Abstract
The squid Loligo (Heterololigo) bleekeri uses two distinct insemination sites, inside or outside the female’s body, which links to the mating behavior of two distinct types of males, consort or sneaker, respectively. We found that sperm release a self-attracting molecule, which causes only sneaker sperm to swarm. We identified respiratory CO2 as the sperm chemoattractant and its sensor, membrane-bound flagellar carbonic anhydrase. Downstream signaling results from generation of an extracellular proton gradient, intracellular acidosis, and concomitant recovery from acidosis. This cycle in turn elicits Ca2+-dependent flagellar turning/tumbling, resulting in chemotactic swarming.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Results
1.1 Sperm from Sneaker Males Swarm in Response to Respiratory CO2 Emission
Sperm chemotaxis , widely recognized in metazoa (Miller 1975; Sun et al. 2009; Kaupp et al. 2008; Guerrero et al. 2010) and plants (Okuda et al. 2009), is the phenomenon in which sperm direct their movement in response to chemicals released from eggs or accessory cells, facilitating sperm−egg encounters. In addition to the well-known biological context in egg-derived chemical guidance for sperm attraction, spermatozoa often form motile conjugates that may be beneficial in competing with sperm from other males in polyandrous species (Moore et al. 2002; Fisher and Hoekstra 2010). Despite extended arguments on the evolutionary adaptation of sperm cooperation for reproductive success (Immler 2008; Foster and Pizzari 2010), little is known about how sperm form functional conjugates (Moore et al. 2002). Previously, we found that each male of the coastal squid Loligo bleekeri produces one of two types of morphologically distinct euspermatozoa , the two types being linked to distinctly different male mating behaviors (Iwata et al. 2011). Consort males produce spermatozoa with short flagella and transfer sperm capsules (spermatophores ) to internal locations of the females, inside the oviduct, whereas sneaker males produce long-flagellum sperm and transfer spermatophores to the outer body wall of the same females (Fig. 2.1, Iwata et al. 2011). The evolutionary consequences by which such phenotypic dimorphism arose remain elusive; however, each type of sperm is expected to face different sperm competitiveness and different fertilization modes.
Mating behaviors in Loligo bleekeri. In L. bleekeri, male individuals conduct one of two alternative reproductive tactics associated with body size. Consort males are relatively larger than females in body size, struggle physically with each other, and copulate predominately with the female to pass their sperm. Sneaker males, on the other hand, display maturity at a relatively smaller size than females and do not participate in male–male competition for mating. Instead, they access females by “sneaking” behavior to transfer their sperm in the course of the consort’s mating
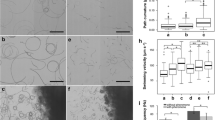
We tested whether squid sperm show any swarming behavior by drawing sperm suspensions into glass capillary tubes. Within 3 min, sneaker, but not consort, sperm became concentrated and formed a regular striped pattern along the longitudinal axis of the capillary. The formation of this pattern was transient, although motility appeared unchanged for the duration of the experiment. To ascertain that swarming is an intrinsic trait specific to sneaker sperm, a mixture of sneaker and consort sperm, each labeled with different mitochondrial dyes, was introduced into the capillary tube. Only sneaker sperm formed swarms, and both mitochondrial dyes yielded the same result. Swarming did not involve reduced motility or physical binding among sperm, but rather each sperm in the swarm moved independently by actively swimming, a phenomenon similar to chemotactic swarming. A filter assay was used to determine whether sperm swarming resulted from a chemical cue. We transferred a small amount of labeled sperm and a large amount of nonlabeled sperm into the lower and upper chambers. We then observed changes in swim-up sperm numbers by confocal microscopy. Swim-up numbers doubled when sneaker, but not consort, sperm were placed in the lower and upper chambers, indicating that the sneaker sperm upward migration is caused by a chemical stimulus that elicits chemotaxis or chemokinesis or both. Unexpectedly, sneaker sperm swam up when consort or even starfish (Asterina pectinifera) sperm were placed in the upper chamber, suggesting that the sperm attractant may be a ubiquitous molecule generated by sperm respiration.
Because Dictyostelium discoideum (Kimmel and Parent 2003) and Escherichia coli (Budrene and Berg 1991) release the chemoattractants cAMP and l-aspartate, respectively, that promote self-organization, we tested each and found no effect on either type of squid sperm. Chemoattractants known in other cell types, such as dicarboxylic acids for fern spermatozoids (Brokaw 1957), l-tryptophan for abalone sperm (Riffell et al. 2002), and sugars or amino acids for bacteria, were all inactive for squid sperm. Finally, we tested various gases and found that only CO2 attracts sneaker, but not consort, sperm. From these observations, we hypothesized that temporal swarming observed in the capillary tube was mediated by chemosensation in response to respiratory CO2 (CO2 taxis) emitted by sperm.
1.2 Flagellar Membrane-Localized Carbonic Anhydrase Serves as a Primary CO2 Sensor
We reasoned that the transient character of the swarming could result from the instability of the formation of a chemical gradient and speculated that the nature of the chemical gradient could be CO2 hydration products (protons or bicarbonate ions) rather than CO2 itself. Because carbonic anhydrases (CAs) serve as primary CO2 sensors in many biological systems (Wang et al. 2010; Chandrashekar et al. 2009; Sun et al. 2009; Ziemann et al. 2009), we tested broad and specific CA inhibitors and found an inhibitory effect on swarming by several of these compounds. We cloned a full-length cDNA encoding CA from sneaker testes and found that it is most similar to membrane-anchored CA isoforms. This transcript was also found in consort testis; therefore, we generated an antibody against a synthetic peptide to confirm protein expression in both types of sperm. Western blots of whole-cell extracts identified a ~31.7-kDa band (the calculated molecular mass of 28.8 kDa) in both sneaker and consort sperm. The flagella of both sneaker and consort sperm were equally stained by the antibody, and immunoreactivity was diminished by treating live sperm with proteinase K, indicating that CA localizes on the cell surface. We examined CO2 metabolism and found that both sneaker and consort sperm converted their respiratory CO2 to H+ and HCO3 – by CA and acidified the pH of the medium (pHe). Notably, only sneaker sperm acidified intracellular pH (pHi) concomitantly with pHe acidification.
1.3 An Extracellular Proton Gradient Establishes and Maintains Swarming
We hypothesized that sneaker sperm sense a proton gradient by which swarming is enabled. We first measured pHe using a pH-sensitive dye during swarm formation. Development of a proton gradient from the central part of the swarm was evident, although estimation of the precise pHe values was precluded by the spatiotemporal alternation of sperm density that affects concentrations of the pH indicator. Next, when the pHe gradient formation was interfered by buffering seawater (10 mM Tris or HEPES), no swarming was observed. Finally, we tested sperm behavior to acid-loaded pipettes. We found that both sneaker (below pH 5.0) and consort (below pH 4.0) sperm showed a chemotactic response to acid (acidotaxis ) and kept swarming in the vicinity of the pipette for a longer period (~30 min). As expected, sperm did not respond to a pipette with 50 mM bicarbonate-containing agarose (pH 8.0), confirming a proton as the inducer of chemotaxis.
Why do consort sperm show acidotaxis, but not CO2 taxis, despite the presence of CA? The acidotaxis assay revealed that the sensitivity of acid detection in sneaker sperm is ~1 pH unit higher than that in consort sperm. Given that no apparent pHi decrease occurred in consort sperm, we hypothesized that only sneaker sperm have the acid-induced proton uptake system by which CO2 taxis is driven. To explore this hypothesis, we first examined pHi homeostasis at various pHe using buffered seawater. We found that both sneaker and consort sperm were similar in maintaining their pHi against alkalosis. However, only consort sperm showed pHi homeostasis against acidosis. If swimming up or down the proton gradient is instantly reflected in the pHi values, the pHi changes could be a signaling component that mediates a chemotactic response. Sperm were placed in buffered seawater (pH 8.0 or 6.0) into which a pipette filled with 1 M sodium acetate (NaAc)-soaked agarose gel (pH 8.0 or 6.0) was inserted. In this setup, because NaAc crosses the plasma membrane and causes cytoplasmic acidosis, sperm are allowed to change pHi depending on their swimming direction: sperm swimming toward the pipette will become acidified and those swimming away from the pipette will recover from cytoplasmic acidosis in a constant pHe environment (pH 8.0 or 6.0). Sperm from sneaker males, but not consort males, showed directional movements toward the pipette when pHe was adjusted to 6.0. Conversely, when a pipette loaded with ammonium chloride (pH 5.0) (an alkalosis-inducing agent without pHe changes) was placed in seawater at pH 5.0, sneaker sperm showed chemorepellent behavior from the pipette. These results, together with other data, suggest that an environmental proton gradient enables synchronous changes in the pHi (acidic range) of sperm that facilitate directional movement to establish and maintain the swarm formation.
1.4 A Return from Intracellular Acidosis Evokes Calcium-Dependent Motor Responses for Turn/Tumbling
In sea urchins (Bohmer et al. 2005; Guerrero et al. 2010), ascidians (Shiba et al. 2008), and perhaps other animals (Cosson et al. 1984), sperm exhibit a coordinated transition of straight runs and quick turns, primarily regulated by calcium flux through the plasma membrane, enabling them to approach the chemoattractant source. Similarly, L. bleekeri sperm require extracellular Ca2+ for swarming in both experimental and natural conditions and for acidotaxis. We then analyzed the swimming trajectory of the sperm entering the border zone of the swarming region. We found that sperm ascending into a swarm tend to maintain straight trajectories, whereas sperm descending into a swarm make frequent turns. These results clearly demonstrated that sperm swarming is driven at least by chemotaxis but not by solely chemokinesis or trapping regardless of their possible existence. Two-dimensional swimming trajectory analysis showed that the reorientation consists of the initiation of the turn or tumbling motion followed by straight swimming directed toward the chemical source (straight–turn–straight). We asked whether this turn/tumbling initiation is caused by a pHi-dependent calcium ion uptake. Unfortunately, we were unable to image flagellar [Ca2+]i; therefore, we took an alternative approach. Sperm preincubated in acidic (pH 5.0) or normal (pH 8.0) seawater were placed in Ca2+-free seawater at pH 8.0 and tested to determine whether they would respond to a local Ca2+ release and, as a result, elicit turn/tumbling behavior. Both types of sperm, regardless of preincubation conditions, exhibited mostly straight swimming behavior in Ca2+-free seawater. However, only sneaker sperm that were preincubated in the acidic environment evoked frequent high-turn swimming episodes in the vicinity of the Ca2+-loaded pipette. These results indicate that intracellular acidosis primes the Ca2+ influx capacity in sneaker sperm, and the subsequent recovery from acidosis elicits Ca2+ uptake, which triggers transition of the swimming mode from straight to turn/tumbling (Fig. 2.2).
Model of CO2 chemotaxis in squid sperm. Respiratory CO2 emitted from self and neighboring sperm is hydrated by the flagellar membrane-bound carbonic anhydrase (CA) into bicarbonate ions and protons. Only sneaker, but not consort, sperm influx contains extracellular protons generated from self and neighboring sperm by an unknown mechanism (1), resulting in intracellular acidosis (below pHi 6.0). When sperm swim along a descending proton gradient, recovery from acidosis (above pHi 6.0) occurs (2), which evokes calcium influx (3) that is necessary for turn/tumbling initiation in the flagellum. Mt. mitochondria, Fl. flagellum
2 Discussion
The spear squid L. bleekeri employs alternative mating tactics; large consort males take physical advantage in courtship with females and deposit their spermatophores inside the female’s body. Therefore, fertilization is assumed to occur internally. In contrast, small sneaker males transfer their spermatophores by sneaking behavior at an external location just below the female’s mouth, so that such sperm would encounter eggs when females hold the eggs in their arms during the egg-laying procedure. Although a factor that influences the male-type decision remains to be identified, this system offers extremely a unique situation where internal and external fertilization coexist within a single spawning episode. Previously, we found that sneaker sperm are ~50 % longer than consort sperm (Iwata et al. 2011). Although no such clear within-species dimorphic eusperm had been reported previously, there are many examples of sperm size differences among closely related species, which have largely been explained as the consequences of sperm competition (Gage 1994; Briskie and Montgomerie 1992; Gomendio and Roldan 1991). Unexpectedly, empirical data supported no evidence that larger sperm are favored in sperm competition in this species regarding the swimming velocity and sperm precedence at the storage site (in the seminal receptacle). Alternatively, different fertilization environments might be a prominent factor that could drive the evolution of sperm size (Iwata et al. 2011).
In this study, we found that sperm behavioral traits are also different between sneaker and consort spermatozoa. Sperm from sneaker, but not consort, males have a characteristic of forming motile conjugates when ejaculated into seawater and hold these in the close vicinity of the spermatophore. From an ecological aspect, retaining ability of ejaculates at the buccal region (site of egg deposition) would have a prominent effect on storing into the externally located seminal receptacle (female sperm storage organ) or fertilization success because mating and egg laying are temporally independent (Iwata et al. 2005). Especially, sperm should travel, either actively or passively, for the certain distance from the ejaculation site to the storage site by an unknown mechanism (Iwata et al. 2011; Sato et al. 2010; Lumkong 1992). Therefore, the sperm swarming trait together with the female arm crown architecture (Naud et al. 2005) would provide an effective diffusion-resistant situation against water movement.
The question of why only sneaker sperm have acquired the swarming trait would be intriguing to address in the light of postcopulatory sexual selection (Birkhead and Pizzari 2002) and natural selection (Foster and Pizzari 2010). Theoretically, a risk of sperm diffusion would be much greater on sneaker (externally deposited and stored) than on consort (internally deposited and stored) sperm, which could account for the evolution of complex adaptive traits on precopulatory (mating behavior) and postcopulatory (sperm function) sexual selection. We therefore carried out further investigations with other Loliginidae species that also employ alternative male mating behavior. In Loligo reynaudii and Photololigo edulis, sperm from sneaker individuals, as judged from the sperm mass morphology (Iwata and Sakurai 2007), exhibited self-swarming, whereas no swarming occurred for sperm from consort individuals. Moreover, in species employing only sneaker-type mating behavior, that is, males inseminate the external sites on females, such as Idiosepius paradoxus and Todarodes pacificus, sperm also showed swarming behavior, supporting our hypothesis that the swarming trait tightly associates with the fertilization mode rather than sperm competition between sneaker and consort (Parker 1990).
3 Perspectives
It remains unknown how changes in pHi elicit [Ca2+]i mobilization in this system. However, recent reports identified that CatSper, a mammalian sperm calcium channel essential for flagellum motility, can be activated by either progesterone (Strunker et al. 2011; Lishko et al. 2011) (a sperm chemoattractant) or intracellular alkalization (Kirichok et al. 2006). CO2/acid detection in the mammalian gustatory system (Chandrashekar et al. 2009; Huang et al. 2006; Kawaguchi et al. 2010; Chang et al. 2010; Lahiri and Forster 2003) and central nervous system (Ziemann et al. 2009; Lahiri and Forster 2003) may represent molecular similarity to CO2 taxis found in squid sperm in terms of intracellular acidosis via transcellular proton currents (Chang et al. 2010) and “off-response” (Kawaguchi et al. 2010). Because CO2 emission is the cell’s fundamental property, understanding the molecular pathway in the CO2 taxis will provide a broad impetus to discover similar examples in biological systems.
References
Birkhead TR, Pizzari T (2002) Postcopulatory sexual selection. Nat Rev Genet 3(4):262–273
Bohmer M, Van Q, Weyand I, Hagen V, Beyermann M, Matsumoto M, Hoshi M, Hildebrand E, Kaupp UB (2005) Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J 24(15):2741–2752
Briskie JV, Montgomerie R (1992) Sperm size and sperm competition in birds. Proc Biol Sci 247(1319):89–95
Brokaw CJ (1957) Electro-chemical orientation of bracken spermatozoids. Nature (Lond) 179(4558):525
Budrene EO, Berg HC (1991) Complex patterns formed by motile cells of Escherichia coli. Nature (Lond) 349(6310):630–633
Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS (2009) The taste of carbonation. Science 326(5951):443–445
Chang RB, Waters H, Liman ER (2010) A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci USA 107(51):22320–22325
Cosson MP, Carre D, Cosson J (1984) Sperm chemotaxis in siphonophores. II. Calcium-dependent asymmetrical movement of spermatozoa induced by the attractant. J Cell Sci 68:163–181
Fisher HS, Hoekstra HE (2010) Competition drives cooperation among closely related sperm of deer mice. Nature (Lond) 463(7282):801–803
Foster KR, Pizzari T (2010) Cooperation: the secret society of sperm. Curr Biol 20(7):R314–R316
Gage MJG (1994) Associations between body size, mating pattern, testis size and sperm lengths across butterflies. Proc R Soc Lond B Biol Sci 258:247–254
Gomendio M, Roldan ER (1991) Sperm competition influences sperm size in mammals. Proc Biol Sci 243(1308):181–185
Guerrero A, Nishigaki T, Carneiro J, Yoshiro T, Wood CD, Darszon A (2010) Tuning sperm chemotaxis by calcium burst timing. Dev Biol 344(1):52–65
Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS (2006) The cells and logic for mammalian sour taste detection. Nature (Lond) 442(7105):934–938
Immler S (2008) Sperm competition and sperm cooperation: the potential role of diploid and haploid expression. Reproduction 135(3):275–283
Iwata Y, Sakurai Y (2007) Threshold dimorphism in ejaculate characteristics in the squid Loligo bleekeri. Mar Ecol Prog Ser 345:141–146
Iwata Y, Munehara H, Sakurai Y (2005) Dependence of paternity rates on alternative reproductive behaviors in the squid Loligo bleekeri. Mar Ecol Prog Ser 298:219–228
Iwata Y, Shaw P, Fujiwara E, Shiba K, Kakiuchi Y, Hirohashi N (2011) Why small males have big sperm: dimorphic squid sperm linked to alternative mating behaviours. BMC Evol Biol 11:236
Kaupp UB, Kashikar ND, Weyand I (2008) Mechanisms of sperm chemotaxis. Annu Rev Physiol 70:93–117
Kawaguchi H, Yamanaka A, Uchida K, Shibasaki K, Sokabe T, Maruyama Y, Yanagawa Y, Murakami S, Tominaga M (2010) Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells. J Biol Chem 285(23):17277–17281
Kimmel AR, Parent CA (2003) Dictyostelium discoideum cAMP chemotaxis pathway. Sci STKE 2003:cm1
Kirichok Y, Navarro B, Clapham DE (2006) Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature (Lond) 439(7077):737–740
Lahiri S, Forster RE 2nd (2003) CO2/H+ sensing: peripheral and central chemoreception. Int J Biochem Cell Biol 35(10):1413–1435
Lishko PV, Botchkina IL, Kirichok Y (2011) Progesterone activates the principal Ca2+ channel of human sperm. Nature (Lond) 471(7338):387–391
Lumkong A (1992) A histological study of the accessory reproductive-organs of female Loligo forbesi (Cephalopoda, Loliginidae). J Zool (Lond) 226:469–490
Miller RL (1975) Chemotaxis of the spermatozoa of Ciona intestinalis. Nature 254:244–245
Moore H, Dvorakova K, Jenkins N, Breed W (2002) Exceptional sperm cooperation in the wood mouse. Nature (Lond) 418(6894):174–177
Naud MJ, Shaw PW, Hanlon RT, Havenhand JN (2005) Evidence for biased use of sperm sources in wild female giant cuttlefish (Sepia apama). Proc Biol Sci 272(1567):1047–1051
Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, 1 Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, Kawano N, Sakakibara T, Namiki S, Itoh K, Otsuka K, Matsuzaki M, Nozaki H, Kuroiwa T, Nakano A, Kanaoka MM, Dresselhaus T, Sasaki N, Higashiyama T (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458:357–361
Parker GA (1990) Sperm competition games: sneaks and extra-pair copulations. Proc Biol Sci 242:127–133
Riffell JA, Krug PJ, Zimmer RK (2002) Fertilization in the sea: the chemical identity of an abalone sperm attractant. J Exp Biol 205(pt 10):1439–1450
Sato N, Kasugai T, Ikeda Y, Munehara H (2010) Structure of the seminal receptacle and sperm storage in the Japanese pygmy squid. J Zool (Lond) 282:151–156
Shiba K, Baba SA, Inoue T, Yoshida M (2008) Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc Natl Acad Sci USA 105(49):19312–19317
Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB (2011) The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature (Lond) 471(7338):382–386
Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M (2009) Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA 106(6):2041–2046
Wang YY, Chang RB, Liman ER (2010) TRPA1 is a component of the nociceptive response to CO2. J Neurosci 30(39):12958–12963
Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA 3rd, Welsh MJ, Wemmie JA (2009) The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 139(5):1012–1021
Acknowledgments
This study was supported by Narishige Zoological Science Award, Research Institute of Marine Invertebrates, Yamada Science Foundation, Grant-in-aid for Scientific Research on Innovative Areas from MEXT and Japanese Association for Marine Biology (JAMBIO) to N.H. and National Research Foundation to W.H.H.S.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Hirohashi, N., Iwata, Y., Sauer, W.H.H., Kakiuchi, Y. (2014). Respiratory CO2 Mediates Sperm Chemotaxis in Squids. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_2
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_2
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)