Abstract
We have developed a real-time radioisotope imaging system (RRIS) to visualize ion transport in plants, and to measure radioactivity in living plants. To know the mechanisms of ion transport in plants, the use of living plants allows us to visualize ion movement in real time. In addition, the RRIS can analyze how a change to the plant environment affects ion transport. In this chapter, we will introduce some of the applications of the RRIS. We analyzed the effect of light on cesium, potassium, magnesium, phosphate, and calcium transport in plants using the RRIS. The results show that magnesium, potassium, and calcium transport in plants were not influenced by light. On the other hand, the amount of cesium and phosphate absorption in roots decreased after light-off. Moreover, the amount of phosphate transport from root to shoot also decreased after light-off.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
After the accident of Fukushima Daiichi nuclear power plant, it is necessary to reduce radiocesium (137Cs) in crops. To solve this problem, it is important to know how much 137Cs is accumulated in each tissue and to understand in detail the mechanism of 137Cs transport in plants. There are two pathways responsible for elemental transport in plants, namely, the symplastic and apoplastic pathways. The symplastic pathway transports water and ions via the plasmodesma, whereas transport by the apoplastic pathway is via cell walls. In addition, transporters within membranes also affect elemental transport. Elements within the vascular bundle are distributed between tissues through vessels and sieve tubes. Water and nutrients are mainly transported via vessels and the driving force is root pressure and transpiration. Water, nutrients, and photosynthetic products are transported via sieve tubes and the driving force depends on the concentration gradient of sucrose. The environmental changes, such as weather, affect ion transport because the rate of transpiration and photosynthesis is influenced by light. Various techniques are available to researchers to analyze transport and distribution of 137Cs in plants. In particular, we often use imaging techniques. One such imaging technique is the real-time radioisotope imaging system (RRIS) which was developed in our laboratory (Nakanishi et al. 2009). The RRIS can visualize transport and distribution of various radioisotopes including 137Cs in living plants. In addition, we can analyze the amount of cesium using the images obtained by the RRIS. To analyze ion transport in plants using the RRIS with living plants is a big advantage because ions in plants are continuously moving. Some of the findings related to Cs transport include the following: Kobayashi visualized 137Cs in rice plants grown in water culture and soil, and found minimal 137Cs translocation to rice grain when grown in soil because of 137Cs adsorption to soil particles (Kobayashi 2013). In addition, Sugita et al. reported that the transport manner of 137Cs and 42K from roots to above-ground parts of Arabidopsis is similar for 137Cs and 42K (Sugita et al. 2016). Kobayashi et al. demonstrated that 137Cs was retained in the root tissues with high efficiency, while 42K was easily exchanged and transported towards the shoots (Kobayashi et al. 2016). Moreover, RRIS can analyze how changes in the growth environment of living plants affect ion transport, such as temperature or nourishment. In this chapter, we will report the characteristics of the Cs transport compared to other elements in roots of rice plants when environmental conditions change from light to darkness.
19.2 Imaging System and Imaging Methods
19.2.1 Obtaining Images
The characteristics of the Real-time radioisotope imaging system (RRIS) are as follows:
-
It is possible to visualize and quantify ion movements in living plants using radioisotopes;
-
It is possible to detect many types of radiations such as beta-, X-, gamma-rays, and positron (Sugita et al. 2014)
There are two steps to visualize radiation. (1) Radiation is converted to visible light by CsI (Tl) scintillator, and (2) the visible light is captured by CCD camera (Fig. 19.1). RRIS can use living plants for experimentation, so we can get sequential images. The light converted from radiation is very weak, therefore in order to take images, it is necessary to use a dark conditions box.
Overview of the mechanism of real-time radioisotope imaging system
The radiation from nuclide is converted to visible light by CsI (Tl) scintillator, and the visible light is then captured by CCD camera
This figure is quoted from Sugita et al. (2017)
19.2.2 Two Types of View Areas
The RRIS has two types of view areas. One is 10 × 20 cm in size (w × h) and known as the macro-RRIS. The other, the micro-RRIS, has the ability to take images within the μm range using a microscope. The view area is approximately 600 × 600 μm (optical lens: x20 magnification) to 5.2 × 5.2 mm (x2.5 magnification) in size (W × L).
19.2.2.1 macro-RRIS
The macro-RRIS mainly visualizes ion movements in whole plants. Test plants are placed on the CsI (Tl) scintillators (Fig. 19.2a). The dark box’s size is approximately 120 × 150 × 80 cm (W × L × D). We have taken images of rice, Arabidopsis, soy beans, poplar, for example.
19.2.2.2 micro-RRIS
The mechanism of how to take images by micro-RRIS is identical to how images were taken by macro-RRIS. The CsI (Tl) scintillator (1.5 × 1.5 cm) is placed on the plant, and the light converted from radiation by the scintillator is captured by CCD camera through a microscope (Fig. 19.2b).
19.2.3 Radioisotopes
Commercially available radioisotopes are predominantly used for visualizing elements within plants. On the other hand, radioisotopes with short half-lives (e.g., 42K and 28Mg) have to be produced by our laboratory as they are not available commercially. For example, 42K has a half-life of 12 h and 28Mg has a half-life of 20 h.
19.2.3.1 42K
42K is produced by 42Ar-42K generators (Aramaki et al. 2015). The half-life of 42Ar and 42K is about 33 y and 12 h, respectively. The method of producing 42K is described as follows (Fig. 19.3a): (1) a steel electrode is inserted into a generator with a cock which has 42Ar. (2) 42K is produced from 42Ar when the steel electrode has a minus charge and the generator is positively charged. (3) When 60 V is applied to the electrode and generator, 42K is attracted to the steel electrode. (4) the steel electrode is removed from the generator and 42K is collected inside a pipette with water (Fig. 19.3b).
19.2.3.2 28Mg
28Mg was produced using a cyclotron by 27Al (α, 3p) 28Mg reaction (Tanoi et al. 2013).
19.2.4 Applying Radioisotopes to Plants
Radioisotopes can be applied to plants either in gas or liquid form. Gas radioisotopes such as 14C-labeled CO2 (Sugita et al. 2013) can be applied to the whole plant or to only specific tissues (e.g., leaves, shoots, stems). Liquid radioisotopes such as 137Cs and 42K are applied to roots when test plants are grown in culture solutions, gels or soils. In addition, it is possible to visualize ion movement from a leaf to other tissues (Sugita et al. 2016).
19.2.5 Applying Light to Plants
The CCD camera in the RRIS has a high sensitivity, but its detector can become damaged if exposed to strong light. In addition, visible light converted from radiation is very weak, and therefore it is necessary to have complete dark conditions while taking images. Because plants need light for growth, an intermittent lighting system was developed (Hirose et al. 2013). This lighting system ensures dark conditions when images are being taken and light conditions for the remaining time. For example, one dark/light cycle is 3 min/7 min and the RRIS gets an image for 3 min in the dark condition. Moreover, the cycle can be set for 24 h, allowing the RRIS to visualize ion movements for 24 h. The micro-system has the same type of system.
19.3 Impact of Light for Ion Transport in Plants
19.3.1 Experimental
19.3.1.1 Test Plants
Rice seeds (Oryza sativa. L. ‘Nipponbare’) were germinated in 0.5 mM CaCl2 solution under dark conditions for 2 days. The plants were transplanted into half-strength Kimura-B nutrient solution (270 mM K+) for 6 days. Next, four plants were placed in a root chamber with half-strength Kimura-B nutrient solution. For 137Cs imaging, 0.1 μM CsCl2 was added to the Kimura-B nutrient solution. The root chamber was made of a polyethylene bag and polyurethane sheet (Fig. 19.4a). The plant roots were fixed inside the bag using the polyurethane sheet, and the bag was fixed onto the scintillator (Fig. 19.4b).
The mounting arrangement of test plants in the real-time radioisotope imaging system
(a) Four plants were placed in a root chamber. The roots were fixed using the polyurethane sheet
(b) The root chamber was pressed onto the scintillator using acrylic resin plates
This figure is quoted from Sugita et al. (2017)
19.3.1.2 Visualization of Element Movement Using RRIS
The following radioisotopes were applied to the roots: 28Mg: 17 kBq mL−1; 32P: 30 kBq mL−1; 42K: 7 kBq mL−1; 45Ca: 830 kBq mL−1; and 137Cs: 25 kBq mL−1. Their characteristics are shown in Table 19.1. The total length of time for taking images was 10 h. During the first 5 h, the dark/light cycle of 3 min was set up using LED lights (100 μmols−1 m−2), and then the RRIS took images using 3 min of exposure time for each 3 min dark condition. During the next 5 h, the RRIS took images using 3 min of exposure time for each 10 min in the dark condition.
19.3.1.3 Transpiration Rate
Transpiration rate was calculated as the decreased level of the culture water under light for 10 h or under light condition for 5 h followed by 5 h of dark condition in the dark box of the RRIS.
19.3.2 Results and Discussion
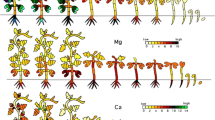
RRIS was used to visualize the response of ion movement between light and dark conditions. At first, each radioisotope (137Cs, 42K, 28Mg, 32P, and 45Ca) was supplied to roots and visualized for 5 h under light (0–5 h) and dark conditions (5–10 h) (Fig. 19.5a-19.5d). To analyze the changes during uptake in roots and translocation from root to shoot, the radioisotope activities were measured in the region of interest (ROI) of the shoot and root based on the RRIS images (Fig. 19.6a); the time course of signal intensity within the ROI was then obtained. The signal intensity value was shown relative to the 5 h after the radioisotope absorption (Fig. 19.6b–e). In addition, the slopes of the calibration curves were calculated for 3–5 h and 5–7 h to compare the radioisotope accumulation velocities (Fig. 19.6f). The results show that the 137Cs activity in roots only increased slightly after turning the light off (Fig. 19.6b) and the slope of the calibration curve showed a statistically significant decrease (Fig. 19.6f). On the other hand, the activities in the shoots of 137Cs were not influenced by light and continuously increased after turning the light off. These results suggest that Cs uptake from the root is largely influenced by light and Cs transport from root to shoot is not influenced by light. In regards to K, there was no influence of light on either shoot or root (Fig. 19.6b). These results suggest that in Cs absorption from roots, it is possible that transporters which are different to K transporter greatly contributes to Cs transport and the activity of those transporters are affected by light. Both 45Ca and 28Mg accumulation in shoot and root were not influenced by the change from light to dark conditions (Fig. 19.6c, e, f). For 32P, the reaction from light to dark condition was prompt, with 32P accumulation rate in both shoot and root decreasing (Fig. 19.6d). Considering the response time of transpiration from light to dark condition is several minutes (Ishikawa et al. 2011), it was assumed that transpiration greatly influenced 32P transport. After 5 h from light to dark condition, transpiration decreased 57% in comparison with continuous light (Fig. 19.7). This result suggests that P was absorbed and transported with water, while Mg, Ca, and K were not affected by the flow of water. On the other hand, the signal level of 45Ca in the root only increased slightly after 3 h after its addition (Fig. 19.6e). This result shows that the replacement of Ca2+ with 45Ca in roots is quick. In addition, 45Ca accumulation in shoot continuously increased, suggesting that surplus 45Ca is transported to the shoot.
Serial images under light/dark conditions in rice, taken by RRIS
After the radioisotope was added, the serial images were taken under light conditions for the first 5 h and dark conditions for the following 5 h
(a) 42K-137Cs, (b) 28Mg, (c) 32P, and (d) 45Ca
This figure is quoted from Sugita et al. (2017)
The influence of ion movement under light/dark conditions
(a) Photograph of test plants. The blue, red, and green lines indicate the region of interest (ROI) of shoot, root, and background, respectively. Time-course analysis of the radioactivity of (b) 42K-137Cs, (c) 28Mg, (d) 32P, and (e) 45Ca. The relative signal intensity was normalized at 5 h. (f) The slope of the calibration curve between 3–5 h (light) and 5–7 h (dark) based on Fig. b-e. Data represent means ± standard deviation (n = 4 plants)
This figure is quoted from Sugita et al. (2017)
Transpiration rate was calculated under the light condition for 10 h (circles), or under light condition for 5 h followed by 5 h of dark condition (crosses) at 3, 5, 7 and 10 h. Data represent means ± standard deviation (n = 4 plants)
This figure is quoted from Sugita et al. (2017)
As shown in the examples above, RRIS is a powerful tool to analyze responses in plants to their environment. To decrease 137Cs accumulation in plants, we will continue to research the uptake and translocation of 137Cs in plants using RRIS.
Abbreviations
- Ca:
-
Calcium
- Cs:
-
Cesium
- K:
-
Potassium
- Mg:
-
Magnesium
- P:
-
Phosphate
- RRIS:
-
real-time radioisotope imaging system
References
Aramaki T, Sugita R, Hirose A, Kobayashi NI, Tanoi K, Nakanishi TM (2015) Application of 42K to Arabidopsis tissues using real-time radioisotope imaging system (RRIS). Radioisotopes 64:169–176
Hirose A, Yamawaki M, Kanno S, Igarashi S, Sugita R, Ohmae Y et al (2013) Development of a C-14 detectable real-time radioisotope imaging system for plants under intermittent light environment. J Radioanal Nucl 296:417–422
Ishikawa JS, Hatano MM, Hayashi H, Ahamed A, Fukushi K, Matsumoto T, Kitagawa Y (2011) Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant, Cell Environ 34:1150-1163
Kobayashi NI (2013) Time-course analysis of radiocesium uptake and translocation in rice by radioisotope imaging. In: Nakanishi TM, Tanoi K (eds) Agricultural implications of the Fukushima nuclear accident. Springer, Tokyo, pp 37–48
Kobayashi NI, Sugita R, Nobori T, Tanoi K, Nakanishi TM (2016) Tracer experiment using 42K+ and 137Cs+ revealed the different transport rates of potassium and caesium within rice roots. Funct Plant Biol 43:151–160
Nakanishi TM, Yamawaki M, Kannno S, Nihei N, Masuda S, Tanoi K (2009) Real-time imaging of ion uptake from root to above-ground part of the plant using conventional beta-ray emitters. J Radioanal Nucl 282:265–269
Sugita R, Kobayashi NI, Hirose A, Ohmae Y, Tanoi K, Nakanishi TM (2013) Nondestructive real-time radioisotope imaging system for visualizing C-14-labeled chemicals supplied as CO2 in plants using Arabidopsis thaliana. J Radioanal Nucl 298:1411–1416
Sugita R, Kobayashi NI, Hirose A, Tanoi K, Nakanishi TM (2014) Evaluation of in vivo detection properties of 22 Na, 65 Zn, 86 Rb, 109 Cd and 137 Cs in plant tissues using real-time radioisotope imaging system. Phys Med Biol 59:837–851
Sugita R, Kobayashi NI, Hirose A, Saito T, Iwata R, Tanoi K et al (2016) Visualization of uptake of mineral elements and the dynamics of photosynthates in arabidopsis by a newly developed real-time radioisotope imaging system (RRIS). Plant Cell Physiol 57:743–753
Sugita R, Kobayashi NI, Hirose A, Iwata R, Suzuki H, Tanoi K et al (2017) Visualization of how light changes affect ion movement in rice plants using a real-time radioisotope imaging system. J Radioanal Nucl 312:717–723
Tanoi K, Kobayashi NI, Saito T, Iwata N, Hirose A, Ohmae Y et al (2013) Application of 28Mg to the kinetic study of Mg uptake by rice plants. J Radioanal Nucl 296:749–751
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2019 The Author(s)
About this chapter
Cite this chapter
Sugita, R., Kobayashi, N.I., Hirose, A., Tanoi, K., Nakanishi, T.M. (2019). Visualization of Ion Transport in Plants. In: Nakanishi, T., O`Brien, M., Tanoi, K. (eds) Agricultural Implications of the Fukushima Nuclear Accident (III). Springer, Singapore. https://doi.org/10.1007/978-981-13-3218-0_19
Download citation
DOI: https://doi.org/10.1007/978-981-13-3218-0_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3217-3
Online ISBN: 978-981-13-3218-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)