Abstract
Objective: The objective of this study was to investigate the regulating role of NFIC in odontoblastic differentiation of human stem cells from apical papilla (hSCAPs).
Materials and methods: The expression of NFIC in young permanent tooth was observed by immunohistochemical (IHC) staining and its expression levels both in young and mature permanent teeth were detected by western blot. hSCAPs were transplanted into the dorsum of immunocompromised mice, and immunohistochemical analysis was performed after 8 weeks. Real-time polymerase chain reaction and western blot were used to explore the expression pattern of NFIC and other odontogenic related genes during in vitro hSCAPs osteogenic differentiation.
Results: During molar root formation, NFIC expression was restricted within the odontoblasts and preodontoblasts of human molars. The expression of NFIC in apical papilla was at a very low level, and the amounts of NFIC protein in coronal pulp were more than that in root pulp in both young and mature permanent teeth. Odontoblast-like cells were positive to NFIC immunohistochemistry staining in dentin-pulp complexes formed after hSCAPs transplantation. NFIC expression was concomitant to dentin sialoprotein (DSP) at early stage of osteogenic differentiation of hSCAPs.
Conclusion: Our results suggest that NFIC is involved in the regulation of hSCAPs differentiation into odontoblasts during root development of the young permanent teeth and could be used as an early marker of odontoblast differentiation.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The formation of a dental root is the result of the interaction between the epithelial root sheath, dental papilla and dental follicle. Dental ectomesenchymal cells from the dental papilla differentiate into odontoblasts to produce a dentin layer that forms the bulk of the tooth. However, the molecular mechanisms underlying ectomesenchymal cells differentiation into odontoblast are not well understood.
The nuclear factor I (NFI) family of transcription-replication factors [1] encodes four members (Nfia, Nfib, Nfic, Nfix) in mammals, which are expressed in almost every tissue and organ [2]. However, mice with disruptions in each of the NFI genes have been found to display distinct phenotypes and developmental defects primarily in the nervous system (Nfia) [3], lung and brain (Nfib) [4, 5], and brain and skeleton (Nfix) [6], indicating different functions for each NFI subtype. Specifically, loss of Nfic is the first mouse mutation to affect the development of dental roots. Studies have demonstrated that Nfic null mice exhibit abnormal roots of molar teeth containing aberrant odontoblasts and abnormal formation of dentin, but normal crowns. NFIC should be necessary for root development [7–10]. However, the mechanism by which disruption of the Nfic gene leads to abnormal root formation remains unclear. Also, the relationship between NFIC and the formation of human dental roots has not been reported.
In present study, we focused on the root development of human molars and the differentiation of stem cells from the apical papilla (SCAPs). We sought to determine NFIC expression in young permanent human teeth and in the mineralization of human SCAPs.
2 Materials and Methods
2.1 Ethical Approval of the Study Protocol and Acquisition of Samples
The procedure to obtain healthy extracted teeth was approved by the Ethical Committee of the Health Science Center of Peking University (Beijing, China) (IRB00001052-11060). Patients provided written informed consent. Normal human impacted third molars with an open apical foramen and closed apical foramen were collected at a clinic in Peking University School and Hospital of Stomatology. The study protocol of animal experimentation was approved by the Animal Ethics Committee of Peking University (LA2012-58).
2.2 Cell Culture and Induction of Mineralization
Apical papilla tissues separated gently from the end of human teeth were digested in a solution of 3 mg/mL collagenase type I (Sigma-Aldrich, USA) and 4 mg/mL dispase (Sigma-Aldrich) for 1 h at 37 °C. Cultures were maintained in α-modified Eagle’s minimum essential medium (α-MEM; Gibco, USA) supplemented with 10 % fetal bovine serum (FBS; Hyclone, USA) in 5 % carbon dioxide at 37 °C. The cells used were at passages 1–4.
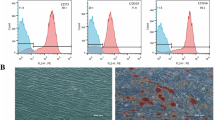
Seventy to eighty percent confluent hSCAPs were cultured in differentiation medium supplemented with 10 % FBS, 10 mmol/L β-glycerolphosphate, 50 mg/mL ascorbate phosphate, 10 nmol/L dexamethasone, and 10 nmol/L 1,25-dihydroxyvitamin D3 for 3 weeks. Cultures were fixed in 4 % paraformaldehyde. Calcium deposition of the extracellular matrix was evaluated by staining with 1 % alizarin red-S (Sigma-Aldrich).
2.3 Transplantation
Approximately 2.0 × 106 in vitro-expanded hSCAPs mixed with 40 mg hydroxyapatite ceramic particles (Bio Osteon, China) were transplanted subcutaneously into the dorsal surfaces of 10-week-old immunodeficient mice (CB-17/SCID; Vitalriver, China) according to a method reported previously [11]. Transplants were harvested 8 weeks after transplantation.
2.4 Immunohistochemical Staining and Immunocytochemistry
Samples were fixed in 4 % paraformaldehyde, then decalcified in 10 % EDTA and processed for embedding in paraffin. Immunohistochemical staining was undertaken on 4 μm-thick tissue sections, which were deparaffinized and subsequently hydrated to water, then quenched endogenous peroxidase activity. Sections were incubated with anti-NFIC primary antibody (1:400 dilution. Abcam, UK), following by consecutive incubations with a Polymer Detection System for IHC Staining kit (Zhongshan Golden Bridge Biotechnology, China). Subsequently, sections were visualized with a 3,3′-diaminobenzidine tetrahydrochloride substrate kit (Zhongshan Golden Bridge Biotechnology). The same passage of hSCAPs grown to 80 % confluence on slides were immunocytochemically strained as described above.
2.5 RT-PCR Analysis
Tissues and cells were harvested and total RNA was isolated using TRIzol reagent (Invitrogen), according to the manufacturer’s protocol. Isolated RNA was purified by removing genomic DNA with a DNase Ι, RNase-free kit (Fermentas, Glen Burnie, MD, USA). One microgram of total RNA from each group was used for synthesis of cDNA using the AMV Reverse Transcriptase kit (Fermentas), according to the manufacturer’s protocol. Semiquantitative real-time PCR was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA) with SYBR Green (Roche). All samples were run in triplicate in 96-well plates, with each well containing 1.0 μL of cDNA diluted 1 in 20 to give a total reaction volume of 20 μL. Reactions were performed at 50 °C for 2 min and then at 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Primers were designed (Table 16.1). For data analysis, the levels of target gene expression in samples relative to the level of expression in the control samples were calculated using the comparative cycle threshold method (ΔΔCT). The expression levels of target gene expression were normalized to the expression of the reference gene GAPDH.
2.6 Western Blotting
The expression level of of NFIC and DSP proteins were measured by western blot. Total protein was extracted from tissues and cells using radioimmunoprecipitation assay (RIPA) lysis buffer containing a protease inhibitor cocktail (Applygen, Beijing, China), according to the manufacturer’s instructions. Protein levels were calculated using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Beijing, China). Equal amounts of protein samples were separated by electrophoresis through a 12 % SDS polyacrylamide gel and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Blots were blocked with 5 % skim milk, followed by incubation with the following primary antibodies: mouse anti-NFIC (Abcam, Cambridge, UK), goat anti-DSP (Santa Cruz Biotechnology, CA), and mouse anti-GAPDH (Abmart, Shanghai, China). Blots were then incubated with goat anti-mouse or anti-goat secondary antibodies conjugated to horseradish peroxidase (Origene, Beijing, China) and visualized by enhanced chemiluminescence (Applygen).
3 Results
3.1 NFIC Expression in Tooth Tissue
To determine the expression pattern of NFIC during molar root formation, we conducted immunohistochemical staining for human molar root. NFIC expression was restricted within odontoblasts and preodontoblasts of the developing root (Fig. 16.1).
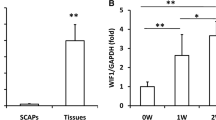
In order to quantify NFIC expression in different parts of human dental pulp, the coronal pulp, root pulp and apical papilla of young permanent teeth, as well as crown pulp and root pulp of mature permanent teeth were isolated and western blot was performed. The results showed that NFIC expressed in apical papilla at a very low level, and the amounts of NFIC protein in coronal pulp were more than that in root pulp in both young and mature permanent teeth. The expression of NFIC in coronal pulp of young permanent teeth was extremely strong (Fig. 16.2).
3.2 NFIC Expression in hSCAPs Transplantation
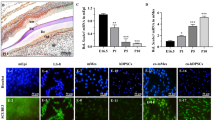
To study the expression of NFIC during odontoblast differentiation in vivo, the hSCAPs with hydroxyapatite carrier were transplanted into immunocompromised mice. Eight weeks after transplantation, the hSCAPs generated dentin-like structures. The odontoblast-like cells lined a layer along the surface of dentin-like structures and displayed protruding cytoplasmic processes into the dentinal matrix, which interfaced with a pulp-like interstitial tissue infiltrated with blood vessels. Odontoblast-like cells were positive to NFIC immunohistochemistry straining in dentin-pulp complex formed ex vivo (Fig. 16.3).
Immunohistochemical straining of NFIC in dentin-pulp complex ex vivo at 8 weeks after hSCAPs transplantation into the dorsum of immunocompromised mice (brown indicated positive staining). (a) The dentin-like structure (d) surfaces are lined with a layer of odontoblast-like cells (od), surrounding pulp-like tissue with blood vessels (bv). (b) Odontoblast-like cells displayed protruding cytoplasmic processes into the dentinal matrix
3.3 The Expression of NFIC and Odontogenic Related Genes During Osteogenic Differentiation of hSCAPs
During tooth development, SCAPs differentiate into odontoblasts and form root dentin. In order to determine how the related genes express when hSCAPs differentiate in vitro and gain insight into the mechanism of differentiation, the cells were cultured in mineralization medium for up to 3 weeks and the expression of odontoblast differentiation markers were analyzed by western blot and real-time polymerase chain reaction (PCR). The formation of mineralized nodules was evaluated by alizarin red-S staining.
Alizarin red-S staining revealed the presence of small, round mineralized nodules from days 6 to 21 after the induction of differentiation (Fig. 16.4a). Expression of NFIC increased from days 6 to 12 then decreased at day 14, and the expression of dentin sialoprotein (DSP) showed similar pattern as NFIC by western blot (Fig. 16.4b). The results of our real-time PCR analysis revealed that the expression levels of ALP, OCN increased significantly from days 12 and continued to increase through day 25. The expression of DMP-1 increased significantly at day 6 and kept at high level throughout the process, while the expression level of COLLA I increased significantly after days 21 (Fig. 16.4c).
Osteogenic differentiation of hSCAPs. (a) Alizarin red-S staining showed mineralized nodule formation from days 6 to 21 after the induction of differentiation. (b) The expression of NFIC and DSP were evaluated by western blot analysis. (c) The expression of ALP, OCN, COLLA I and DMP1 were evaluated by real-time PCR.*P < 0.05. GAPDH used as a loading control
4 Discussion
Previous studies showed that disruption of Nfic caused major defects in postnatal murine tooth development, the most striking defect being loss of molar root formation. It is generally believed that NFIC is one of the molecules known to be required for root formation-the key late event in tooth morphogenesis [12–14]. However most studies obtained conclusions based on experiments with other non-molar root tissues (such as jaw, incisor) or cell lines of mice [11]. In order to further explore the function of Nfic on root development, we use human young molar stem cells from apical papilla (SCAPs) as our research objectives, which can differentiate into the odontoblasts located in root portion to generate root dentin.
In this study, we found that NFIC in situ expression in tooth tissues showed interesting pattern. NFIC expression appeared most strongly within the odontoblasts and preodontoblasts though can be seen in a wide range of tissues, including ameloblasts, periodontal ligament and weakly in the pulp. Moreover the level of NFIC protein in coronal pulp was higher than in root pulp. However it is well known that the main tooth defect of Nfic null mice existed not in the crown but in abnormal roots of molar teeth. Therefore, we speculate that developmental mechanism maybe different between root and crown in human molar, and NFIC is probably essential for root development even though it is not the site of the highest NFIC expression. Moreover, it was predicted that osteodentin formation during dentin repair may be the result of pulp cells that do not express Nfic gene, given the morphological similarities of repair dentin with the abnormal roots dentin found in Nfic-deficient mice [13]. Thus, we proposed that primary dentin and secondary or reparative dentin may have different formational mechanisms.
To study the expression of NFIC during odontoblast differentiation ex vivo, hSCAPs with hydroxyapatite carriers were transplanted into immunocompromised mice. After eight weeks the hSCAPs generated dentin-like structures. Odontoblast-like cells aligned in a layer along the surface of dentin-like structures were positive to NFIC immunohistochemical straining, which conceivably imply that NFIC participates in the hSCAPs differentiation ex vivo and the generation of osteodentin. It’s well established that HERS regulates the formation of organized root dentin through epithelial-mesenchymal interactions. The absence of HERS during the formation of dentin-like structures would cause odontoblast progenitor cells to form osteodentin without organized dentinal tubules, that resembled repair dentin [15]. The mechanisms behind the osteodentin formation and odontoblast-like cells differentiation remain uncertain. In summary, NFIC may work in the common molecular regulation mechanism sharing by the development and repair of dentin. Further research will be required to identify these different pathways at various stages of dentin formation and the relationship between the expression and function of NFIC.
It has previously been reported that Nfic-deficient mice showed abnormal odontoblasts with a round shape, no odontoblastic processes and no polarity [16] and the ectomesenchymal cells (EM) near the abnormal root showed no expression of DSPP mRNA [13]. The odontoblasts of mice incisors exhibited a decreased level of DSP expression that is a product of DSPP-a dentin-specific gene. In the present study, NFIC and DSP had consistent expression pattern, both increased at early stage of hSCAPs osteogenic differentiation, and decreased at late stage. While the expression of osteogenic differentiation markers Alp, OCN and the main component of the dentin collagenous protein framework COLLA I, were gradually increased during later period of differentiation. The marker gene of odontoblast differentiation DMP-1 showed sustained high expression. Based on these findings, we suggest that NFIC and DSPP are likely to have a more compact expression and functional relationship, and NFIC may be another dentin-specific marker during human odontoblast differentiation.
In conclusion, NFIC expression was restricted to the odontoblasts, pre-odontoblasts and predentin of human molars. NFIC is involved in the regulation of hSCAPs differentiation into odontoblasts during root development of the young permanent teeth in a stage- and tissue-specific manner and could be regarded as an early marker of odontoblast differentiation.
References
Nagata K, Guggenheimer RA, Enomoto T, et al. Adenovirus DNA replication in vitro identification of a host factor that stimulates synthesis of the preternal protein-dCMP complex. Proc Natl Acad Sci. 1982;79(21):6438–42.
Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene. 2000;249:31–45.
Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ. Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci. 2003;23:203–12.
Steele-Perkins G, Plachez C, Butz KG, Yang G, Bachurski CJ, et al. The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol Cell Biol. 2005;25:685–98.
Grunder A, Ebel TT, Mallo M, Schwarzkopf G, Shimizu T, et al. Nuclear factor I-B (Nfib) deficient mice have severe lung hypoplasia. Mech Dev. 2002;112:69–77.
Driller K, Pagenstecher A, Uhl M, Omran H, Berlis A, et al. Nuclear factor I X deficiency causes brain malformation and severe skeletal defects. Mol Cell Biol. 2007;27:3855–67.
Steele-Perkins G, Buts KG, Lyons GE, et al. Essential role for NFI-C/CTF transcription replication factor in tooth root development. Mol Cell Biol. 2003;23(3):1075–84.
Xing X, Deng Z, Yang F, et al. Determination of genes involved in the early process of molar root development initiation in rat by modified subtractive hybridization. Biochem Biophys Res Commun. 2007;363(4):994–1000.
Derynck R, Chen RH, Ebner R, Filvaroff EH, Lawler S. An emerging complexity of receptors for transforming growth factor-beta. Princess Takamatsu Symp. 1994;24:264–75.
Massague ´ J. How cells read TGF-β signals. Nat Rev Mol Cell Biol. 2000;1:169–78.
Lee DS, Yoon WJ, Cho ES, Kim HJ, Gronostajski RM, Cho MI, Park JC. Crosstalk between nuclear factor I-C and transforming growth factor-β1 signaling regulates odontoblast differentiation and homeostasis. PLoS One. 2011;6(12):e29160.
Lee DS, Park JT, Kim HM, et al. Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblasted differentiation during tooth root development. J Biol Chem. 2009;254(25):17293–303.
Park JC, Herr Y, Kim HJ, et al. Nfic gene disruption inhibits differentiation of odontoblasts responsible for root formation and results in formation of short and abnormal roots in mice. J Periodontol. 2007;78(9):1795–802.
Kim MY, Reyna J, Chen LS, Zeichner-David M. Role of the transcription factor NFIC in odontoblast gene expression. J Calif Dent Assoc. 2009;37(12):875–81.
Xu L, Tang L, Jin F, et al. The apical region of developing tooth root constitutes a complex and maintains the ability to generate root and periodontium-like tissues. J Periodontal Res. 2009;44:275–82.
Lee TY, Lee DS, Kim HM. Disruption of Nfic causes dissociation of odontoblasts by interfering with the formation of intercellular junctions and aberrant odontoblast differentiation. J Histochem Cytochem. 2009;57(5):469–76.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Zhao, Y., Gao, S., Ge, L. (2015). The Role of NFIC in Regulating Odontoblastic Differentiation of Human Molar Stem Cells from Apical Papilla. In: Sasaki, K., Suzuki, O., Takahashi, N. (eds) Interface Oral Health Science 2014. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55192-8_16
Download citation
DOI: https://doi.org/10.1007/978-4-431-55192-8_16
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55125-6
Online ISBN: 978-4-431-55192-8
eBook Packages: MedicineMedicine (R0)