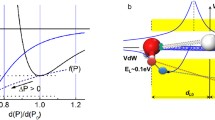
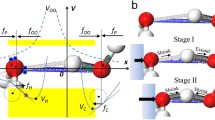
Abstract
Water and ice respond to mechanical compression unusually with numerous anomalies. Regelation, i.e., ice melts under compression and freezes again when the pressure is relieved, evidences that the O:H–O bond extraordinary recoverability and that quasisolid phase boundary dispersivity. An oxygen atom always finds bonding partners to retain its sp3-orbital hybridization once the O:H breaks, which ensures O:H–O bond recoverability to its original state once the pressure is removed. On the other hand, mechanical compression shortens the O:H nonbond and soften its phonon but the H–O bond responds to compression oppositely, lowering the H–O phonon frequency, which offsets the Debye temperature and the boundaries of the quasisolid phase outwardly, which elevates the freezing point and depresses the melting point, so regelation takes place. Reproduction of the Tm(P) profile clarifies that the H–O bond energy EH determines the Tm with derivative of EH = 3.97 eV for bulk water and ice.
• Compression shortens the O:H nonbond and lengthens the H−O bond towards H + centralization with strong polarization that enlarges the band gap.
• Compression depresses the T m by dispersing the quasisolid-phase boundary through ω x (Θ Dx ) relaxation.
• O:H–O bond recovers when the mechanical compression, molecular undercoordination, or thermal excitation is relieved.
• Persistence of sp 3—orbital hybridization of O 2− entitles O:H–O extraordinary recoverability from deformation and dissociation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
J.D. Forbes, Illustrations of the viscous theory of glacier motion. part ii. an attempt to establish by observation the plasticity of glacier ice. Philos. Trans. R. Soc. London 136, 157–175 (1846)
J.D. Forbes, Illustrations of the viscous theory of glacier motion. part i. containing experiments on the flow of plastic bodies, and observations on the phenomena of lava streams. Philos. Trans. R. Soc. London 136, 143–155 (1846)
J. Thomson, Note on professor Faraday’s recent experiments on regelation. Proc. R. Soc. London 10, 151–160 (1859)
M. Faraday, Note on regelation. Proc. R. Soc. London 10, 440–450 (1859)
J.L. Green, D.J. Durben, G.H. Wolf, C.A. Angell, Water and solutions at negative pressure: raman spectroscopic study to -80 megapascals. Science 249(4969), 649–652 (1990)
M. Chaplin, Water Structure and Science: http://www.lsbu.ac.uk/water/
YouTube. Does Pressure Melt Ice?, https://www.youtube.com/watch?v=gM3zP72-rJE
Y. Yoshimura, S.T. Stewart, M. Somayazulu, H.K. Mao, R.J. Hemley, Convergent Raman features in high density amorphous ice, ice VII, and ice VIII under pressure. J. Phys. Chem. B 115(14), 3756–3760 (2011)
Y. Huang, X. Zhang, Z. Ma, Y. Zhou, W. Zheng, J. Zhou, C.Q. Sun, Hydrogen-bond relaxation dynamics: resolving mysteries of water ice. Coord. Chem. Rev. 285, 109–165 (2015)
C.Q. Sun, X. Zhang, X. Fu, W. Zheng, J.-L. Kuo, Y. Zhou, Z. Shen, J. Zhou, Density and phonon-stiffness anomalies of water and ice in the full temperature range. J. Phys. Chem. Lett. 4, 3238–3244 (2013)
C.Q. Sun, X. Zhang, W.T. Zheng, Hidden force opposing ice compression. Chem. Sci. 3, 1455–1460 (2012)
C.Q. Sun, Oxidation electronics: bond-band-barrier correlation and its applications. Prog. Mater. Sci. 48(6), 521–685 (2003)
C.Q. Sun, Relaxation of the chemical bond, in Springer Series in Chemical Physics 108. vol. 108 (Springer, Heidelberg, 2014), 807 pp
T.B. James, Melting and regelation of ice. Nature (London) 5, 185 (1872)
J.M. Thomas, Michael Faraday and The Royal Institution: The Genius of Man and Place (1991)
W. Holzapfel, On the symmetry of the hydrogen bonds in ice VII. J. Chem. Phys. 56(2), 712 (1972)
A.F. Goncharov, V.V. Struzhkin, H.-K. Mao, R.J. Hemley, Raman spectroscopy of dense H2O and the transition to symmetric hydrogen bonds. Phys. Rev. Lett. 83(10), 1998–2001 (1999)
M. Benoit, D. Marx, M. Parrinello, Tunnelling and zero-point motion in high-pressure ice. Nature 392(6673), 258–261 (1998)
P. Loubeyre, R. LeToullec, E. Wolanin, M. Hanfland, D. Husermann, Modulated phases and proton centring in ice observed by X-ray diffraction up to 170 GPa. Nature 397(6719), 503–506 (1999)
A.F. Goncharov, V.V. Struzhkin, M.S. Somayazulu, R.J. Hemley, H.K. Mao, Compression of ice to 210 gigapascals: infrared evidence for a symmetric hydrogen-bonded phase. Science 273(5272), 218–220 (1996)
J. Teixeira, High-pressure physics - the double identity of ice X. Nature 392(6673), 232–233 (1998)
I.A. Ryzhkin, “Symmetrical” phase and collective excitations in the proton system of ice. J. Exp. Theor. Phys. 88(6), 1208–1211 (1999)
F.H. Stillinger, K.S. Schweizer, Ice under compression-transition to symmetrical hydrogen-bonds. J. Phys. Chem. 87(21), 4281–4288 (1983)
L.N. Tian, A.I. Kolesnikov, J.C. Li, Ab initio simulation of hydrogen bonding in ices under ultra-high pressure. J. Chem. Phys. 137(20), 204507 (2012)
Y. Yoshimura, S.T. Stewart, M. Somayazulu, H. Mao, R.J. Hemley, High-pressure x-ray diffraction and Raman spectroscopy of ice VIII. J. Chem. Phys. 124(2), 024502 (2006)
K. Liu, J.D. Cruzan, R.J. Saykally, Water clusters. Science 271(5251), 929–933 (1996)
R. Ludwig, Water: from clusters to the bulk. Angewandte Chemie-International Edition 40(10), 1808–1827 (2001)
D. Kang, J. Dai, Y. Hou, J. Yuan, Structure and vibrational spectra of small water clusters from first principles simulations. J. Chem. Phys. 133(1), 014302 (2010)
T. Yan, S. Li, K. Wang, X. Tan, Z. Jiang, K. Yang, B. Liu, G. Zou, B. Zou, Pressure-induced phase transition in N-H…O hydrogen-bonded molecular crystal oxamide. J. Phys. Chem. B 116(32), 9796–9802 (2012)
B. Santra, J. Klimeš, D. Alfè, A. Tkatchenko, B. Slater, A. Michaelides, R. Car, M. Scheffler, Hydrogen bonds and van der Waals forces in ice at ambient and high pressures. Phys. Rev. Lett. 107(18), 185701 (2011)
J.E. Bertie, E. Whalley, Infrared spectra of Ices II, III, and V in the Range 4000 to 350 cm—1. J. Chem. Phys. 40(6), 1646–1659 (1964)
P.T.T. Wong, E. Whalley, Raman spectrum of ice VIII. J. Chem. Phys. 64(6), 2359–2366 (1976)
J.E. Bertie, F.E. Bates, Mid-infrared spectra of deuterated ices at 10 °K and interpretation of the OD stretching bands of ices II and IX. J. Chem. Phys. 67(4), 1511–1518 (1977)
W.T. Zheng, C.Q. Sun, Underneath the fascinations of carbon nanotubes and graphene nanoribbons. Energy Environ. Sci. 4(3), 627–655 (2011)
J.W. Li, S.Z. Ma, X.J. Liu, Z.F. Zhou, C.Q. Sun, ZnO meso-mechano-thermo physical chemistry. Chem. Rev. 112(5), 2833–2852 (2012)
M.X. Gu, Y.C. Zhou, L.K. Pan, Z. Sun, S.Z. Wang, C.Q. Sun, Temperature dependence of the elastic and vibronic behavior of Si, Ge, and diamond crystals. J. Appl. Phys. 102(8), 083524 (2007)
M.X. Gu, L.K. Pan, T.C.A. Yeung, B.K. Tay, C.Q. Sun, Atomistic origin of the thermally driven softening of Raman optical phonons in group III nitrides. J. Phys. Chem. C 111(36), 13606–13610 (2007)
C. Yang, Z.F. Zhou, J.W. Li, X.X. Yang, W. Qin, R. Jiang, N.G. Guo, Y. Wang, C.Q. Sun, Correlation between the band gap, elastic modulus, Raman shift and melting point of CdS, ZnS, and CdSe semiconductors and their size dependency. Nanoscale 4, 1304–1307 (2012)
M. Song, H. Yamawaki, H. Fujihisa, M. Sakashita, K. Aoki, Infrared absorption study of Fermi resonance and hydrogen-bond symmetrization of ice up to 141 GPa. Phys. Rev. B 60(18), 12644 (1999)
P. Pruzan, J.C. Chervin, E. Wolanin, B. Canny, M. Gauthier, M. Hanfland, Phase diagram of ice in the VII-VIII-X domain. Vibrational and structural data for strongly compressed ice VIII. J. Raman Spectrosc. 34(7–8), 591–610 (2003)
T. Okada, K. Komatsu, T. Kawamoto, T. Yamanaka, H. Kagi, Pressure response of Raman spectra of water and its implication to the change in hydrogen bond interaction. Spectrochim. Acta A 61(10), 2423–2427 (2005)
K. Aoki, H. Yamawaki, M. Sakashita, Observation of Fano interference in high-pressure ice VII. Phys. Rev. Lett. 76(5), 784–786 (1996)
G. Malenkov, Liquid water and ices: understanding the structure and physical properties. J. Phys.-Condens. Matter 21(28), 283101 (2009)
C.Q. Sun, H.L. Bai, B.K. Tay, S. Li, E.Y. Jiang, Dimension, strength, and chemical and thermal stability of a single C-C bond in carbon nanotubes. J. Phys. Chem. B 107(31), 7544–7546 (2003)
C.Q. Sun, Thermo-mechanical behavior of low-dimensional systems: the local bond average approach. Prog. Mater Sci. 54(2), 179–307 (2009)
X.J. Liu, M.L. Bo, X. Zhang, L.T. Li, Y.G. Nie, H. Tian, Y. Sun, S. Xu, Y. Wang, W. Zheng, C.Q. Sun, Coordination-resolved electron spectrometrics. Chem. Rev. 115(14), 6746–6810 (2015)
M. Zhao, W.T. Zheng, J.C. Li, Z. Wen, M.X. Gu, C.Q. Sun, Atomistic origin, temperature dependence, and responsibilities of surface energetics: An extended broken-bond rule. Phys. Rev. B 75(8), 085427 (2007)
C.Q. Sun, X. Zhang, J. Zhou, Y. Huang, Y. Zhou, W. Zheng, Density, elasticity, and stability anomalies of water molecules with fewer than four neighbors. J. Phys. Chem. Lett. 4, 2565–2570 (2013)
X. Zhang, Y. Huang, P. Sun, X. Liu, Z. Ma, Y. Zhou, J. Zhou, W. Zheng, C.Q. Sun, Ice regelation: hydrogen-bond extraordinary recoverability and water quasisolid-phase-boundary dispersivity. Sci. Rep. 5, 13655 (2015)
Air Pressure and Altitude above Sea Level. Engineering toolbox, http://www.engineeringtoolbox.com/air-altitude-pressure-d_462.html
Boiling Points of Water at Various Elevations. Engineering toolbox, http://www.engineeringtoolbox.com/boiling-points-water-altitude-d_1344.html
L.K. Pan, S.Q. Xu, W. Qin, X.J. Liu, Z. Sun, W.T. Zheng, C.Q. Sun, Skin dominance of the dielectric-electronic-phononic-photonic attribute of nanostructured silicon. Surf. Sci. Rep. 68(3–4), 418–455 (2013)
C.Q. Sun, T.P. Chen, B.K. Tay, S. Li, H. Huang, Y.B. Zhang, L.K. Pan, S.P. Lau, X.W. Sun, An extended ‘quantum confinement’ theory: surface-coordination imperfection modifies the entire band structure of a nanosolid. J. Phys. D-Appl. Phys. 34(24), 3470–3479 (2001)
C.Q. Sun, Dominance of broken bonds and nonbonding electrons at the nanoscale. Nanoscale 2(10), 1930–1961 (2010)
A. Hermann, P. Schwerdtfeger, Blueshifting the onset of optical UV absorption for water under pressure. Phys. Rev. Lett. 106(18), 187403 (2011)
S. Luntz, A. Cornell. This Is What The Underside Of An Iceberg Looks Like. 2015, http://www.iflscience.com/environment/underside-iceberg
J.D. Goddard, The viscous drag on solids moving through solids. AlChE J. 60(4), 1488–1498 (2014)
D.T. Möhlmann, Are nanometric films of liquid undercooled interfacial water bio-relevant? Cryobiology 58(3), 256–261 (2009)
T. Hynninen, V. Heinonen, C.L. Dias, M. Karttunen, A.S. Foster, T. Ala-Nissila, Cutting ice: nanowire regelation. Phys. Rev. Lett. 105(8), 086102 (2010)
D. Petely, Our strange desire to find a landslide trigger. http://ihrrblog.org/2013/11/08/our-strange-desire-to-find-a-landslide-trigger/, (2013)
J. Corripio, Spiked Ice (Edinburgh, Scotland, 2001)
V. Bergeron, C. Berger, M. Betterton, Controlled irradiative formation of penitentes. Phys. Rev. Lett. 96(9), 098502 (2006)
Author information
Authors and Affiliations
Corresponding author
Appendix: Featured News
Appendix: Featured News
Unlocking the mysteries of ice
Home/Chemistry World/News/2012/Marc
By Erica Wise, Editor for RSC Press
27 March 2012
The unusual properties of ice under compression are due to Coulomb repulsion between bonding and non-bonding electron pairs, say scientists from Singapore and China.
Frozen water behaves differently from other materials in response to pressure. It has abnormally low compressibility, and applying pressure decreases rather than increases the critical temperature for phase transitions. These anomalies have puzzled scientists for many years and satisfactorily modelling them has proven a great challenge.
Now, Chang Sun at Nanyang Technological University and his colleagues at Jilin and Xiangtan Universities have developed a new method to simulate these properties accurately. Their work has also helped to clarify the physical basis of the behaviour.
The key to their model is in considering O\(\cdots\)H–O as the basic structural unit of ice. The left hand oxygen forms a hydrogen bond using its lone pair of electrons to polarise electron density around the hydrogen. Meanwhile, the hydrogen shares its electron with the right hand oxygen to form a real bond.

Sun’s model works better for the system than commonly used rigid non-polarisable models. Such models ‘have a fixed molecular geometry so they cannot intrinsically account for changes in the molecular geometry,’ according to Jose Abascal, an expert on the theoretical chemistry of water and ice, Universidad Complutense de Madrid, Spain. The rigid models ‘approximate the H2O molecule as two point charges with a fixed bond length and bond angle,’ explains Sun. However, ‘what changes with the applied stimulus are the angle, length and energy of the hydrogen bond and the associated electron polarisation.’
Sun’s results indicate that the repulsion between the lone pair and bonding pair causes the O\(\cdots\)H hydrogen bond to shorten and the O-H real bond to lengthen. At sufficiently high pressure, the hydrogen bond and real bond become equivalent in length. The change in binding energy of the real bond dominates, causing the observed effects on physical quantities as it lengthens and weakens.
Sun now anticipates that there is further work to be done in unravelling the many other anomalies of ice, including why freezing water expands.
Reference
C.Q. Sun, X. Zhang, W Zheng, Chem Sci., 2012, doi:10.1039/c2sc20066j
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Sun, C.Q., Sun, Y. (2016). Mechanical Compression. In: The Attribute of Water. Springer Series in Chemical Physics, vol 113. Springer, Singapore. https://doi.org/10.1007/978-981-10-0180-2_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-0180-2_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-0178-9
Online ISBN: 978-981-10-0180-2
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)