Abstract
Vitellogenin is a precursor of yolk protein that is necessary for embryonic development. Vitellogenin is a large multidomain protein consisting of a signal peptide, a heavy-chain lipovitellin, a phosvitin, a light-chain lipovitellin, a von Willebrand factor type D domain (vWF-D), and a C-terminal coding region (CT), which are processed to respective domains after uptake into oocytes. It is currently believed that only lipovitellin and phosvitin domains are necessary for nutrient supply to oocytes. Thus, molecular species of vitellogenin lacking these domains are not known. We recently found that two novel isoforms of vitellogenin, both of which possess vWF-D and CT domains but not a lipovitellin or phosvitin domain, are expressed in the gonad of the ascidian Halocynthia roretzi. In situ hybridization revealed that mRNAs of these proteins are specifically expressed in oocytes and test cells, accessory cells in the perivitelline space of ascidian eggs. Immunocytochemistry showed that these proteins are localized around the surface of test cells in immature oocytes. Immunoelectron microscopy revealed that vitellogenin associates with vesicles located beneath the vitelline coat (VC) before fertilization but that it dissociates from the VC after fertilization. These results, together with our previous results showing that vWF-D and CT domains are capable of binding to the two sperm proteases HrProacrosin and HrSpermosin led us to propose that novel isoforms of vitellogenin, which are expressed in oocytes and test cells and released to the perivitelline space during oocyte maturation, may participate in gamete interaction upon fertilization.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Vitellogenin Is a Binding Partner of Sperm Proteases
To accomplish successful fertilization, sperm must bind to and penetrate through the extracellular glycoprotein matrix surrounding the egg, which is called the zona pellucida (ZP) in mammals and the vitelline coat (VC) in marine invertebrates (McRorie and Williams 1974; Wassarman 1987; Sawada 2002). In mammals, an acrosomal trypsin-like protease, acrosin [EC 3.4.21.10], had long been believed to be a lytic agent, lysin, which makes a small hole for sperm penetration through the ZP of the ovum (Müller-Esterl and Fritz 1981; Urch et al. 1985a, b). However, because mouse sperm lacking the acrosin gene can penetrate through the ZP, albeit with some delay (Baba et al. 1994), it is currently thought that acrosin is not essential for the penetration of sperm through the ZP but is involved, at least in part, in the dispersal of acrosomal contents during the acrosome reaction (Yamagata et al. 1998) and in the secondary binding of sperm to the ZP (Howes et al. 2001; Howes and Jones 2002). However, more detailed studies on the targets of sperm proteases, including acrosin, are necessary to elucidate the roles of sperm proteases in fertilization.
Sperm trypsin-like proteases have been believed to take some part in fertilization of the ascidian Halocynthia roretzi because sperm–egg interaction was inhibited by protease inhibitors (Hoshi et al. 1981; Lambert et al. 2002). Then, two trypsin-like proteases, HrProacrosin and HrSpermosin, were purified from H. roretzi sperm (Sawada et al. 1984a), and it was also revealed that these proteases play important roles in ascidian fertilization (Sawada et al. 1984b, 1996; Sawada and Someno 1996). Both these proteases possess potential regions for protein–protein interaction: two CUB domains in the C-terminus of HrProacrosin and a proline-rich region in the N-terminus of HrSpermosin. Previously, we explored the binding partners of these proteases using affinity beads immobilized with the CUB domain peptide or a proline-rich region synthesized in Escherichia coli and succeeded in isolating several VC proteins (Kodama et al. 2001, 2002). However, they were not identified because the N-terminal sequences of these proteins showed no significant homology to any protein.
Recently, we carried out 5′- and 3′-rapid amplification of cDNA ends (5′-RACE)-polymerase chain reaction (PCR) on the basis of these N-terminal sequences, showing that some of these VC proteins are identical to the C-terminal coding region (CT) and von Willebrand factor type D (vWF-D) domain, which is located at the C-terminal region of vitellogenin (Akasaka et al. 2010). The binding ability between the vitellogenin C-terminus and CUB domain of HrProacrosin was confirmed by an in vitro pulldown assay (Fig. 12.1a).
(a) Pulldown assay of CUB domain 1 with vitellogenin C-terminus. The [35S]-labeled vitellogenin C-terminus was pulled down with agarose beads immobilized with a recombinant CUB domain 1 protein. (b) Southern blotting. Halocynthia roretzi genome was digested with BamHI (lane 1), EcoRI (lane 2), HindIII (lane 3), or PstI (lane 4), and subjected to agarose gel electrophoresis. Southern blotting was carried out using a DIG-labeled probe corresponding to the vitellogenin C-terminus. (c) Northern blotting. Poly(A)+ RNA obtained from each tissue was subjected to agarose gel electrophoresis followed by Northern blotting using a DIG-labeled probe corresponding to the vitellogenin C-terminus. Lane 1, muscle; lane 2, intestine; lane 3, gonad; lane 4, gill; lane 5, endostyle; lane 6, hepatopancreas. Note that 2.0- and 2.5-kb mRNA signals Fig. 12.1 (continued) were detected in the gonad, and stronger and longer signals were detected in hepatopancreas. (d) Putative gene model of vitellogenin that resulted from the search for the H. roretzi genome database. Exon and intron are represented by a square and a bar, respectively. Based on the gene model, S1 and S2 are predicted to be composed of ten exons and seven exons, respectively. (e) In situ hybridization of vitellogenin. An antisense probe of vitellogenin C-terminus was hybridized with sections of immature oocytes and hepatopancreas. Note that test cells, which are accessory cells of ascidian eggs located in the perivitelline space in mature eggs, are located in the periphery in immature oocytes. Sense probe was also hybridized with immature oocytes and hepatopancreas as a control. A 1-ng probe sample was hybridized in all specimens. (f) Immunohistochemisty of vitellogenin in immature oocytes. A specimen of the section of gonad eggs was treated with anti-vitellogenin C-terminus primary antiserum (upper panels), preimmune serum (middle panels), and anti-HrVC70 (lower panels). Vitellogenin was shown located on the surface of test cells. Anti-HrVC70 was used to distinguish the VC because HrVC70 is a major component of the VC. Test cell and vitelline coat are indicated as TC and VC, respectively. Right bright-field images, left fluorescent images in each panel
2 Novel Isoforms of Vitellogenin are Expressed in the Gonad
Vitellogenin is a major precursor of yolk protein, which is necessary for embryonic development (Wallace and Selman 1985), and a large multidomain molecule that consists of the following regions/domains from the N-terminus to the C-terminus: a signal peptide, lipovitellin-1 (heavy chain), phosvitin, lipovitellin-2 (light chain), von Willebrand factor type D (vWF-D), and C-terminal coding region (CT) (Finn 2007). Gene expression of vitellogenin is induced in the liver by an estrogen-like hormone, and the synthesized protein is then transferred to the ovary via the bloodstream and is taken up into immature oocytes by clathrin-mediated endocytosis (Wallace and Selman 1990). Although the gene encoding vitellogenin in H. roretzi is a single copy (Fig. 12.1b), several lengths of mRNAs were detected mainly in the hepatopancreas and only two shorter species were weakly detected in the gonad by Northern blot analysis (Fig. 12.1c). These results suggest that mRNAs of vitellogenin were expressed in a tissue-specific manner, and it is inferred that shorter isoforms may play a specific role in the gonad.
To clarify the domain composition of vitellogenin expressed in the gonad, we determined the full-length cDNA sequences of two isoforms of vitellogenin by 5′-RACE-PCR using an H. roretzi gonad cDNA library and named vitellogenin S1 and S2 (Akasaka et al. 2013). A BLAST search of the deduced amino-acid sequences of vitellogenin S1 and S2 against genomic data of H. roretzi showed that S1 and S2 are alternatively spliced isoforms from a gene model of vitellogenin named Hr.Aug-120507.S000436.g02527, and the difference between S1 and S2 appears to be caused by the presence or absence of three exons (personal communication) (Fig. 12.1d). Unexpectedly, neither of these contains lipovitellin and phosvitin, which are necessary for vitellogenin functioning as a nutrient source, but contain vWF-D and CT domains. Concerning the vWF-D and CT domains, the CGXC motif of vWF-D and polycysteine residues of CT appear to participate in the folding of vitellogenin by forming a disulfide linkage (Mayadas and Wagner 1992; Mouchel et al. 1996). To the best of our knowledge, there is no report about the occurrence of vitellogenin isoform consisting of only vWF-D and CT domains that lacks both lipovitellin and phosvitin.
3 Localization of Vitellogenin in Immature Oocytes
Although there are several reports showing the expression of vitellogenin in the gonad, there have been few experiments examining the detailed expression site of vitellogenin mRNA. To clarify the detailed expression site of vitellogenin, in situ hybridization against the sectioned gonad and hepatopancreas was carried out. As a positive control, the expression of vitellogenin mRNA in the hepatopancreas was confirmed, which coincided well with the expression pattern of vitellogenin mRNA in the hepatopancreas as revealed by Northern blot analysis (Fig. 12.1c). In immature oocytes, vitellogenin mRNA was detected in the cytosol (Fig. 12.1e): In particular, it appears to be concentrated on the surface of the test cells. Test cells are accessory cells in ascidian eggs that are embedded in immature oocytes and released to the perivitelline space during oocyte maturation. Several functions of test cells have been proposed from the results of ultrastructural observations; however, those functions are still controversial and poorly understood.
Because vitellogenin appears to be synthesized in oocytes and test cells, we carried out immunohistochemistry of vitellogenin in immature oocytes in the gonad using a mouse antiserum against a C-terminal region of vitellogenin. The results showed that vitellogenin appears to be specifically localized on the surface of test cells or the border between oocytes and test cells (Fig. 12.1f). As a marker for the VC, anti-HrVC70 antiserum was used to distinguish the outer structure of oocytes because HrVC70 is a major component of the VC in H. roretzi (Sawada et al. 2002). It was clarified that vitellogenin exists in neither the VC nor yolk but specifically on the surface of test cells or the intermembrane space between test cells and immature oocytes in the gonad. These results indicate that vitellogenin S1 and S2 are expressed and synthesized in immature oocytes, and probably also in test cells at the stage of immature oocytes, and are localized at the surface or intermembrane space between immature oocytes and test cells.
4 Localization of Vitellogenin in Mature Eggs
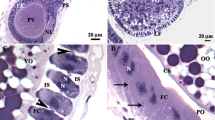
It is known that test cells move out from the egg periphery to the perivitelline space during ovulation (Tucker 1942). To investigate the localization of vitellogenin in terms of the movement of test cells, whole-mount immunohistochemistry was first carried out in mature eggs (unfertilized eggs) and the fertilized eggs using antiserum against vitellogenin C-terminus. The results showed that vitellogenin exists in the VC of unfertilized eggs, but not in the VC of fertilized eggs (Fig. 12.2a). Then, detailed observation on the localization of vitellogenin in the perivitelline space was attempted, but nonspecific autofluorescence within the egg was too strong to observe fluorescence in the perivitelline space (data not shown). Therefore, observation by immunoelectron microscopy was performed using the fixed unfertilized and fertilized eggs. As a result, gold particles detecting vitellogenin were observed in vesicles in the perivitelline space in both unfertilized and fertilized eggs, and the vesicles containing vitellogenin adhered to the VC of unfertilized eggs and dissociated from the VC after fertilization (Fig. 12.2b). It is suggested that vitellogenin appears to be incorporated into vesicles and secreted to the perivitelline space on ovulation or oocyte maturation, most of which is located beneath the VC in unfertilized eggs.
(a) Fluorescence immunohistochemistry of vitellogenin C-terminus on unfertilized and fertilized eggs. Both unfertilized and fertilized eggs were treated with anti-vitellogenin C-terminus antiserum and control preimmune serum. Note that vitellogenin is present on the VC of unfertilized eggs (arrow) and disappears in fertilized eggs. Right bright-field images, left fluorescent images in each panel. (b) Immunoelectron micrographs of the perivitelline spaces of unfertilized and fertilized eggs. Unfertilized and fertilized eggs were sectioned and treated with anti-vitellogenin C-terminus antiserum and visualized by using a 10-nm gold particle-conjugated secondary antibody. In the unfertilized egg (left), gold particles are mainly observed in vesicles (arrow), which adhere to the inner side of the VC. After fertilization, vesicles with vitellogenin detach from the VC and localize in the perivitelline space. The VC becomes thinner after fertilization. Vitelline coat and perivitelline space are indicated as VC and PS, respectively
5 Future Perspective
Our results shown here indicate that the novel isoform of vitellogenin is synthesized in test cells as well as in immature oocytes and is released to the perivitelline space, associating with vesicles. Although the biological roles of the novel type of vitellogenin remain to be elucidated, it should be emphasized that the expression, localization, and behavior of novel truncated vitellogenin isoforms during oocyte maturation and fertilization are new findings in this study. Because vitellogenin S1 and S2 contain vWF-D and CT domains, which we previously identified as binding proteins of the sperm proteases HrProacrosin and HrSpermosin, these novel vitellogenin species may play an important role in gamete interaction, in particular in the process of sperm passage through the perivitelline space or gamete fusion, although it remains to be determined whether these vitellogenin species are exposed to the surface of the small vesicles. Further studies are necessary to elucidate the biological roles of these novel vitellogenin species during ascidian fertilization.
Interestingly, neither of the novel vitellogenin species possesses a typical signal sequence. Therefore, it could be presumed that these proteins synthesized in oocytes and test cells are incorporated into vesicles and accumulated in the boundary between immature oocytes and test cells. During oocyte maturation, these vesicles may be secreted into the perivitelline space and mostly locate beneath the VC. The detailed transport of vitellogenin from test cells to vesicles in mature eggs is still unclear. Further observation of vitellogenin in the perivitelline space of mature eggs is necessary.
In connection with these vesicles, it is notable that CD9, which is a tetra-spanning membrane protein expressed in mouse oocytes that is essential for membrane fusion with sperm, was recently found to be secreted to the perivitelline space with the aid of small vesicles secreted from oocytes called “exosomes,” and it was revealed that association of exosomes and sperm in the perivitelline space is essential for sperm–oocyte membrane fusion (Miyado et al. 2008). Moreover, the incorporation of vitellogenin into exosomes has been reported in the fruit fly and in humans (Brasset et al. 2006; Von Wald et al. 2010). These results led us to speculate that vitellogenin-associating vesicles may behave as an exosome in the mouse egg and play some important roles in gamete fusion or other fertilization processes. Further studies are necessary to elucidate the functions of the novel isoforms of vitellogenin in ascidian fertilization. Possible involvement of vitellogenin in gamete fusion should also be considered by analogy of the aforementioned recent discovery in CD9.
References
Akasaka M, Harada Y, Sawada H (2010) Vitellogenin C-terminal fragments participate in fertilization as egg-coat binding partners of sperm trypsin-like proteases in the ascidian Halocynthia roretzi. Biochem Biophys Res Commun 392(4):479–484
Akasaka M, Kato KH, Kitajima K, Sawada H (2013) Identification of novel isoforms of vitellogenin expressed in ascidian eggs. J Exp Zool B Mol Dev Evol 320(2):118–128
Baba T, Azuma S, Kashiwabara S, Toyoda Y (1994) Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem 269(59):31845–31849
Brasset E, Taddei A, Arnaud F, Faye B, Fausto A, Mazzini M, Giorgi F, Vaury C (2006) Viral particles of the endogenous retrovirus ZAM from Drosophila melanogaster use a pre-existing endosome/exosome pathway for transfer to the oocyte. Retrovirology 3:26
Finn RN (2007) Vertebrate yolk complexes and the functional implications of phosvitins and other subdomains in vitellogenins. Biol Reprod 76(6):926–935
Hoshi M, Numakunai T, Sawada H (1981) Evidence for participation of sperm proteinases in fertilization of the solitary ascidian, Halocynthia roretzi: effects of protease inhibitors. Dev Biol 86(1):117–121
Howes L, Jones R (2002) Interactions between zona pellucida glycoproteins and sperm proacrosin/acrosin during fertilization. J Reprod Immunol 53(1-2):181–192
Howes E, Pascall JC, Engel W, Jones R (2001) Interactions between mouse ZP2 glycoprotein and proacrosin; a mechanism for secondary binding of sperm to the zona pellucida during fertilization. J Cell Sci 114(Pt 22):4127–4136
Kodama E, Baba T, Yokosawa H, Sawada H (2001) cDNA cloning and functional analysis of ascidian sperm proacrosin. J Biol Chem 276(27):24594–24600
Kodama E, Baba T, Kohno N, Satoh S, Yokosawa H, Sawada H (2002) Spermosin, a trypsin-like protease from ascidian sperm: cDNA cloning, protein structures and functional analysis. Eur J Biochem 269(2):657–663
Lambert CC, Someno T, Sawada H (2002) Sperm surface proteases in ascidian fertilization. J Exp Zool 292(1):88–95
Mayadas TN, Wagner DD (1992) Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc Natl Acad Sci USA 89(8):3531–3535
McRorie RA, Williams WL (1974) Biochemistry of mammalian fertilization. Annu Rev Biochem 43:777–803
Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A (2008) The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci USA 105(35):12921–12926
Mouchel N, Trichet V, Betz A, Le Pennec JP, Wolff J (1996) Characterization of vitellogenin from rainbow trout (Oncorhynchus mykiss). Gene (Amst) 174(1):59–64
Müller-Esterl W, Fritz H (1981) Sperm acrosin. Methods Enzymol 80(pt C):621–632
Sawada H (2002) Ascidian sperm lysin system. Zool Sci 19(2):139–151
Sawada H, Someno T (1996) Substrate specificity of ascidian sperm trypsin-like proteases, spermosin and acrosin. Mol Reprod Dev 45(2):240–243
Sawada H, Yokosawa H, Ishii S (1984a) Purification and characterization of two types of trypsin-like enzymes from sperm of the ascidian (Prochordata) Halocynthia roretzi. Evidence for the presence of spermosin, a novel acrosin-like enzyme. J Biol Chem 259(5):2900–2904
Sawada H, Yokosawa H, Someno T, Saino T, Ishii S (1984b) Evidence for the participation of two sperm proteases, spermosin and acrosin, in fertilization of the ascidian, Halocynthia roretzi: inhibitory effects of leupeptin analogs on enzyme activities and fertilization. Dev Biol 105(1): 246–249
Sawada H, Iwasaki K, Kihara-Negishi F, Ariga H, Yokosawa H (1996) Localization, expression, and the role in fertilization of spermosin, an ascidian sperm trypsin-like protease. Biochem Biophys Res Commun 222(2):499–504
Sawada H, Sakai N, Abe Y, Tanaka E, Takahashi Y, Fujino J, Kodama E, Takizawa S, Yokosawa H (2002) Extracellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proc Natl Acad Sci USA 99(3):1223–1228
Tucker GH (1942) The histology of the gonads and development of the egg envelopes of an ascidian (Styela plicata Lesueur). J Morphol 70(1):81–113
Urch UA, Wardrip NJ, Hedrick JL (1985a) Limited and specific proteolysis of the zona pellucida by acrosin. J Exp Zool 233(3):479–483
Urch UA, Wardrip NJ, Hedrick JL (1985b) Proteolysis of the zona pellucida by acrosin: the nature of the hydrolysis products. J Exp Zool 236(2):239–243
Von Wald T, Monisova Y, Hacker M, Yoo S, Penzias A, Reindollar R, Usheva A (2010) Age-related variations in follicular apolipoproteins may influence human oocyte maturation and fertility potential. Fertil Steril 93(7):2354–2361
Wallace RA, Selman K (1985) Major protein changes during vitellogenesis and maturation of Fundulus oocytes. Dev Biol 110(2):492–498
Wallace RA, Selman K (1990) Ultrastructural aspects of oogenesis and oocyte growth in fish and amphibians. J Electron Microsc Tech 16(3):175–201
Wassarman PM (1987) Early events in mammalian fertilization. Annu Rev Cell Biol 3:109–142
Yamagata K, Murayama K, Okabe M, Toshimori K, Nakanishi T, Kashiwabara S, Baba T (1998) Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. J Biol Chem 273(17):10470–10474
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Akasaka, M., Kato, K.H., Kitajima, K., Sawada, H. (2014). Novel Isoform of Vitellogenin Expressed in Eggs Is a Binding Partner of the Sperm Proteases, HrProacrosin and HrSpermosin, in the Ascidian Halocynthia roretzi . In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_12
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_12
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)