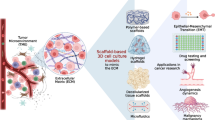
Abstract
The conventional two-dimensional (2D) cell culture models, although providing considerable information about the cellular dynamics, often fail in vivo. In the area of oncology drug discovery and development process, the tumor microenvironment poses a significant challenge owing to the complexity of tumor stroma, and the traditional 2D in vitro systems fail to mimic the extracellular matrix (EC) and cell-to-cell interaction-based modulations. Three-dimensional (3D) cell culture systems/matrices, also designated as scaffolds, offer an excellent platform to study the tumor microenvironment in a more realistic way. Alginate matrices are widely used for cellular encapsulation, cell transplantation, and tissue engineering. Alginate-based 3D gels and scaffolds have emerged as a prime matrix to simulate tumor microenvironment closer to physiological condition. Alginate being hydrophilic provides uniform matrix for growth and proliferation. The alginate scaffolds and hydrogels have been used to investigate the cytotoxicity, apoptosis, and penetration of conventional drugs as well as various formulations into the in vitro tumor scaffolds/spheroids. Another advantage of alginate-based matrices for 3D cultures is that the cancer stem cell (CSC) niches can be better understood owing to the inherent 3D nature of CSCs. Moreover, the use of 3D systems gives a better impression of the physiological architecture; unique cellular interactions can occur and improve the functional properties. In this chapter, initially we compare and contrast 2D and 3D cell culture systems and the pitfalls of the conventional in vitro tumor models. Then a brief introduction of alginate-based 3D scaffolds is provided. Various in vitro models based on alginate scaffolds (AlgiMatrix™) and hydrogels are briefed with the parameters studied and the associated advantages. Future improvements in the 3D cell culture based on alginate matrices are thought through, and the possible future directions are provided. In conclusion, results from different studies give an indication that high-throughput in vitro 3D tumor models based on alginate can be prepared to study the effect of various anticancer agents and various molecular pathways affected by the anticancer drugs and formulations.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
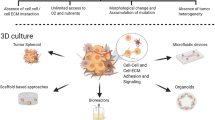
Similar content being viewed by others
References
Sharma SV, Haber DA, Settleman J (2010) Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer 10(4):241–253
Maltman DJ, Przyborski SA (2010) Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitro analyses. Biochem Soc Trans 38(4):1072–1075
Smalley KS, Lioni M, Herlyn M (2006) Life isn’t flat: taking cancer biology to the next dimension. In Vitro Cell Dev Biol Anim 42(8-9):242–247
Hongisto V, Jernstrom S, Fey V et al (2013) High-throughput 3D screening reveals differences in drug sensitivities between culture models of JIMT1 breast cancer cells. PLoS One 8(10):e77232
Gillet JP, Calcagno AM, Varma S et al (2011) Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. PNAS 108(46):18708–18713
Wrzesinski K, Fey SJ (2015) From 2D to 3D-a new dimension for modelling the effect of natural products on human tissue. Curr Pharm Des 21(38):5605–5616
Kim JB (2005) Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 15(5):365–377
Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8(10):839–845
Breslin S, Driscoll LO (2013) Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18(5):240–249
Misfeldt DS, Hamamoto ST, Pitelka DR (1976) Transepithelial transport in cell culture. PNAS 73(4):1212–1216
Santini M, Rainaldi G, Indovina P (1999) Multicellular tumour spheroids in radiation biology. Int J Radiat Biol 75(7):787–799
Van Wezel A (1967) Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 216:64–65
Hutmacher DW (2001) Scaffold design and fabrication technologies for engineering tissues state of the art and future perspectives. J Biomater Sci Polym Ed 12(1):107–124
Bolz J, Novak N, Staiger V (1992) Formation of specific afferent connections in organotypic slice cultures from rat visual cortex cocultured with lateral geniculate nucleus. J Neurosci 12(8):3054–3070
Nirmalanandhan VS, Duren A, Hendricks P et al (2010) Activity of anticancer agents in a three-dimensional cell culture model. Assay Drug Dev Technol 8(5):581–590
Fitzgerald KA, Malhotra M, Curtin CM et al (2015) Life in 3D is never flat: 3D models to optimise drug delivery. J Control Release 215:39–54
Godugu C, Patel AR, Desai U et al (2013) AlgiMatrix™ based 3D cell culture system as an in-vitro tumor model for anticancer studies. PLoS One 8(1):e53708
Thoma CR, Zimmermann M, Agarkova I et al (2014) 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 69-70:29–41
Xu X, Sabanayagam CR, Harrington DA et al (2014) A hydrogel-based tumor model for the evaluation of nanoparticle-based cancer therapeutics. Biomaterials 35(10):3319–3330
Rohwer N, Cramer T (2011) Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 14(3):191–201
Imamura Y, Mukohara T, Shimono Y et al (2015) Comparison of 2D-and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep 33(4):1837–1843
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37(1):106–126
Chen L, Xiao Z, Meng Y et al (2012) The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 33(5):1437–1444
Cardoso T, Sakamoto SS, Stockman D et al (2016) A three-dimensional cell culture system as an in vitro canine mammary carcinoma model for the expression of connective tissue modulators. Vet Comp Oncol 2016:1–9
XX X, Liu C, Liu Y et al (2014) Enrichment of cancer stem cell-like cells by culture in alginate gel beads. J Biotechnol 177:1–12
Nakaoka R, Hirano Y, Mooney DJ et al (2013) Study on the potential of RGD-and PHSRN-modified alginates as artificial extracellular matrices for engineering bone. J Artif Organs 16(3):284–293
Hahn MS, Teply BA, Stevens MM et al (2006) Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials 27(7):1104–1109
Akeda K, Nishimura A, Satonaka H et al (2009) Three-dimensional alginate spheroid culture system of murine osteosarcoma. Oncol Rep 22(5):997–1003
Leung M, Kievit FM, Florczyk SJ et al (2010) Chitosan-alginate scaffold culture system for hepatocellular carcinoma increases malignancy and drug resistance. Pharm Res 27(9):1939–1948
Kievit FM, Florczyk SJ, Leung MC et al (2010) Chitosan–alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials 31(22):5903–5910
Florczyk SJ, Liu G, Kievit FM et al (2012) 3D porous chitosan–alginate scaffolds: a new matrix for studying prostate cancer cell–lymphocyte interactions in vitro. Adv Healthc Mater 1(5):590–599
Wang K, Kievit FM, Florczyk SJ et al (2015) 3D porous chitosan–alginate scaffolds as an in vitro model for evaluating nanoparticle-mediated tumor targeting and gene delivery to prostate cancer. Biomacromolecules 16(10):3362–3372
Ginzberg DM, Konson A, Cohen S et al (2007) Entrapment of retroviral vector producer cells in three-dimensional alginate scaffolds for potential use in cancer gene therapy. J Biomed Mater Res B Appl Biomater 80(1):59–66
Wang JZ, Zhu YX, Ma HC et al (2016) Developing multi-cellular tumor spheroid model (MCTS) in the chitosan/collagen/alginate (CCA) fibrous scaffold for anticancer drug screening. Mater Sci Eng C 62:215–225
Wenzel C, Riefke B, Gründemann S et al (2014) 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp Cell Res 323(1):131–143
Meli L, Jordan ET, Clark DS et al (2012) Influence of a three-dimensional, microarray environment on human cell culture in drug screening systems. Biomaterials 33(35):9087–9096
XX X, Liu C, Liu Y et al (2013) Encapsulated human hepatocellular carcinoma cells by alginate gel beads as an in vitro metastasis model. Exp Cell Res 319(14):2135–2144
Shakibaei M, Kraehe P, Popper B et al (2015) Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 15(1):1–15
Buhrmann C, Shayan P, Kraehe P et al (2015) Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem Pharmacol 98(1):51–68
Buhrmann C, Shayan P, Popper B et al (2016) Sirt1 is required for resveratrol-mediated chemopreventive effects in colorectal cancer cells. Nutrients 8(3):145–166
Malarvizhi GL, Retnakumari AP, Nair S et al (2014) Transferrin targeted core-shell nanomedicine for combinatorial delivery of doxorubicin and sorafenib against hepatocellular carcinoma. Nanomedicine 10(8):1649–1659
Lan SF, Mroczka BS, Starly B (2010) Long-term cultivation of HepG2 liver cells encapsulated in alginate hydrogels: a study of cell viability, morphology and drug metabolism. Toxicol in Vitro 24(4):1314–1323
Zhou S, Li F, Xiao J et al (2010) Isolation and identification of cancer stem cells from human osteosarcom by serum-free three-dimensional culture combined with anticancer drugs. J Huazhong Univ Sci Technol Med Sci 30:81–84
Agarwal P et al (2013) One-step microfluidic generation of pre-hatching embryo-like core–shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip 13(23):4525–4533
Rao W, Zhao S, Bielecki P et al (2014) Enhanced enrichment of prostate cancer stem-like cells with miniaturized 3D culture in liquid core-hydrogel shell microcapsules. Biomaterials 35(27):7762–7773
Kievit FM, Florczyk SJ, Leung MC et al (2014) Proliferation and enrichment of CD133+ glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials 35(33):9137–9143
Ordikhani F, Kim Y, Zustiak SP (2015) The role of biomaterials on cancer stem cell enrichment and behavior. JOM 67(11):2543–2549
Dai X, Ma C, Lan Q et al (2016) 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 8(4):1–11
Qiao SP, Zhao YF, Li CF et al (2016) An alginate-based platform for cancer stem cell research. Acta Biomater 37:83–92
Acknowledgments
The authors would like to thank the Department of Biotechnology (DBT), Government of India for the financial support to Dr. CG via North East Twinning Grant, MAP/2015/58; Indo-Brazil Grant, DBT/IC-2/Indo-Brazil/2016-19/01; and Science and Engineering Board Early Career Research Award (SERB-ECR) Grant, ECR/2016/000007. In addition, the authors would also like to thank the Department of Pharmaceuticals, the Ministry of Chemicals and Fertilizers, the Government of India, and the Project Director, NIPER-Hyderabad.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Khurana, A., Godugu, C. (2018). Alginate-Based Three-Dimensional In Vitro Tumor Models: A Better Alternative to Current Two-Dimensional Cell Culture Models. In: Rehm, B., Moradali, M. (eds) Alginates and Their Biomedical Applications. Springer Series in Biomaterials Science and Engineering, vol 11. Springer, Singapore. https://doi.org/10.1007/978-981-10-6910-9_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-6910-9_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6909-3
Online ISBN: 978-981-10-6910-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)