenaline release [14, 15]. Many adrenergic nerve terminals were shown in the muscular walls of the human DA, and fluorometric results revealed the presence of noradrenaline in the human DA [16]. A previous report demonstrated that noradrenaline induced significant constriction of the DA even in the 20-week human fetus [16]. Moreover, a recent study demonstrated that glutamate induces noradrenaline release through glutamate inotropic receptor alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) type subunit 1 (GluR1) [17]. As a result, we investigated the role of glutamate in DA closure.
In the rat, GluR1 mRNA was highly expressed in the DA compared to the aorta on gestational day 19 (preterm) and gestational day 21 (term). GluR1 proteins were co-localized with tyrosine hydroxylase-positive autonomic nerve terminals in the rat and human DA. Intraperitoneal administration of glutamate promoted noradrenaline production in the rat DA. Glutamate administration induced DA contraction in both preterm (gestational day 20) and term rat fetuses in vivo. Glutamate-induced DA contraction was attenuated by the Ca2+-sensitive GluR receptor antagonist NASPM or the adrenergic receptor α1 blocker, prazosin.
These data suggest that glutamate induces DA contraction through GluR-mediated noradrenaline production (Fig. 37.2) [18].
3 Future Direction and Clinical Implications
PDA often causes serious problems in very premature infants, depending on the degree of left-to-right shunting. It has been reported that more than 50% of infants with birth weights <1000 g receive pharmacological therapy consisting of cyclooxygenase (COX) inhibitors or surgical ligation of the DA. Because the current pharmacological and surgical therapies for treating PDA are not always ideal, it is highly desirable to find a relatively simple and noninvasive strategy that would prevent PDA from occurring.
Our study demonstrated that serum osmolality is transiently decreased after birth, and this decrease may be a physiological mechanism to facilitate DA contraction. The significance of this transient decrease in serum osmolality has been largely overlooked. It may be time to reconsider the importance of serum osmolality. Keeping their serum osmolality at the proper, but not elevated, level may be important among preterm infants at risk for PDA.
Our data also suggest that glutamate promoted DA contraction in preterm infants. The composition of amino acid mixtures varies between commercially available solutions. Of note, glutamate concentration is markedly different compared to the other components among commercially available amino acid solutions (0.8–10.0 g/L). Supplementation of glutamate might help to prevent PDA in extremely preterm infants, and the importance of amino acid composition, i.e., glutamate, may need to be considered in postnatal circulatory adaptation.
References
Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacologic closure of patent ductus arteriosus in the premature infant. N Engl J Med. 1976;295:526–9.
Kiefer AS, Wickremasinghe AC, Johnson JN, Hartman TK, Hintz SR, Carey WA, Colby CE. Medical management of extremely low-birth-weight infants in the first week of life: a survey of practices in the United States. Am J Perinatol. 2009;26:407–18.
Yokoyama U. Prostaglandin E-mediated molecular mechanisms driving remodeling of the ductus arteriosus. Pediatr Int. 2015;57:820–7.
Feldman W, Drummond KN. Serum and urine osmolality in normal full-term infants. Can Med Assoc J. 1969;101:73–4.
Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, Parenteral Nutrition G, Guidelines Working N. European Society for Clinical, Metabolism, H. European Society of Paediatric Gastroenterology, Nutrition, R. European Society of Paediatric, 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr. 2005;41(Suppl 2):S1–87.
Bourque CW, Oliet SH. Osmoreceptors in the central nervous system. Annu Rev Physiol. 1997;59:601–19.
Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–703.
Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–501.
Oberwinkler J, Phillipp SE. TRPM3. Handb Exp Pharmacol. 2007;179:253–67.
Nakanishi T, Gu H, Hagiwara N, Momma K. Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ Res. 1993;72:1218–28.
Akaike T, Jin MH, Yokoyama U, Izumi-Nakaseko H, Jiao Q, Iwasaki S, Iwamoto M, Nishimaki S, Sato M, Yokota S, Kamiya Y, Adachi-Akahane S, Ishikawa Y, Minamisawa S. T-type Ca2+ channels promote oxygenation-induced closure of the rat ductus arteriosus not only by vasoconstriction but also by neointima formation. J Biol Chem. 2009;284:24025–34.
Yokoyama U, Minamisawa S, Adachi-Akahane S, Akaike T, Naguro I, Funakoshi K, Iwamoto M, Nakagome M, Uemura N, Hori H, Yokota S, Ishikawa Y. Multiple transcripts of Ca2+ channel alpha1-subunits and a novel spliced variant of the alpha1C-subunit in rat ductus arteriosus. Am J Physiol Heart Circ Physiol. 2006;290:H1660–70.
Aoki R, Yokoyama U, Ichikawa Y, Taguri M, Kumagaya S, Ishiwata R, Yanai C, Fujita S, Umemura M, Fujita T, Okumura S, Sato M, Minamisawa S, Asou T, Masuda M, Iwasaki S, Nishimaki S, Seki K, Yokota S, Ishikawa Y. Decreased serum osmolality promotes ductus arteriosus constriction. Cardiovasc Res. 2014;104:326–36.
Pittaluga A, Raiteri M. N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors regulating hippocampal norepinephrine release. I. Location on axon terminals and pharmacological characterization. J Pharmacol Exp Ther. 1992;260:232–7.
Wang JK, Andrews H, Thukral V. Presynaptic glutamate receptors regulate noradrenaline release from isolated nerve terminals. J Neurochem. 1992;58:204–11.
Aronson S, Gennser G, Owman C, Sjoberg NO. Innervation and contractile response of the human ductus arteriosus. Eur J Pharmacol. 1970;11:178–86.
Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hortnagl H, Riva MA, Sprengel R, Gass P. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 2008;22:3129–34.
Fujita S, Yokoyama U, Ishiwata R, Aoki R, Nagao K, Masukawa D, Umemura M, Fujita T, Iwasaki S, Nishimaki S, Seki K, Ito S, Goshima Y, Asou T, Masuda M, Ishikawa Y. Glutamate promotes contraction of the rat ductus arteriosus. Circ J. 2016;80:2388–96.
Acknowledgments
The authors are grateful to Yuka Sawada for technical assistance. This study was funded by MEXT/JSPS KAKENHI (U.Y., JP17K19403, JP16H05358, JP15H05761; S.I., JP17K16276; J.S., JP16H07107; Y.I., JPH1605300), and the Yokohama Foundation for the Advancement of Medical Science (J.S.), the Japan Agency for Medical Research and Development (AMED) (Y.I., 66890007, 56689113), and the Kitsuen Research Foundation (Y.I., 71890005).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this paper
Cite this paper
Yokoyama, U. et al. (2020). New Insights on How to Treat Patent Ductus Arteriosus. In: Nakanishi, T., Baldwin, H., Fineman, J., Yamagishi, H. (eds) Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension. Springer, Singapore. https://doi.org/10.1007/978-981-15-1185-1_37
Download citation
DOI: https://doi.org/10.1007/978-981-15-1185-1_37
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1184-4
Online ISBN: 978-981-15-1185-1
eBook Packages: MedicineMedicine (R0)