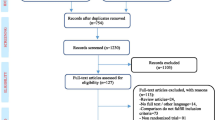
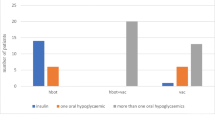
Abstract
This paper presents a study to assist of a Decision Support Systems and Clinical Engineering Health Technology Management. The methodology is based on methodological guideline for the evaluation of medical equipment addressing its main domains (Clinical, Admissibility, Technical, Operational and Economic) in order to verify the incorporation of Hyperbaric Chambers in comparison to the outsourcing service for the injuries treatment diabetes carriers in Santa Catarina, Brazil. The HBOT application is still very controversial, often generating doubts and making it difficult to make decisions about its incorporation for the public health services. As a result, the Systematic Review, Randomized Controlled Trials and clinical reports were selected in the clinical domain and the operational and technical domain, it is performed a comparative of equipment with its technological resources and service, seeking to analyze parameters that influence in its performance. In the economic domain, through the total cost of ownership, it was estimated it’s direct and indirect costs related to the equipment’s acquisition and inherent to the life cycle sustainability. HTA for medical equipment present several barriers due to the lack of evidence and quality information, it is expected that this work can generate scientific evidences of knowledge and instruments to enable new research involving hyperbaric chambers, as well as contribute to decision-making or other concomitant programs, due to the application of resources in a planned and adequate decision.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR ISO 14971 – Associação Brasileira de Normas Técnicas. Produtos para saúde – aplicação de gerenciamento de riscos a produtos para saúde. Rio de Janeiro, 2009, 86 p.
AUGUSTOVISKI, F.; PICHON-RIVIERE, A.; RUBINSTEIN, A. Critérios utilizados pelos sistemas de saúde para a incorporação de tecnologias. In: NITA, M. E. et al. Avaliação de tecnologias em saúde: evidência clínica, análise econômica e análise de decisão. Porto Alegre: Artmed, 2010.
Brasil, MINISTÉRIO DA SAÚDE, 2001b. Indicadores e Dados Básicos – IDB/SUS. Disponível em: <http://www.datasus.gov.br>. Accessin: Nov 2017.
BRASIL, Ministério da Saúde. Agência Nacional de Vigilância Sanitária.Tecnovigilância. 2001. Disponível em: <http://www.anvisa.gov.br/>. Access in: Sep 2017.
BRASIL. Ministério da Saúde. Departamento de Ciência e Tecnologia. Diretrizes metodológicas: elaboração de estudos para avaliação de equipamentos médicos assistenciais. Brasília: Ministério da Saúde, 2013b. 96 p.
BRASIL. Ministério da Saúde. Avaliação de tecnologias em saúde: ferramentas para a gestão do SUS/ Ministério da Saúde, Secretaria-Executiva, Área de Economia da Saúde e Desenvolvimento. – Brasília: Editora do Ministério da Saúde, 2009.
CALIL, S. J.; TEIXEIRA, M. S. Gerenciamento de Manutenção de Equipamentos Hospitalares, Série Saúde & Cidadania, v.11. São Paulo: Faculdade de Saúde Pública da Universidade de São Paulo, 1998.
FERNANDES, I. A. D. Judicialização Da Saúde: Estudos De Casos – Oxigenoterapia Hiperbárica Lesões Refratárias: lesões pé- diabético. Pouso Alegre – MG: FDSM, 2016.
GOODMAN. C S. HTA 101 Introduction to Health Technology Assessment. National Information Center on Health Services Research & Health Care Technology, USA, 2004.
MARGOTTI, A. E. Metodologia para incorporação de equipamento médico assistencial em hospitais utilizando a avaliação de tecnologias em saúde na engenharia clínica. Master’s thesis, Universidade Federal de Santa Catarina, 2012.
PHILLIPS B, BALL C, SACKETT D, et al. Oxford Centre for Evidence-based Medicine - Levels of evidence. Grades of recommendation. Available from: http://www.cebm.net/index.aspx?o=1025.
SA’AID, H. B.; STEWART, D.; ENGLAND, I. Decision Making Processes for Introducing New Health Technology at Institutional Level: Decision Makers’ Perspective. World Review of Business Research, v.1, n.2, p. 10–19, Mai. 2011.
SANTOS, F. A., GARCIA, R., Decision Process Model to the Health Technology Incorporation, In Proc. 32nd Annual International Conference of the IEEE EMBS, Buenos Aires - Argentina, pp. 414–417, 2010.
SÔNEGO, F. S. Estudo de Métodos de Avaliação de Tecnologias em Saúde aplicada a Equipamentos Eletromédicos. 2007. 92 f. Dissertação (Mestrado em Engenharia Elétrica) – Centro Tecnológico, Universidade Federal de Santa Catarina, Florianópolis, 2007.
WANG, B. Strategic Health Technology Incorporation. In: ENDERLE, J. D. Synthesis lectures on biomedical engineering. Morgan &Claypool, Princeton NJ, 2009.
WHO. World Health Organization. Medical Devices: managing the mismatch: an outcome of the priority devices project. Suiça, 2010.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors of this article declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Pezzolla, F.M.G., Avelar, P., Maciel, J., Garcia, R. (2019). Support in the Medical Equipment Incorporation Decision: Hyperbaric Oxygen Therapy Adjunct for Diabetic Foot Ulcers Therapy. In: Lhotska, L., Sukupova, L., Lacković, I., Ibbott, G. (eds) World Congress on Medical Physics and Biomedical Engineering 2018. IFMBE Proceedings, vol 68/3. Springer, Singapore. https://doi.org/10.1007/978-981-10-9023-3_60
Download citation
DOI: https://doi.org/10.1007/978-981-10-9023-3_60
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-9022-6
Online ISBN: 978-981-10-9023-3
eBook Packages: EngineeringEngineering (R0)