Abstract
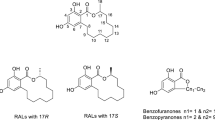
The resorcylic acid lactones (RALs) are a family of benzannulated macrolides, which are produced by a variety of fungi and show versatile biological activities (6). According to their name, they consist structurally of a partially substituted β-resorcylic acid scaffold, which is linked to a 12- or 14-membered macrolactone moiety. Selected members of this group are shown in Fig. 9.1.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Winssinger N, Barluenga S (2007) Chemistry and biology of resorcylic acid lactones. Chem Commun 22
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16:497
Bräse S, Encinas A, Keck J, Nising CF (2009) Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev 109:3903
Mirrington RN, Ritchie E, Shoppee CW, Taylor WC, Aternhell S (1964) The constitution of radicicol. Tetrahedron Lett 7:365
McCapra F, Scott AI, Delmotte P, Delmotte-Plaquee J, Bhacca NS (1964) The constitution of monorden, an antibiotic with tranquilising action. Tetrahedron Lett 15:869
Delmotte P, Delmotte-Plaquee J (1953) A new antifungal substance of fungal origin. Nature 171:344
Stob M, Baldwin RS, Tuite J, Andrews FN, Gilette KG (1962) Isolation of an anabolic, uterotropic compound from corn infected with Gibberella zeae. Nature 196:1318
Urry WH, Wehrmeister HL, Hodge EB, Hidy PH (1966) The structure of zearalenone. Tetrahedron Lett 27:3109
Hagler WM, Mirocha CJ, Pathre SV, Behrens JC (1979) Identification of the naturally occurring isomer of zearalenol produced by Fusarium roseum ‘Gibboseum’ in rice culture. Appl Environ Microbiol 37:849
Agatsuma T, Takahashi A, Kabuto C, Nozoe S (1993) Revised structure and chemistry of hypothemycin. Chem Pharm Bull 41:373
Nair MSR, Carey ST (1980) Metabolites of pyrenomycetes XӀӀӀ: structure of (+) hypothemycin, an antibiotic macrolide from Hypomyces trichothecoides. Tetrahedron Lett 21:2011
Isaka M, Suyarnsestakorn C, Tanticharoen M, Kongsaeree P, Thebtaranonth Y (2002) Aigialomycins a-E, new resorcylic macrolodes from the marine mangrove fungus Aigialus parvus. J Org Chem 67:1561
Hellwig V, Mayer-Bartschmid A, Müller H, Greif G, Kleymann G, Zitzmann W, Tichy H-V, Stadler M (2003) Pochonins a-F, new antiviral and antiparasitic resorcylic acid lactones from Pochonia chlamydosporia var. Catenulata. J Nat Prod 66:829
Xu L, He Z, Xue J, Chen X, Wei X (2010) β-resorcylic acid lactones from a Paecilomyces fungus. Nat Prod 73:885
Shao C-L, Wu H-X, Wang Ch-Y, Liu Q-A, Xu Y, Wei M-Y, Qian P-Y, Gu Y-C, Zheng C-J, She Z-G, Lin Y-C (2011) Potent antifouling resorcylic acid lactones from the gorgonian-derived fungus Cochliobolus lunatus. J Nat Prod 74:629
Oyama H, Sassa T, Ikeda M (1978) Structured of new plant growth inhibitors, trans- and cis-resorcylide. Agric Biol Chem 42:2407
Aldridge DC, Galt S, Giles D, Turner WB (1971) Metabolites of Lasiodiplodia theobromae. J Chem Soc 1623
Miksicek RJ (1994) Interaction of naturally occuring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol 49:153
Shier WT, Shier AC, Xie W, Mirocha CJ (2001) Stucture-activity relationship for human estrogenic activity in zearalenone mycotoxins. Toxicon 39:1435
Hodge EG, Hidy PH Wehrmeister HJ (1966) Estrogenic compounds and animal growth promotors. US Patent 3239345
Utian WH (1973) Comparative trial of P1496, a new non-steroidal oestrogen analogue. Br Med J 1:579
Kwon HJ, Yoshida M, Fukui Y, Horinouchi S, Beppu T (1992) Potent and specific inhibition of p60v-src protein kinase both in vivo and in vitro by radicicol. Cancer Res 52:6926
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407
Barluenga S, Dakas P, Boulifa M, Moulin E, Winssinger N (2008) Resorcylic acid lactones: a pluripotent scaffold with therapeutic potential. C R Chim 11:1306
Schirmer A, Kennedy J, Sumati M, Reid R, Santi AV (2006) Target covalent inactivation of protein kinases by resorcylic acid lactone polyketides. Proc Natl Acad Sci USA 103:4234
Zhao A, Lee SH, Jenkins RG, Patrick DR, Huber HE, Goetz MA, Hensens OD, Zink DL, Vilella D, Dombrowski AW, Lingham RB, Huang L (1999) Resorcylic acid lactones: naturally occurring potent and selective inhibitors of MEK. J Antibiot 52:1086
Masamune S, Bates GS, Corcoran JW (1977) Macrolides. Recent progress in chemistry and biochemistry. Angew Chem Int Ed Engl 16:585
Corey EJ, Nicolaou KC (1974) An efficient and mild lactonization method for the synthesis of macrolides. J Am Chem Soc 96:5614
Masamune S, Kamata S, Schilling W (1975) Syntheses of macrolide antibiotics. ӀӀӀ. Direct ester and lactone synthesis from S-tert-butyl tioate (thiol ester). J Am Chem Soc 97:3515
Fürstner A, Thiel OR, Kindler N, Bartkowska B (2000) Total syntheses of (S)-(‒)-zearalenone and lasiodiplodin reveal superior metathesis activity of ruthenium carbene complexes with imidazol-2-ylidene ligands. J Org Chem 65:7990
Srihari P, Mahankali B, Rajendraprasad K (2012) Stereoselective total synthesis of paecilomycin E. Tertrahedron Lett 53:56
Taub D, Girotra NN, Hoffsommer RD, Kuo CH, Slates HL, Weber S, Wendler NL (1967) Total synthesis of the macrolide, zearalenone. Chem Commun 225
Vlattas I, Harrison IT, Tökés L, Fried JH, Cross AD (1978) The synthesis of dl-zearalenone. J Org Chem 33:11
Takahashi T, Kasuga K, Takahashi M, Tsuji J (1979) A simple total synthesis of (±)-zearalenone by intramolecular alkylation using a butadiene telomer as building block. J Am Chem Soc 101:5072
Keinan E, Sinha SC, Sinha-Bagchi A (1991) Thermostable enzymes in organic synthesis, Part 6. Total synthesis of (S)-(‒)-zearalenone using a TBADH-generated trifunctional chiron. J Chem Soc Perkin Trans 1:3333
Hurd RN, Shah DH (1973) Total synthesis of the macrolide (R, S)-zearalenone. J Med Chem 16:543
Takahashi T, Ikeda H, Tsuji J (1981) New synthetic method for orsellic acid type macrolides by intramolecular alkylation of protected cyanohydrin. The synthesis of (±)-zearalenone. Tetrahedron Lett 22:1363
Hitchcock SA, Pattenden G (1990) Synthesis of macrocycles via allylic radical intermediates. A total synthesis of (±)-zearalenone. Tetrahedon Lett 31:3641
Kalivretenos K, Stille JK, Hegedus LS (1991) Synthesis of β-resorcylic macrolides via organopalladium chemistry. Application to the total synthesis of (S)-zearalenone. J Org Chem 56:2883
Nicolaou KC, Winssinger N, Pastor J, Murphy F (1998) Solid-phase synthesis of macrocyclic systems by a cyclorelease strategy: application of the stille coupling to a synthesis of (S)-zearalenone. Angew Chem Int Ed 37:2534
Navarro I, Basset J-F, Hebbe S, Major SM, Werner T, Howsham C, Bräckow J, Barrett AGM (2008) Biomimetic synthesis of resorcylic natural products utilizing large stage aromatizitaion: concise total syntheses of the marine antifungal agents 15G253ι and 15G256β. J Am Chem Soc 130:10293
Yadav JS, Murphy AV (2011) A concise synthesis of (S)-zearalenone and zeranol. Synthesis 13:2117
Hodge EB (1974) Reduction of zearalenone. DE Patent 2328605
Ley SV, Burckhardt S (2000) The use of π-allyltricarbonyliron lactone complexes in the synthesis of the resorcylic macrolides α- and β-zearalenol. J Chem Soc Perkin Trans 1:3028
Lampilas M, Lett R (1992) Convergent stereospecific total synthesis of monochiral monocillin I related macrolides. Tetrahedron Lett 33:773
Lampilas M, Lett R (1992) Convergent stereospecific total synthesis of monocillin I and monorden (or radicicol). Tetrahedron Lett 33:777
Tinchkowski I, Lett R (2002) Convergent stereospecific total synthesis of monocillin I and radicicol: some simplifications and improvements. Tetrahedron Lett 43:3997
Tinchkowski I, Lett R (2002) Improvements of the total synthesis of monocillin I and radicicol via Miyaura-Suzuki couplings. Tetrahedron Lett 43:4003
Garbaccio RM, Stachel SJ, Baeschlin DK, Danishefsky SJ (2001) Concise asymmetric syntheses of radicicol and monocillin Ӏ. J Am Chem Soc 123:10903
Sellès P, Lett R (2002) Convergent stereospecific synthesis of C292 (or LL-Z1640-2) and hypothemycin. Part 1. Tetrahedron Lett 43:4621
Sellès P, Lett R (2002) Convergent stereospecific synthesis of LL-Z1640-2 (or C292), hypothemycin and related macroliodes. Part 2. Tetrahedron Lett 43:4627
Dakas PY, Jogireddy R, Valot G, Barluenga S, Winssinger N (2009) Divergent syntheses of resorcylic acid lactones: L-783277, LL-Z1640-2, and hypothemycin. Chem Eur J 15:1149
Geng X, Danishefsky SJ (2004) Total synthesis of aigialomycin D. Org Lett 6:413
Barluenga S, Dakas PY, Ferandin Y, Meijer L, Winssinger N (2006) Modular asymmetric synthesis of aigialomycin D, a kinase-inhibitory scaffold. Angew Chem Int Ed 45:3951
Lu J, Ma J, Xie X, Chen B, She X, Pan X (2006) Enantioselective total synthesis of aigialomycin D. Tetrahedron: Asymm 17:1066
Baird LJ, Timmer MSM, Teesdale-Spittle PH, Harvey JE (2009) Total synthesis of aigialomycin D using a ramberg-bäcklund/RCM strategy. J Org Chem 74:2271
Barluenga S, Lopez P, Moulin E, Winssinger N (2004) Modular asymmetric synthesis of pochonin C. Angew Chem Int Ed 43:3467
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2013 Springer-Verlag Wien
About this chapter
Cite this chapter
Bräse, S. et al. (2013). Resorcylic Acid Lactones. In: The Chemistry of Mycotoxins. Progress in the Chemistry of Organic Natural Products, vol 97. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1312-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1312-7_9
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1311-0
Online ISBN: 978-3-7091-1312-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)