Abstract
Bats are the only mammals with the capacity of powered flight. Nearly 1,000 species can be found all over the world except in the northern and southern polar areas. They perform important ecosystem services such as control of insects, reseeding of cut forests and pollination of plants, which provide food for humans and animals. On the other side, they are also recognized to be natural reservoir hosts of a large variety of zoonotic diseases with the ability to cross species barriers. To date, more than 80 virus species of different groups and various parasites, which can cause several diseases have been isolated or detected in bats. Especially their high population density and gregarious roosting behaviour increase the likelihood of intra- and inter-species transmission of infections. Another important factor, which enables pathogens to spread long distances, is the migratory habit of some bat species, resulting in a great dispersal capacity. The transmission of pathogens from bats to humans or other animals occurs by direct contact with infected animals, their blood and tissue or through vector species. One of the most important vector groups are insects. With more than a million described species, they are the most diverse group of animals. Especially haematophagous groups such as Cimicidae, Culicidae or Phlebotominae are known as vectors for a variety of diseases. These include bacteria, protozoan and metazoan parasites as well as viruses. We focused on blood-feeding insects, because the presence of certain viruses in them as well as in bats comprises a potential virus transmission from bats to humans through mosquitoes or other blood-feeding insects. For this chapter, we could find 20 viruses from four different families and two parasitic pathogens detected in all three groups of haematophagous insects.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Bats (order Chiroptera) are one of the most diverse, abundant and widely distributed groups of mammals and the only one with the capacity of powered flight (Li et al. 2010; Omatsu et al. 2007). Nearly 1,000 species are found worldwide, except in the northern and southern polar areas, representing approximately 20 % of all mammalian species (Omatsu et al. 2007; Teeling et al. 2005). Chiroptera can be divided into the two suborders Megachiroptera (old world fruit bats) and Microchiroptera (echolocating bats) (Jones et al. 2002). Analysis of 17 nuclear genes dated the origin of chiropterans up to 50 million years ago (Calisher et al. 2006). One major lineage of Microchiroptera was traced to Laurasia and one to Gondwana (Teeling et al. 2005). Derived ancient origins for certain zoonotic viruses in bats, such as lyssa and henipa viruses, suggest a long history of coevolution and cospeciation (Calisher et al. 2006). On the one hand, bats perform important ecosystem services, such as control of insects (Reiskind and Wund 2009, 2010; Rydell et al. 2002), reseeding of cut forests and pollination of plants that provide food for humans and animals. On the other hand, bats are recognized to be a natural reservoir of a large variety of zoonotic viruses, which can cross species barriers to infect humans and other domestic or wild animals (Li et al. 2010). To date, more than 80 virus species of different groups have been isolated or detected in bats. Bats have a great dispersal capacity and the migratory habits of some species provide a good opportunity for pathogens to spread long distances (Messenger et al. 2003). Further, different migration patterns can often be found within one species. These different patterns may allow the exchange of viruses or virus variants between subpopulations as well as members of other species, because several species may roost in the same place. Also, their high population densities and gregarious roosting behaviour increase the likelihood of intra- and inter-species transmission of viral infections (Calisher et al. 2006). The extreme longevity of bats may help to maintain the viruses, resulting in chronic infections, and increase the chances of transmission to other mammals or vertebrates. Calisher et al. (2006) suggest an explanation why some viruses, which are deadly for humans and other mammals, can persist in bats without proving being fatal: because bats form a very ancient lineage amongst the mammals, it is possible that their immune system with its innate acquired immune responses may differ significantly from those of other mammals. So far very little is known about bat immune systems, although some studies indicate similarities between bats immune responses and those of other mammals (Chakravarty and Sarkar 1994; McMurray et al. 1982; Sarkar and Chakravarty 1991). The studies of Halpin et al. (2000), Lau et al. (2005) and Leroy et al. (2005) show the occurrence of virus-specific B- and T-cell responses despite persistent virus infection. One possible pathway of virus transmission involves the animal’s ecology. Due to their flying habits, bats are constrained by the aerodynamics of flight and cannot therefore ingest huge amounts of food. Instead of swallowing whole fruits, bats chew these to extract sugars and other substances. The partially digested fruit is spat out and falls to the ground, where these remnants are fed on by other animals. Residual virus particles in the bat saliva on the fruit remnants may cause infection of the latter animals. Heavier body parts of insect prey are discarded in the same way and are also eaten by terrestrial foraging species (Dobson 2005).
Insects comprise the most diverse group of animals with more than a million described species. The estimated number of extant species is between four and six million (Chapman 2009; Novotny et al. 2002). Insects can be found in nearly all terrestrial environments. Some species have become specialized feeders on blood. Depending on the species, this haematophagous behaviour can be observed either in both males and females or just in females. In the latter case, blood proteins are essential for egg production. All haematophagous insects use modified extremities of the head and extensions of the head capsule as piercing-sucking mouthparts to obtain and feed on blood (Krenn and Aspöck 2012; Lehane 2005). Convergent evolution has led to the development of piercing proboscides in various haematophagous insect groups (Krenn and Aspöck 2010, 2012).
One group of haematophagous insects with a worldwide distribution is the family Cimicidae (bed and bat bugs), which contains more than 100 species. Balvín (2008) mentions that bats are generally considered to be the original hosts of the family. The same had long been thought about the bedbug Cimex lectularius (e.g. Sailer 1952; Usinger 1966), but new results suggest an early sympatric speciation on humans and bats for different populations of bed bugs (Balvín et al. 2012). Although various pathogens have been identified from Cimicidae (e.g. Burton 1963; Delaunay et al. 2011; Goddard and deShazo 2009), their role as vectors is still unclear.
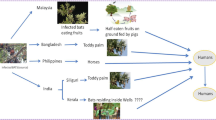
Mosquitoes (Diptera: Culicidae) are a second group of blood-sucking insects. They are regarded worldwide as the major vectors of vector-borne diseases. Especially, species of the genus Anopheles, which are well known as vectors of malaria, can also impact human and animal health by their ability to transmit arboviruses (arthropod-borne viruses) as well as filarial parasites such as the elephantiasis-causing Wuchereria bancrofti and Brugia malayi (Gillies and Coetzee 1987; Sallum et al. 2000; Service 1993). Apart from Anopheles spp., numerous other species of mosquitoes are pests or vectors of pathogens that cause diseases in humans and animals (Reinert et al. 2004). Because of their adaptive abilities, mosquitoes are capable of inhabiting and surviving in a wide range of habitats. Worldwide, they colonize nearly every aquatic habitat. As a result of their large flight range, some floodwater mosquito species can become pests even in places located far away from their breeding sites (Mohrig 1969; Schäfer et al. 1997). Additionally, flood plains along coastal areas as well as tree holes are used as breeding sites by certain species. Also impressive is the adaptive capacity of mosquitoes to extremes of, or changes in, climatic factors. This ecological flexibility is one of the reasons for the success of mosquitoes (Becker et al. 2010). With the exception of the tropical genus Toxorhynchites, in which both sexes subsist on carbohydrate-rich materials such as honeydew, nectar and plant fluids (Snodgrass 1959), the females consume blood, e.g. to obtain proteins necessary for egg production. The host is located mainly by their olfactory senses, by the odour of carbon dioxide or the use of visual contact (Becker et al. 2010). While some mosquito species have specialized on birds, amphibians and other animal groups, others feed on mammals. Especially species with the last feeding habits, or possibly hybrids between different species, can be responsible for zoonoses, because they act as vectors between reservoir hosts and humans. Phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae) are small (ca. 3 mm) nematoceran dipterans (Ready 2013) and a third group of haematophagous insects. They are mainly distributed in the tropics, but there is also an important Palaearctic element (Lewis 1974). The group acts as vector of leishmaniasis, Bartonella bacilliformis as well as some arboviruses of the three different genera: Phlebovirus (family Bunyaviridae), Vesiculovirus (family Rhabdoviridae) and Orbivirus (family Reoviridae) (Depaquit et al. 2010). Known disease agents, who can be transmitted by insects and have a potential impact on bats include bacteria, viruses, fungi as well as protozoan and metazoan parasites. With a few exceptions, in this chapter we concentrate on viruses, which have been detected in bats, haematophagous insects and humans (short information see Table 3.1).
3.2 Pathogens in Bats, Humans and Haematophagic Insects
The most important pathogens causing viral infections in bats, humans and haematophagic insects belong to the families Togaviridae (genera Alphavirus and Rubivirus), Flaviviridae (genus Flavivirus), Bunyaviridae (genera Orthobunyavirus and Phlebovirus) and Arenaviridae (genus Arenavirus).
3.2.1 Bunyaviridae
The Bunyaviridae is the largest family of RNA viruses with over 300 serologically or molecular-genetically distinguishable strains (Elliott 1997; Soldan and González-Scarano 2005). It was characterized in 1975 (Soldan and González-Scarano 2005) and is now considered to contain five genera: Tospovirus (the only plant-infecting viruses in the group), Hantavirus, Nairovirus, Phlebovirus and Orthobunyavirus (Bowen et al. 2001; LeDuc and Kahlon 2012; Weidmann et al. 2003). The medically most important pathogens within this family are not only transmitted through the bite of infected mosquitoes but also by sand flies or possibly by bedbugs (Darai et al. 2011; Elliott and Blakqori 2011). The largest of the five genera is the genus Orthobunyavirus, containing 174 known viruses (Elliott and Blakqori 2011). Within this group, up to half of the potential 60 Bunyaviridae viruses that cause disease in humans belong to Orthobunyavirus (Soldan and González-Scarano 2005) including the prototype bunyavirus, Bunyamwera virus. It was first isolated in 1943 from Aedes mosquitoes in Uganda and gave its name to the family Bunyaviridae and the genus Bunyavirus. In 2005, Bunyavirus was renamed Orthobunyavirus (Bowen et al. 2001; Elliott and Blakqori 2011). The first virus within this genus is the Bwamba virus (BWAV), which is a member of the Bwamba serogroup (Lambert and Lanciotti 2008). It is transmitted by mosquitoes including Aedes furcifer, Anopheles coustani, Anopheles funestus, Anopheles gambiae and Mansonia uniformis (Lee et al. 1974; Lutwama et al. 2002). According to Gonzales and Georges (1988), the principal anthropophilic vector species are An. funestus and An. gambiae. The first encounter with Bwamba fever was in 1937 among construction workers in Western Uganda (Smithburn et al. 1941). Today, Bwamba virus is endemic in Nigeria, Cameroon, Central African Republic, Kenya, Tanzania and South Africa, but due to the mild symptoms it is often mistaken for malaria (Lutwama et al. 2002; Moore et al. 1975; Smithburn et al. 1941). In a laboratory study by Reagan et al. (1955), the cave bat Myotis lucifugus was successfully infected after intraperitoneal, intradermal, intracerebral and intrarectal injection of the virus, although the bats were not susceptible to the virus after intranasal exposure. The second virus is the Kaeng Khoi virus (KKV), which was first isolated in Thailand in 1969 from the bat species Chaerephon plicata (wrinkle-lipped bat) and Taphozous theobaldi (Theobald’s bat). Both species can be found across the Indian subcontinent and Southeast Asia. (Hutson et al. 2001) In 1976 and 2003, the virus was found again in C. plicata in Thailand and for the first time in Cambodia (Osborne et al. 2003; Williams et al. 1976). Apart from bats, Williams et al. (1976) found the virus also in bedbugs (Stricticimex parvus and Cimex insuetus) that inhabit caves together with other haematophagous arthropods, which attack humans. The virus might be a public health concern, because serum analysis found neutralizing antibody in 29 % of the population. The symptoms of the virus in bats and humans are unknown, but a survey of the population indicated that they believe that bedbug bites were the cause of an influenza-like illness, which is typical of infection by members of Orthobunyavirus (Osborne et al. 2003). Also known to occur in bats and humans are the Guamá and Catú viruses, which are members of the Guamá serotype group and are transmitted by species of Culex mosquitoes (Darai et al. 2011; Löscher and Burchard 2008). Both were isolated from humans and mosquitoes in the Amazon area (Causey et al. 1961) and Catú virus also from humans in Trinidad (Tikasingh et al. 1974). However, Catú virus was also isolated in Brazil from the bat Molossus currentium and Guamá virus from an unidentified bat (Calisher et al. 2006; Karabatsos 1985). The symptoms of both viruses show considerable variation such as fever, headache, general body pains, weakness or dizziness and photophobia (Causey et al. 1961).
Another group of viruses that may be transmitted by bats is the genus Phlebovirus, including nine species with 37 viruses (Bouloy 2011), distributed in Africa, Asia, North and South America and the Mediterranean region (McMullan et al. 2012). Many phleboviruses are transmitted by sandflies or other arthropods such as mosquitoes or ticks. The first pathogen in this genus that has to be considered in connection with bats is the Rift Valley fever virus (RVFV). It is primarily transmitted by mosquitoes of the genus Aedes (e.g. Ae. cumminsii, Ae. circumluteolus, Ae. mcintoshi or Ae. vexans) or Culex (e.g. Cx. pipiens, Cx. tritaeniorhynchus or Cx. neavei), but it has been shown that sandflies (Phlebotomus duboscqi and P. papatasi) might also be potential vectors (Dohm et al. 2000; Fontenille et al. 1998; Pepin et al. 2010). RVFV can be transmitted into mosquito offspring transovarially (Ikegami and Makino 2011). Outbreaks of RVF are associated with heavy rainfalls during the El Niño/Southern Oscillation (ENSO) phenomenon (Miller et al. 2002; WHO 2013a) because the floods create optimal breeding conditions (Bowen et al. 2001; Fontenille et al. 1998; Woods et al. 2002). After the first identification of the virus in the Rift Valley of Kenya in 1930, numerous outbreaks of RVF have been reported in many regions of Africa (Fig. 3.1a) (Daubney and Hudson 1931; Fontenille et al. 1998). The largest occurred in Egypt in 1977–1978 with 200,000 estimated human infections, 18,000 cases of illness and 600 deaths (Ikegami 2012). The first detected outbreak of RVF outside the African continent was in 2000 in Saudi Arabia and Yemen. The latest outbreak of RVF was 2012 in Mauritania with 34 cases and 17 deaths reported (WHO 2012). The clinical symptoms range from flu-like fever, muscle pain or headache to neck stiffness, retinal lesions, loss of memory and even death (Ikegami 2012; WHO 2013b). However, RVF occurs not only in humans. Outbreaks can also result in devastating economic losses when livestock is infected (Woods et al. 2002). In 1991, the virus was also isolated from bats in West Africa (Fontenille et al. 1998). Calisher et al. (2006) suggest the bat species Micropteropus pusillus, Epomops franqueti, Hipposideros abae, H. caffer, Miniopterus schreibersii and Glauconycteris argentata as potential hosts for the RVFV. They are all distributed in West, East and Central Africa except for M. schreibersii, which occurs primarily in southern and middle Europe (e.g. Portugal, Italy (Sardinia, Sicily), Turkey, Switzerland and Serbia) (IUCN red list 2012). The effect on potential vectors (e.g. Aedes vexans) of climate changes may enable this bat to spread the virus to European countries. The second pathogen within the Phlebovirus genus that has been linked with bats is the Toscana virus, a serotype of Sand fly fever distributed in the Mediterranean region (Fig. 3.1b) (Charrel et al. 2005; Cusi et al. 2010). In 1971, the Toscana virus was isolated for the first time from the sand fly Phlebotomus perniciosus in Monte Argentario, Toscana (Braito et al. 1997; Cusi et al. 2010; Valassina et al. 2003; WHO 2004). Later the virus was also isolated from the sand fly P. perfiliewi (Valassina et al. 2003). However, P. perniciosus is probably the most common insect vector of the Toscana virus, being one of the most abundant sand flies in southern Europe (Maroli et al. 1994; Sanbonmatsu-Gámez et al. 2005). With tourists, returning from Mediterranean countries, the virus has been imported to further European countries such as Germany, Sweden and Switzerland (Cusi et al. 2010). The only known vertebrate hosts beside humans are bats. Accordingly, the virus has been isolated from the bat Pipistrellus kuhlii in regions where the insect vectors were present (Charrel et al. 2005; Valassina et al. 2003). The role of bats as virus reservoirs is not yet definitely clarified (Valassina et al. 2003). Most infections are recorded during summertime and cause high fever, headaches, muscle aches, neck stiffness and aseptic meningitis with a non-fatal course or a mild meningoencephalitis, but asymptomatic infections are also possible (Baldelli et al. 2004; Braito et al. 1997; Hemmersbach-Miller et al. 2004). Studies in central Italy showed that 52 % of aseptic meningitis cases were caused by Toscana virus (Charrel et al. 2005). Studies of Sanbonmatsu-Gámez et al. (2005) in Spain, where 979 persons were probed for their seroprevalence, indicated that Toscana virus occurs more often in rural areas (26.7 %) than in urban (20.6 %).
Virus distribution (a) Rift Valley fever: dark orange (do)—epizootic and epidemic areas; light orange (lo)—serological evidence or virus isolation (Ikegami 2012). (b) Toscana virus: do—native infections; middle orange (mo)—imported cases; lo—seropositive cases in population (Cusi et al. 2010). (c) Chikungunya virus: do—endemic or epidemic areas; lo—imported cases (Powers and Logue 2007). (d) Eastern equine encephalitis: reported cases between 1964 and 2010 (CDC 2010). (e) Venezuelan equine encephalitis: major outbreaks regions (Weaver et al. 2004)
3.2.2 Togaviridae
Within this second large virus family, the two genera Alphavirus and Rubivirus are known (Rolle and Mayr 2007). It can be characterized as a group of positive stranded RNA viruses, which possess a cubically symmetric capsid. All vector-borne togaviruses, which are medically important, belong to Alphavirus that contains 26 different viruses (Laine et al. 2004). The first of five viruses associated with bats, human and haematophagous insects is the Western equine encephalitis virus, which is eponymous for the western equine encephalitis antigenic complex and an important pathogen not only in human but also in veterinary medicine (Reisler et al. 2012). It was isolated in 1930 from a horse brain although epidemics had already been described in 1912 and 1919 (Johnson 1964). The natural vertebrate hosts of WEEV are passerine and domestic birds (Eklund 1954) where it maintains an enzootic cycle with the mosquito vectors. The main vector is Culex tarsalis, of which hybrid strains have evolved that are highly resistant to WEEV infection (Hardy et al. 1978). Other vectors are Aedes campestris, Ae. dorsalis and Ochlerotatus melanimon (Zacks and Paessler 2010). In early experiments, transmission could also be shown in Ae. aegypti (Kelser 1933; Merrill and Ten Broeck 1935; Merrill et al. 1934), Ae. albopictus (Simmons et al. 1936), Ae. nigromaculis (Madsen and Knowlton 1935; Madsen et al. 1936), Ae. sollicitans (Merrill et al. 1934), Ae. taeniorhynchus (Kelser 1937, 1938) and Ae. vexans (Kelser 1937, 1938; Knowlton and Rowe 1935). Culiseta spp. is mentioned as vector by Whitley and Gnann (2002) without mentioning the species or the source of the information.
The connection between Western equine encephalitis and human disease was proven in 1938, when the virus was recovered from a child’s brain that died of encephalitis (Griffin 2001). Nevertheless in North America, WEEV is a rare cause of encephalitis and only seven cases were reported from 1987 to 2002 (CDC 2002; Romero and Newland 2003). The virus has caused encephalitis epidemics in emus, humans and horses with fatality rates of 10 % for emus and humans and 20–40 % for horses (Ayers et al. 1994; Nalca et al. 2003). The patients suffer a 2- or 3-day period with fever, headache, vomiting, nausea, somnolence and irritability before manifestations in the CNS begin (Nalca et al. 2003). Constantine (1970) mentions that WEEV has been isolated from bats and Western equine encephalitis N antibodies were detected in Artibeus jamaicensis from Haiti (McLean et al. 1979) and Artibeus lituratus from Tikal, Guatemala (Ubico and McLean 1995). The latter authors postulate that bats may become infected, especially during epizootics in other hosts. The prototype virus of the genus Alphavirus is the Sindbis virus (SINV), which belongs together with the Highland J, Fort Morgan, Buggy Creek and Aura virus to the Western equine encephalomyelitis antigenic complex (Hubálek 2008; Lundström and Pfeffer 2010; Netolitzky et al. 2000). It was first recognized and isolated from Culex pipiens and Cx. univittatus in 1952 in Egypt (Jöst et al. 2010; Kurkela et al. 2008; Laine et al. 2004). The first record of Sindbis virus in European countries was in 1975 (Laine et al. 2004). Today, Sindbis virus is one of the most widely distributed viruses, having been isolated in Europe (e.g. Sweden, Finland and Italy (Sicily)), Africa (e.g. Egypt, Kenya and South Africa), Asia (e.g. China, Malaysia and Lebanon) and Australia (Lundström and Pfeffer 2010; Norder et al. 1996; Tesh 1982). Nucleotide sequencing of SINV strains isolated around the globe has shown that the majority of SINV strains are geographically distinct genotypes and that migrating birds may carry the virus over long distances (Modlmaier et al. 2002; Strauss and Strauss 1994). Together with a wide distribution, the virus also has a broad host range and has been isolated from frogs, ticks, humans and numerous mosquito species (Kurkela et al. 2008; Modlmaier et al. 2002). Furthermore, Blackburn et al. (1982) isolated the virus from the organs of the Microchiropteran bats Hipposideros spp. and Rhinolophus spp. Vectors of SINV are ornithophilic mosquitoes Cx. torrentium or Cx. pipiens, Culiseta morsitans and Ochlerotatus spp. or Aedes spp. (Hubálek 2008; Jöst et al. 2010). The first description of symptoms caused by Sindbis virus infection is from Uganda in 1961 (Laine et al. 2004). They range from itchy exanthema, mild fever and joint pain in wrists, hips, knees and ankle to nausea, headache and muscle pain (Kurkela et al. 2005). Together with other mosquito-borne diseases like the Ross River virus, Mayaro-, onyong-nyong-, Bebaru-, Getah and Semliki forest virus, the Chikungunya virus is a member of the Semliki forest antigenic complex (Burt et al. 2012). The first isolation of the virus from an infected patient was carried out by Ross in 1952/1953 during an outbreak in Tanzania (Diallo et al. 1999; Tesh 1982; Tiawsirisup 2011). Today, the geographic distribution of this enzootic virus includes the tropical and subtropical areas of Africa, and southern or southeast Asia, including India, Sri Lanka, Myanmar, Thailand, Indonesia, Malaysia and the Philippines (Fig. 3.1c) (Burt et al. 2012; Krishna et al. 2006; Sam et al. 2006). The virus is transmitted by Aedes spp. mosquitoes and exhibits two different transmission cycles. Aedes aegypti as well as Ae. albopictus are the main vectors in Asia and transmit the virus to humans directly in an urban transmission cycle. Virus isolation from non-human primates or vertebrates like bats in Africa suggests the occurrence of a sylvatic transmission cycle. The main vectors of CHIKV in Africa are Ae. furcifer-taylori, Ae. africanus, Ae. luteocephalus and Ae. aegypti (Higgs 2006; Krishna et al. 2006). In the last 50 years, numerous outbreaks of CHIKV were reported (Schwartz and Albert 2010). One of the last large-scale epidemics began in 2004 in Kenya and spread to several islands in the Indian Ocean (Powers and Logue 2007). On La Réunion, nearly 34 % of the total island population was infected and 237 people died (Schwartz and Albert 2010; Tsetsarkin et al. 2007). It was a noteworthy outbreak because Ae. albopictus was recognized as the major vector for the first time (Reiter et al. 2006). In July to September 2007, the first autochthonous epidemic outbreak of CHIKV with 248 cases was reported in Italy. The vector responsible for this outbreak was also Ae. albopictus (Rezza et al. 2007; Sambri et al. 2008). An infection with CHIKV can cause acute, subacute and chronic diseases. Especially in areas that also suffer outbreaks of Dengue virus, CHIKV can easily be mistaken for Dengue and both viruses can occur in one patient. Dengue has much more potential for causing infections with serious outcomes (PAHO 2011; Tiawsirisup 2011). An acute disease is characterized by fever and joint pain, while other symptoms may include headache, myalgia, nausea, vomiting, polyarthritis, rash and conjunctivitis (PAHO 2011). Apart from humans, bats can also be carriers of the virus. Diallo et al. (1999) isolated it from bats of the genus Scotophilus sp. in Senegal. Other surveys suggest Rousettus aegyptiacus (Egyptian rousette), Hipposideros caffer (Sundevall’s leaf-nosed bat) as well as Chaerephon pumilus (little free-tailed bat) to be vertebrate hosts for the virus (Calisher et al. 2006). Ross River virus (RRV) causes a disease known as epidemic polyarthritis, which is regarded with 2,000–8,000 notified cases per annum as the most common cause of arboviral disease in humans in Australia (Russell 2002; Smith et al. 2011). The first reported outbreak of an infection with RRV was in 1928 during epidemics in New South Wales (Mackenzie et al. 1994; Russell 2002). Today, human infections are also documented for New Guinea, Solomon Islands, Fiji and American Samoa (Meyer 2007; Tesh 1982). It is believed that the virus was introduced to these islands by viraemic air travellers from Australia (Smith et al. 2011). The first isolation of the virus from a mosquito (Ae. vigilax) was by Doherty et al. in 1959 (Harley et al. 2001). In 1979, the virus was isolated for the first time from the serum of a patient with epidemic polyarthritis (Mackenzie et al. 1994). The virus was also isolated from a Pteropus bat in Australia (Doherty et al. 1966; Messenger et al. 2003). Serological surveys and virus isolation by Harley et al. (2000) from mosquitoes trapped near a flying fox camp suggested the flying fox Pteropus conspicillatus as a potential reservoir host. These authors also suggest that other vertebrates living in the flying fox camp could also be important reservoir hosts (Harley et al. 2000, 2001). On the other hand, Ryan et al. (1997) showed that the grey-headed flying fox (Pteropus poliocephalus) does not produce a viraemia of sufficient magnitude to be very competent vertebrate hosts of RRV (Ryan et al. 1997). Within 9 years (1991–2000), more than 47,000 laboratory-notified cases were reported by national authorities (Russell 2002). Typical symptoms are various combinations of arthralgia and arthritis, muscle and joint pains, myalgia, lethargy, headache or fever. To return to full physical activity, most of the patients need up to 6 months (Mackenzie et al. 1994; Smith et al. 2011; Weinstein et al. 2011). So far, RRV has been isolated from 27 mosquito species. In Australia, these include the major vectors Aedes vigilax, Ae. camptorhynchus and Cx. annulirostris (Harley et al. 2000; Hu et al. 2006; Mackenzie et al. 1994). There is also evidence that Ae. aegypti can be infected with and transmit the virus, but it has not been isolated from this species in the field (Harley et al. 2000). The Eastern equine encephalitis virus (EEEV) is placed as the only species in the Eastern equine encephalitis complex (EEE) and is distributed in North America (Fig. 3.1d) (Calisher et al. 1980). Originally, it was divided into North and South American varieties based on antigenic properties (Casals 1964). Following further antigenic studies four different subtypes have been distinguished, which correspond to four genetic lineages (I–IV) (Arrigo et al. 2010; Brault et al. 1999). EEEV was first recognized as a horse disease in the northeastern USA in 1831 (Hanson 1957; Nalca et al. 2003; Scott and Weaver 1989). The most severe outbreak of EEEV was recorded from Texas and Louisiana in 1947, causing 14,344 cases with 11,722 horse deaths (Chang and Trent 1987; Nalca et al. 2003). However, suspected EEEV could not be linked to humans till an outbreak in 1938 (Fothergill et al. 1938; Getting 1941). In humans, the virus causes severe meningoencephalitis, sometimes causing focal brain lesions. Morse et al. (1992) report the fatality rate in humans of all ages as 30 %, and in affected children up to 75 %. A prodrome of fever, headache, confusion, lethargy, myalgias, vomiting and abdominal pain, which lasts 1–3 weeks, precedes the onset of neurologic symptoms. The onset of illness is characterized by fever, altered mental condition, seizures, vomiting and cyanosis (Deresiewicz et al. 1997; Hart et al. 1964; Romero and Newland 2003). In a study made in Alabama, several mosquito species were found to be carriers of the virus: Culiseta melanura, Aedes vexans, Coquillettidia perturbans, Culex erraticus and Uranotaenia sapphirina. Interestingly, these species were infected at different times: Aedes vexans at the beginning of the season; Cx. erraticus and Cs. melanura from June till mid-September (Cupp et al. 2003). In most publications, the ornithophilic mosquito Cs. melanura is mentioned as the main vector of EEEV, but Cx. erraticus is an important bridge vector between birds and mammals in the mid-south USA, because of frequent virus isolations and abundance of this mosquito species in bottomland swamps, flood plains, permanent standing water, recreation areas near rivers or ponds and water impoundments (Jacob et al. 2010). However, EEEV has been isolated from a wide range of species of Aedes, Anopheles, Coquillettidia, Culex, Ochlerotatus and Uranotaenia, though not all of the species involved can be regarded as competent vectors (more specific e.g. in Armstrong and Andreadis 2010; Arrigo et al. 2010). In surveying for the natural vector of EEEV, Merrill et al. (1934) mention Ae. cantator and even more A. sollicitans and A. aegypti. Regarding the latter species, they conclude with Kelser (1933) that it could not be the transmitting species at it did not occur sufficiently far northwards. Generally, epizootics of EEE occur every 5–10 years and are associated with increased mosquito populations resulting from heavy rainfall and warm weather (Grady et al. 1978; Letson et al. 1993; Mahmood and Crans 1998; Nalca et al. 2003). Although birds seem to be the main reservoir hosts of EEEV, the virus has also been detected in bats. During 1969 and 1970, it was found in ten bat individuals caught in the wild in New Jersey, belonging to three species: one Lasiurus cinereus, two Myotis lucifugus and seven Eptesicus fuscus (Main 1979a). Antibodies have been detected in the previously mentioned species, Tadarida brasiliensis and an unidentified bat (Daniels et al. 1960; Hayes et al. 1964; Karstad and Hanson 1958). The survey by Main (1979a) showed that EEE neutralizing antibodies were detectable in a small percentage of the tested adult hibernating animals (0.3 % of Myotis keenii) but significantly more in non-hibernating animals (3.4 % in M. lucifugus, M. keenii and E. fuscus). In experimentally infected bats, the virus could be detected in the blood, mammary glands, brown fat, lung, kidney, brain, pancreas, heart, salivary glands, liver and ovary, with the highest percentage in blood and mammary glands (Main 1979b). In a serological survey of Guatemalan bats, antibodies neutralizing EEEV were found in Artibeus intermedius, A. jamaicensis, A. lituratus, Glossophaga soricina, Rhynchonycteris naso and Sturnira lilium (Ubico and McLean 1995). The Venezuelan equine encephalitis virus (VEEV) belongs to the VEE complex, which consists of six subtypes which have been identified in North, Central and South America (Fig. 3.1e) (Fine et al. 2007; Weaver et al. 1992). The first isolation of VEEV was in 1938 from the brain of a Venezuelan animal (Beck and Wyckhoff 1938). It is the most important pathogen among the New World alphaviruses affecting humans and horses. It not only remains a naturally emerging disease but is also a highly developed biological weapon (Colpitts et al. 2007), because it can be also spread by aerosol infection. Since 1938, sporadic outbreaks have involved hundreds of thousands of people (Weaver et al. 2004). During an epidemic in Colombia, more than 75,000 human cases were reported (Rivas et al. 1997). The symptoms in humans include malaise, sore throats, headaches, fever and chills, persisting for 4–6 days and followed by 2 or 3 weeks of generalized weakness. Encephalitis occurs mainly in children (in about 4 % of cases). Other symptoms range from mild nausea, vomiting with decreased sensorial capability, nuchal rigidity, ataxia and convulsions, to coma and paralysis. The fatality rate in humans is about 1 % (Johnson and Martin 1974; Johnson et al. 1968; Nalca et al. 2003; Pittman et al. 1996). Currently, no vaccine for VEEV is publicly available and the experimental military vaccine has poor efficacy (Colpitts et al. 2007; Russell 1999). In nature, VEEV is maintained in a cycle between mosquitoes and small rodents (Grayson and Galindo 1968; Nalca et al. 2003; Scherer et al. 1972). Epidemic outbreaks of the epizootic strains occur in 10–20-year intervals in the ranch areas in Peru, Venezuela, Colombia and Ecuador after heavy rainfall, which lead to increased mosquito populations (Rivas et al. 1997). Enzootic strains are transmitted by Culex species, whereas the main vector of the epizootic strains seems to be Ochlerotatus taeniorhynchus and possibly also Psorophora confinnis, but nearly all mosquito species have been found to be infected during epizootics (Rivas et al. 1997; Smith et al. 2008; Zacks and Paessler 2010). Weaver et al. (1992) list Culex cedeci, Cx. ocossa, Cx. panocossa, Cx. portesi, Cx. taeniopus and cliff swallow bugs Oeciacus vicarius as vectors. Other natural enzootic vectors are Cx. accelerans, Cx. adamesi, Cx. amazonicus, Cx. ferreri, Cx. nigripalpus, Cx. pedroi, Cx. spissipes, Cx. vomerifer, Aedes serratus and Mansonia titillans (Ferro et al. 2003). In the laboratory, Aedes aegypti and Culex aikenii could additionally be infected (Kramer and Scherer 1976; Sudia et al. 1971). By testing haemagglutination inhibition antibody titres in Guatemalan bats, antibodies against enzootic VEEV strains were detected in seven bat species: Artibeus jamaicensis, A. lituratus, A. phaeotis, Desmodus rotundus, Glossophaga commissarisi, Myotis nigricans and Uroderma bilobatum, and it was noted that the insectivorous M. nigricans may become infected by eating infected mosquitoes and the vampire bat D. rotundus by feeding on viraemic cattle (Seymour et al. 1978a). Experimental infection studies showed that bat genera respond differently to infection, e.g. Artibeus jamaicensis and A. lituratus showed longer VEEV viraemias than Phyllostomus discolor, which had a faster, higher and longer lasting immune response to epizootic strains than to enzootic ones. Phyllostomus discolor may not serve as a host for enzootic VEEV strains, while the circulating virus levels in Artibeus jamaicensis, A. lituratus and Sturnira lilium seemed to be high enough to permit the infection of Culex vectors (Seymour et al. 1978b).
3.2.3 Flaviviridae : Flavivirus
The family Flaviviridae can be divided into the three genera Pestivirus, Hepacivirus and Flavivirus (Cook and Holmes 2006). The last named contains the largest number of viruses with the potential to cause diseases in bats and humans and may be spread by haematophagous insects. Flavivirus currently consists of 70 serologically related, single-strand, positive-sense RNA viruses (Hoshino et al. 2009; Mackenzie and Williams 2008; Tajima et al. 2005). All members of the genus can be found around the world and are most often transmitted by arthropods. The most important mosquito-borne diseases caused by flaviviruses are Yellow fever, Japanese Encephalitis, West Nile fever, Dengue fever and St. Louis encephalitis (Mackenzie and Williams 2008). Yellow fever is an acute haemorrhagic disease and is endemic in tropical areas of Africa and Latin America with an incidence of 200,000 cases and 30,000 deaths each year (Fig. 3.2b). The symptoms range from mild to severe illness (WHO 2000). Yellow fever transmission depends on arthropods as vectors, e.g. several mosquito (Culicidae) species belonging to Aedes in Africa and Haemogogus in South America (Ellis and Barrett 2008; WHO 2000). As long ago as the first half of the twentieth century, scientists tried to detect a connection between the yellow fever virus and bats. Williams et al. (1964) emphasized the potential and importance of bats in the wild cycle of arboviruses, e.g. yellow fever virus. In the experiments of Kumm (1932), Brazilian bats (Molossus molossus obscurus and M. rufus) were exposed to unfed Aedes aegypti. The mosquitoes willingly took blood, but no transmission of the disease from the infected mosquitoes to the bats resulted. The author concluded that bats play little part in the life cycle of this disease. Contrastingly, Simpson and O’Sullivan (1968) stated that in East Africa, yellow fever virus circulated in the fruit bat genera Eidolon and Rousettus. However, the virus did not produce demonstrable viraemia in the tested fruit bats (Simpson and O’Sullivan 1968). Marinkelle and Grose (1972) reviewed organisms, which act as vectors between bats, humans and domestic animals. They listed six references involving yellow fever records from two continents.
Occurrence of viruses (a) West Nile virus: detected in human sera or antibodies in birds (Hubálek and Halouzka 1999; Gubler 2007); (b) Yellow fever: dark orange (do)—risk area (Travel approved 2010); (c) Japanese encephalitis virus (CDC 2012; Van der Hurk et al. 2009b); (d) Dengue fever: transmission risk areas (WHO 2009)
Oelofsen and van der Ryst (1999) stated that bats can be infected orally by ingesting a single mosquito and that experiments with bats and yellow fever virus produced positive results. The virus was recovered from several organs of the bats (Oelofsen and van der Ryst 1999). Furthermore, serological prevalence of bats for yellow fever virus had been demonstrated in Uganda, Kenya and Sudan and in previous publications the laboratory capacity had been tested in the genera Eidolon and Rousettus (Ellis and Barrett 2008). The Japanese encephalitis virus (JEV) is eponymous for the JEV antigenic complex. Other members of this group are the West Nile virus, Murray Valley encephalitis virus, St. Louis encephalitis virus and Kunjin virus (Bengis et al. 2004; Campbell et al. 2002). It is the leading cause of viral encephalitis in rural regions of eastern, southeastern and southern Asia (Fig. 3.2c). The central nervous system may be affected, leading to severe complications and even death (Agarwal 2006; Mackenzie et al. 2006). Up to 50,000 cases of JEV are estimated to occur annually worldwide (Ravanini et al. 2012; Van der Hurk et al. 2009a). According to the sequence of its genomic RNA, JEV is classified into five genotypes (Nabeshima et al. 2009; Solomon et al. 2003). The majority of infections are subclinical, but the fatality rate is nearly 25 % (Ravanini et al. 2012; Van der Hurk et al. 2009b). Over the last 60 years, it has been estimated that the virus has been responsible in humans for more than ten million infections, three million deaths and four million cases of long-term disability. It is also calculated that today nearly two billion people live in JEV-prone areas. Historically, epidemics had been recorded in Japan since 1871 (Mackenzie et al. 2006; Van der Hurk et al. 2009a), but the first isolation of JEV in Japan was not until 1935 in Japan (Tiawsisirsup et al. 2012). A sequence closely related to JEV strains from Japan was isolated for the first time in Europe from the mosquito Culex pipiens (Ravanini et al. 2012). Birds and mosquitoes play the major role in the life cycle of JEV, but the virus can also infect a wide range of other vertebrates such as humans, domestic animals, bats, snakes or frogs (Agarwal 2006; Ravanini et al. 2012; Tiawsisirsup et al. 2012). The main vectors seem to be species of Culex mosquitoes such as Cx. pipiens (Korea), Cx. annulirostris (Australia) or Cx. tritaeniorhynchus (Nabeshima et al. 2009; Van der Hurk et al. 2009b), but JEV has also isolated from Cx. gelidus, Cx. vishnui, Cx. fucocephala, Cx. pseudovishnui, Mansonia uniformis, Anopheles subpictus and Ochlerotatus japonicus (Mackenzie et al. 2006; Van der Hurk et al. 2009a). Sulkin et al. (1970) isolated the virus in Japan from the bats Miniopterus schreibersii and Rhinolophus cornutus. Other bats, which have yielded antibodies or JEV, were Myotis mystacinus, Pipistrellus abramus, Plecotus auritus, Vespertilio superans, Myotis macrodactylus and M. nattereri bombinus (Banerjee et al. 1984). In China, JEV has been isolated from Rousettus lechenaultii and Murina aurata (Wang et al. 2009). West Nile virus, the disease causing agent of the West Nile fever, was first discovered in the blood of a native woman of the West Nile district of Uganda in 1937 (Garmendia et al. 2001; George et al. 1984). In South Africa, one of the biggest outbreaks, with nearly 18,000 human infections, was reported in 1974 (Dauphin et al. 2004). Molecular epidemiological survey indicates that WNV spread from Africa to the Mediterranean and southern European regions and then to India as well as Central and South Asia (Fig. 3.2a) (Buckley et al. 2003; Hayes 2006). In 1999, the virus was inadvertently introduced into North America (Pilipski et al. 2004). Up to now, over 12,000 human cases of meningitis or encephalitis and 1,100 deaths caused by WNV have been documented in the USA (Murray et al. 2010). Phylogenetic studies have identified several genetic lineages of the virus in different geographical locations (Campbell et al. 2002; Rappole et al. 2000). West Nile virus is transmitted in natural cycles between birds and mosquitoes (Mackenzie et al. 2004). So far, the virus has been isolated from at least 300 bird and 43 mosquito species from 11 genera. The major vectors for Africa and the Middle East are Culex univittatus, Cx. poicilipes, Cx. neavei or Aedes albocephalus. For Asia, it is Cx. quinquefasciatus, Cx. tritaeniorhynchus and Cx. vishnui. In Europe, Cx. pipiens, Cx. modestus as well as Coquillettidia richardii may act as important vectors. A huge number of infections (nearly 80 % symptomless) occur during summer, early fall and during the rainy season in the tropics (Campbell et al. 2002). The symptoms reach from fever, headache, tiredness or swollen lymph glands to neck stiffness, disorientation, coma and paralysis (Mackenzie et al. 2004; WHO 2011; Zeller and Schuffenecker 2004). The first identification of WNV in Chiroptera was in Rousettus aegyptiacus (fruit bat) in Uganda and Israel. Nearly 8 % of the surveyed R. aegyptiacus in Israel tested positive for WNV antibodies (Bunde et al. 2006). In India, the virus was isolated from Rousettus leschenaultia (Davis et al. 2005; Paul et al. 1970) and in 2,000 antibodies were isolated again from live Eptesicus fuscus (big brown bat) as well as from Myotis lucifugus (little brown bat) in New York City (Bunde et al. 2006). Pilipski et al. (2004) found antibodies again in M. lucifugus and in M. septentrionalis, whereas Davis et al. (2005) determined neutralizing antibodies for WNV from Tadarida brasiliensis. The St. Louis encephalitis virus is the etiological agent of St. Louis encephalitis and is a member of the Japanese encephalitis antigenic complex. It was first detected in 1933 during an outbreak of human encephalitis in St. Louis in the US State Missouri (Auguste et al. 2009; Flores et al. 2010; Rodrigues et al. 2010). Today, the virus is found all over the USA and Canada as well as Central and South America (Diaz et al. 2006; Pires and Gleiser 2010). The largest outbreak of SLEV among humans so far was in 2005 in Argentina (Diaz et al. 2006). The first detection of SLEV in Argentina was in 1957 (Flores et al. 2010). Studies have indicated Cx. tarsalis, Cx. nigripalpus and Cx. quinquefasciatus to be the major vectors in the USA (Reisen 2003). Diaz et al. (2012) isolated the virus from eight different mosquito species: Cx. quinquefasciatus, Cx. interfor, Cx. apicinus, Ae. scapularis, Ae. aegypti, Ae. albifasciatus, An. albitarsis and Ps. ferox. The primary transmission cycle is between mosquitoes and birds, but serological evidence of infection has also been found in horses, cattle and goats (Calisher 1994; Spinsanti et al. 2003). In a survey by Bunde et al. (2006), the bats Eptesicus fuscus (big brown bat) and Myotis lucifugus (little brown bat) were tested positive for SLEV antibodies. An infection with SLEV can cause a slight illness with fever and headache or serious illness with meningoencephalitis and death. Dengue is a viral infection and characterized by symptoms like fever, severe headache, orbital pain and general indisposition and start 5–7 days after infection. Haemorrhages and an increase in vascular permeability are the consequences of the Dengue haemorrhagic fever, which frequently leads to death. There is good evidence that sequential infection with different serotypes increases the risk of developing this dangerous form of dengue fever (Aguilar-Setién et al. 2008; Becker et al. 2010; Kalayanarooj et al. 1997). Dengue infections increased worldwide during the last decades and about one-fifth of the world population lives in Dengue risk zones (Fig. 3.2d) (Thomas et al. 2011). Dengue virus has been detected worldwide in tropical and subtropical regions, especially not only in the Southeast and South Asia but also in Central and South America and with an ongoing transmission risk in Africa (e.g. Chen and Wilson 2005). The first reported cases occurred at the end of the eighteenth century in Asia, Africa and North America, while Dengue haemorrhagic fever first occurred in the 1950s in the Philippines and Thailand (Becker et al. 2010). In the first half of the twentieth century in Europe (Austria, Greece, Italy and Spain), the virus caused epidemics. Primary vectors are species of the mosquito genus Aedes (Aedes aegypti and Ae. albopictus). Hypothetically, insectivore bats might become infected by ingestion of virus-infected mosquitoes, while fructivore species have to be infected by a mosquito bite (Aguilar-Setién et al. 2008). Wong et al. (2007) categorized the risk of bat to human transmission for the families Pteropodidae and Phyllostomidae as low because of low prevalence of pathogens in bats or inefficient vectorial capacity. However, de Thoisy et al. (2009) detected dengue viral RNA in 4 % of Chiroptera samples (Carollia perspicillata) from French Guiana. Platt et al. (2000) detected antibodies against Dengue virus in 22.6 % of examined bats from Costa Rica (n = 53) and 30 % of those from Ecuador (n = 10), mainly not only in bats of the genera Artibeus and Uroderma but also in four species of Molossus. In laboratory experiments, Ae. aegypti from Costa Rica fed on bats (Platt et al. 2000), but Scott (2001) doubted that this feeding is consistent with bat involvement in Dengue transmission and considered that there is no proof of interactions in natural conditions. In Mexico, Dengue virus is transmitted between humans by mosquitoes of the genus Aedes. Bat samples (n = 162) from five families (Emballonuridae, Mormoopidae, Phyllostomidae, Natalidae and Vespertilionidae) contained nine individuals of four species that were seropositive according to ELISA (Artibeus jamaicensis, Myotis nigricans, Pteronotus parnellii and Natalus stramineus). This is the first definite evidence of Dengue virus in Myotis species. These results support the contention that Dengue virus is present in bats from the Pacific and gulf coasts of Mexico (Aguilar-Setién et al. 2008). The first isolation of Ilheus virus (ILHV) was from mosquitoes of the genera Ochlerotatus and Psorophora from Brazil, especially Psorophora ferox, which is considered its main vector (da Silva Azevedo et al. 2010; Laemmert and Hughes 1947). Later it was isolated also from the genera Culex, Haemagogus, Sabethes and Trichoprospon (Venegas et al. 2012). In Brazil, it has been isolated also from birds, sentinel monkeys and horses (Iversson et al. 1993). Only a few reports of isolation from humans are available (Johnson et al. 2007; Spence et al. 1962; Srihongse and Johnson 1967; Venegas et al. 2012). Results of infection are widely variable, ranging from asymptomatic to encephalitis, but most of the cases are accompanied by fever, headache, chills, photophobia, arthralgia, myalgia and asthenia (da Silva Azevedo et al. 2010). Price (1978) found sera in bats from Trinidad that protect against Ilheus. According to da Silva Azevedo et al. (2010), it has been isolated also from bats, but unfortunately no details of which species were involved were mentioned by the authors. Zika virus is known from Africa and Southeast Asia (Dick et al. 1952; Duffy et al. 2009; Hayes 2009). It is related to West Nile, Dengue and Yellow fever viruses (Duffy et al 2009). The first isolation of Zika virus was in 1947 from a rhesus monkey (Simpson 1964) in 1948 was the first isolation from a mosquito (Aedes africanus) and in 1968 from humans in Nigeria (Hayes 2009). Other serological studies showed human Zika virus infection in Africa also e.g. in Senegal, Uganda, Central African Republic and Egypt and for Asia e.g. in India, Malaysia, Vietnam or Indonesia (Duffy et al. 2009; Hayes 2009). Zika virus has been isolated from Ae. aegypti, Ae. africanus, Ae. furcifer, Ae. luteocephalus and Ae. vittifer (Dick 1952; Haddow et al. 1964; Hayes 2009; Lee and Moore 1972; Marchette et al. 1969). So far, no natural infections of bats with Zika virus have been documented, but in the laboratory the cave bat Myotis lucifugus was infected successfully when the virus was injected intraperitoneal, intradermal, intracerebral and intrarectal. But the bats were not susceptible to the virus after intranasal exposure (Reagan et al. 1955). Tacaribe virus (TCRV) belongs to the Arenaviridae (genus Arenavirus) (Bowen et al. 1996; Rossi et al. 1996). Diseases caused by the Tacaribe virus complex of the new world are Argentine haemorrhagic fever, Brazilian haemorrhagic fever, Venezuelan haemorrhagic fever and another yet unnamed haemorrhagic fever, induced by Junín, Sabiá, Guanarito, Machupo and Chapare viruses (Carballal et al. 1987; Cogswell-Hawkinson et al. 2012; Tesh et al. 1994). In contrast to the other arenaviruses, which have all been isolated from rodents, Tacaribe virus was originally isolated from two bat species: great fruit-eating bats (Artibeus lituratus) and Jamaican fruit bats (A. jamaicensis) (Downs et al. 1963; Price 1978). Furthermore, Price (1978) was able to detect antibodies against TCRV in the little yellow-shouldered bat (Sturnira lilium), Heller’s broad-nosed bat (Platyrrhinus helleri) and in the vampire bat (Desmodus rotundus). Nevertheless, the study of Cogswell-Hawkinson et al. (2012) does not support the hypothesis that A. jamaicensis is a natural reservoir host for TCRV, because the injection of high doses resulted in significant and fatal disease including pneumonia, pathological changes in liver and spleen and brain lesions. One study (Downs et al. 1963) however revealed close correspondence between a strain isolated from a mosquito pool and one isolated from a bat. Unfortunately, the pool consisted of 18 mosquito species, so that no precise information about the possible vector can be given.
Besides viruses, some parasites are known, which occur in bats and humans, and can be transmitted through haemorrhagic insects. The first one is the Chagas disease an infection with the flagellate Trypanosoma cruzi (Zeledón and Rabinovich 1981). The disease occurs in Central and South America and is transmitted by the intestinal content of triatomine kissing bugs (Mehlhorn 2001). It is the leading cause of heart disease in South America with the major vector species Triatoma infestans, T. dimidiata and Rhodnius prolixus (Reduviidae) (Dorn et al. 2003). Flagellates of the genus Trypanosoma are parasitic in nearly all mammalian species. In more than 100 species of bats more than 30 trypanosome species are recorded, while the subgenus Schizotrypanum comprises species restricted to bats as well as T. cruzi (Cavazzana et al. 2010). Kissing bugs live for example in caves, burrows, nests of wild animals on which they feed during the night. During the blood meal infected faeces is set free and after the bite scratched by the victim in the itching wound. During the life cycle in man or other reservoir hosts, amastigotes reproduce in the cytoplasm of different host cell types, which appear as “pseudocysts” when they are completely filled with parasites. The most important lesions are in the heart and a myocardial failure results to death years after the infection (Mehlhorn 2001). The symptoms range from fever to inflammation of heart, muscles and brain (CDC 2010).
Beneath the usual triatomine vector, several wild animals are associated with the Chagas disease. Among others Marsupialia, Rodentia, Edentata, Primates and Chiroptera had been positive investigated for T. cruzi (Coura et al. 2002). Common species of neotropical bats, including those of the genera Artibeus, Noctilio, Mormoops, Nautilus, Pteronotus, Myotis, Carollia, Desmodus, Glossophaga, Phyllostomus and Molossus, have been reported to be susceptible to T. cruzi infection under natural as well as under experimental conditions (Añez et al. 2009). Bats can get infected by the blood meal of the kissing bugs or through the ingestion of infected arthropods. So it is not astonishing that most infected bats are insectivorous. The prevalence in South American bats varied widely. In Colombia and in the Amazonia of Brazil, it is approximately 9.0 %, respectively 2.4–4.6 % (García et al. 2012).
Añez et al. (2009) detected in Molossus molossus in western Venezuela a congenital transmission from pregnant female bats to their foetus. Trypomastigotes had been found in 100 % of all examined foetus. In their natural habitat, M. molossus is associated with R. prolixus kissing bugs. The insectivore M. molossus shows a high susceptibility for T. cruzi, due to the fact that 80 % of the examined bats are infected. These results emphasize the role of Chiroptera as host for Chagas disease in endemic areas and their impact for the sylvatic cycle of T. cruzi (Añez et al. 2009).
Recent examinations detected new genotypes of T. cruzi associated with bats, which indicate that the complexity of T. cruzi is larger than known and confirmed bats as important reservoir for infections to humans (e.g. Maeda et al. 2011; Marcili et al. 2009) and the strong association between bats and, for instance, Schizotryphanum suggests a long shared evolutionary development (García et al. 2012). Furthermore, the molecular examination of Chagas virus strains reveals the movement of bats, naturally or by human transport, between the Old and the New World (Hamilton et al. 2012b). Hamilton et al. (2012a) suggested that T. cruzi evolved from bat trypanosomes and have successful switched into other mammalian hosts.
Coccidia (Apicomplexa) are characterized by intracellular life cycles consisting of the three phases: schizogony, gamogony and sporogony. The coccidian genus Plasmodium is the pathogenic agent of malaria, a mosquito-borne infectious disease of humans and animals, which causes fever, headache and in severe cases death (Mehlhorn 2001). Worldwide 3.3 billion people live in risk areas (Africa, Southeast Asia region and the Eastern Mediterranean) of malaria transmission and each year at least one million people die after infection (Snow et al. 2005). The order Haemosporidia consists of the five genera: Plasmodium, Hepatocystis, Polychromophilus, Nycteria and Rayella. The vectors of the first three genera are respectively haematophagous Diptera of the families Culicidae (Anopheles spp.), Ceratopogonidae and Nycteribiidae (Witsenburg et al. 2012). With the exception of Rayella, all haemosporidia genera are known to infect insectivorous bats in temperate and tropical regions (Duval et al. 2012). Megali et al. (2011) investigated 237 bats of four species from Switzerland to obtain a better understanding of the complex co-evolutionary processes between hosts and parasites. A total of 34 % (n = 70) was infected with Plasmodium murinus. In detail, Myotis daubentonii was the most parasitized species (51 %), followed by Eptesicus serotinus (11 %), Nyctalus noctula (7 %) and Myotis myotis (4 %) (Megali et al. 2011). The prevalence (P) of P. murinus in M. daubentonii was twice as great as found by Gardner and Molyneux (1988) in England and Scotland. Duval et al. (2007) examined 530 bat individuals (Pteropodidae, Rhinolophidae, Hipposideridae, Megadermatidae, Emballonuridae, Vespertilionidae and Molossidae) from Madagascar and Cambodia. In Madagascar haemosporidian infections were found in Hipposideridae (Triaenops furculus, P 4 %) and Vespertilionidae (Miniopterus gleni, P 23 %; Myotis goudoti, P 24 % and Miniopterus manavi, P 38 %). In Cambodia, infections were found in the Hipposideridae (Hipposideros larvatus, P 8 %), Megadermatidae (Megaderma spasma, P 80 %) and Vespertilionidae (Kerivoula hardwickii, P 20 %) (Duval et al. 2007). In Pteropus poliocephalus from Australia (P 36 %), Landau et al. (1980) described Hepatocystis levinei, which under laboratory conditions, used Culicoides nubecolusus (Diptera and Ceratopogonidae) to complete its life cycle. Landau et al. (2012) listed bat hosts of six families in which different types of gametocytes were detected. The authors concluded that the Microchiroptera harbour mainly parasites of the falciparum and malariae groups, while Megachiroptera harbour parasites of the vivax group (Landau et al. 2012). Duval et al. (2012) sampled 164 bats from Gabon in Central Africa of which only Miniopterus inflatus was positively tested for haemosporidian parasites. The prevalences ranged from 17.6 % to 66.7 % (blood smear examinations), while the molecular prevalence ranged from 63.2 % to 88.9 %. The nycteribiid Polychromophilus fulvida was found infected with Polychromatophilia sp. in Faucon Cave in Gabon (Landau et al. 1980). Miniopterus inflatus and other bat species (e.g. H. gigas, H. caffer and C. afra) are potentially exposed to this blood parasite (Duval et al. 2012). The five host specific Polychromophilus species are restricted, regarding their vertebrate hosts, to insectivorous bats of the order Microchiroptera. Vectors are Nycteribiidae (Diptera, Hippoboscoidea) (Witsenburg et al. 2012).
3.3 Conclusion
In the past, a high number of viruses and parasites has been detected in bats, which are important reservoir hosts. On the contrary, many haematophagous insects serve as vectors for numerous arboviruses and parasites, with mosquitoes (Culicidae) being the most important vectors worldwide. So the presence of certain viruses in both mosquitoes and bats is not really surprising and a transmission cycle between bats, mosquitoes and humans is thinkable. However, it is not possible to say whether the mosquitoes served as vector for the bats or the bats as reservoir both options must be taken into consideration.
In the present chapter, we describe 20 viruses from four different families as well as two parasitic pathogens, which have been detected in bats, in haematophagous insects and in humans (see also Table 3.1, Fig. 3.3). Therefore, for these pathogens is a probability to be transmitted from bats to humans via insects. However, because of the amount of publications in these fields and the quantity of described viruses it is difficult to give a definite number for the possible diseases and the chapter makes no claim to be complete. So far, this way of transmission between bats–mosquito–humans could not be proven for any of the diseases, but vectors being in relation with bat infections (e.g. Cx. quinquefasciatus, Ae. vexans or Ae. aegypti and Ae. albopictus) are also known to bite humans. Climate change as well as global trade could not only increase the risk for such way of transmission e.g. when potential hosts but also competent vectors expand their distribution. Apart from the listed viruses and parasites (see Table 3.1, Fig. 3.3) are some pathogens often described only in humans or bats but has been detected already also in mosquitoes or is, at least, suspected to be transmitted by arthropods, like Bimiti, Oriboca, Mayaro or Yokose virus. With climate change range swift of vectors and possible spontaneous mutations, new hosts and/or vector competences can occur. For some diseases like WNV, Dengue or Yellow fever, the way of transmission is already well known, while this is not the case for others like Kaeng Khoi, Catu, Guama, Zika or Bwamba virus, but knowledge about these things is essential as epidemics cause high social as well as economic impact.
References
Agarwal AK (2006) Post viral encephalitis sequelae and their rehabilitation. Indian J Phys Med Rehabil 17:39–40
Aguilar-Setién A, Romero-Almaraz ML, Sanchez-Hernández C, Figuero R, Juárez-Palma LP, Garcia-Flores MM, Vázquez-Salinas C, Salas-Rojas M, Hidalgo-Martinez AC, Aguilar Pierle S, Garcia-Estrada C, Ramos C (2008) Dengue virus in Mexico bats. Epidemiol Infect 136:1678–1683
Añez N, Crisantea G, Soriano PJ (2009) Trypanosoma cruzi congenital transmission in wild bats. Acta Trop 109:78–80
Armstrong PM, Andreadis TG (2010) Eastern equine encephalitis virus in mosquitoes and their role as bridge vectors. Emerg Infect Dis 16:1869–1874
Arrigo NC, Adams AP, Weaver SC (2010) Evolutionary patterns of eastern equine encephalitis virus in North versus South America suggest ecological differences and taxonomic revision. J Virol 84:1014–1025
Auguste AJ, Pybus OG, Carrington CVF (2009) Evolution and dispersal of St. Louis encephalitis virus in the Americas. Infect Genet Evol 9:709–715
Ayers JR, Lester TL, Angulo AB (1994) An epizootic attributable to western equine encephalitis virus infection in emus in Texas. J Am Vet Med Assoc 205:600–601
Baldelli F, Ciufolini MG, Francisci D, Marchi A, Venturi G, Fiorentini C, Luchetta ML, Bruto L, Pauluzzi S (2004) Unusual presentation of life-threatening Toscana virus Meningoencephalitis. Clin Infect Dis 38:515–520
Balvín O (2008) Revision of the West Palaerarctic Cimex species. Preliminary report. Bull Insectol 61:129–130
Balvín O, Munclinger P, Kratochvil L, Vilímová J (2012) Mitochondrial DNA and morphology show independent evolutionary histories of bedbug Cimex lectularius (Heteroptera: Cimicidae) on bats and humans. Parasitol Res 111:457–469
Banerjee K, Ilkal MA, Deshmukh PK (1984) Susceptibility of Cynopterus sphinx (frugivorus bat) & Suncus murinus (house shrew) to Japanese encephalitis virus. Indian J Med Res 79:8–12
Beck CE, Wyckhoff RWG (1938) Venezuelan equine encephalomyelitis. Science 88:530
Becker N, Petrić D, Zgomba M, Boase C, Dahl C, Lane J, Kaiser A (2010) Mosquitoes and their control. Kluwer Academic/Plenum, New York, NY, 578 pp
Bengis RG, Leighton FA, Fischer JR, Artois M, Mörner T, Tate CM (2004) The role of wildlife in emerging and re-emerging zoonoses. Rev Sci Tech 23:497–511
Blackburn NK, Foggin CM, Searle L, Smith PN (1982) Isolation of Sindbis virus from bat organs. Cent Afr J Med 28:201
Bouloy M (2011) Molecular biology of phleboviruses. In: Plyusnin A, Elliott RM (eds) Bunyaviridae, molecular and cell biology. Caister Academic, Norwich, pp 95–128
Bowen MD, Peters CJ, Nichol ST (1996) The phylogeny of New World (Tacaribe complex) arenaviruses. Virology 219:285–290
Bowen MD, Trappier SG, Sanchez AJ, Meyer RF, Goldsmith CS, Zaki SR, Dunster LM, Peters CJ, Ksiazek TG, Nichol ST, Task Force RVF (2001) A reassortant Bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology 291:185–190
Braito A, Corbisiero R, Corradini S, Marchi B, Sancascian N, Fiorentini C, Ciufolini MG (1997) Evidence of Toscana virus infections without central nervous system involvement: a serological study. Eur J Epidemiol 13:761–764
Brault AC, Powers AM, Chavez CL, Lopez RN, Cachon MF, Gutierrez LF, Kang W, Tesh RB, Weaver SC, Carrington CVF (1999) Genetic and antigenic diversity among eastern equine encephalitis viruses from North, Central and South America. Am J Trop Med Hyg 61:579–586
Buckley A, Dawson A, Moss SR, Hinsley SA, Bellamy PE, Gould EA (2003) Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J Gen Virol 84:2807–2817
Bunde JM, Heske EJ, Mateus-Pinilla NE, Hofmann JE, Novak RJ (2006) A survey for West Nile virus in bats from Illinois. J Wildl Dis 42:455–458
Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT (2012) Chikungunya: a re-emerging virus. Lancet 379:662–671
Burton GJ (1963) Bedbugs in relation to transmission of human diseases. Review of the literature. Public Health Rep 78:513–524
Calisher CH (1994) Medically important arboviruses of the United States and Canada. Clin Microbiol Rev 7:89–116
Calisher CH, Shope RE, Brandt W, Casals J, Karabatsos N, Murphy FA, Tesh RB, Wiebe ME (1980) Proposed antigenic classification of registered arboviruses I. Togaviridae, Alphavirus. Intervirology 14:229–232
Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545
Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ (2002) West Nile virus. Lancet Infect Dis 2:519–529
Carballal G, Calello MA, Laguens RP, Weissenbacher MC (1987) Tacaribe virus: a new alternative for Argentine hemorrhagic fever vaccine. J Med Virol 23:257–263
Casals J (1964) Antigenic variants of eastern equine encephalitis virus. J Exp Med 119:547–565
Causey OR, Causey CE, Maroja OM, Macedo DG (1961) The isolation of arthropod-borne viruses, including members of two hitherto undescribed serological groups, in the Amazon region of Brazil. Am J Trop Med Hyg 10:227–249
Cavazzana Jr. M, Marcili A, Lima L, Maia da Silva F, Junqueira ACV, Veludo HH, Viola LB, Campaner M, Nunes VLB, Paiva F, Coura JR, Camargo EP, Teixeira MMG (2010) Phylogeographical, ecological and biological patterns shown by nuclear (ssrRNA and gGAPDH) and mitochondrial (Cyt b) genes of trypanosomes of the subgenus Schizotrypanum parasitic in Brazilian bats. Int J Parasitol 40:345–355
CDC (2002) Summary of notifiable disease-United States, 2000. MMWR Morb Mortal Wkly Rep 49:1–100
CDC (2010) Parasites-American trypanosomiasis (also known as Chagas disease). http://www.cdc.gov/parasites/chagas/disease.html
CDC (2012) Geographic distribution of Japanese encephalitis virus. http://www.cdc.gov/japaneseencephalitis/maps/index.html
Chakravarty AK, Sarkar SK (1994) Immunofluorescence analysis of immunoglobulin bearing lymphocytes in the Indian fruit bat: Pteropus giganteus. Lymphology 27:97–104
Chang G-JJ, Trent DW (1987) Nucleotide sequence of the genome region encoding the 26S mRNA of eastern equine encephalomyelitis virus and the deduced amino acid sequence of the viral structural proteins. J Gen Virol 68:2129–2142
Chapman AD (2009) Numbers of living species in Australia and the world. Australian Biological Resources Study, Canberra, 60 pp
Charrel RN, Gallian P, Navarro-Marí J-M, Nicoletti L, Papa A, Sánchez-Seco MP, Tenorio A, de Lamballerie X (2005) Emergence of Toscana virus in Europe. Emerg Infect Dis 11:1657–1663
Chen LH, Wilson ME (2005) Non-vector transmission of Dengue and other mosquito-borne flaviviruses. Dengue Bull 29:18–31
Cogswell-Hawkinson A, Bowen R, James S, Gardiner D, Calisher CH, Adams R, Schountza T (2012) Tacaribe virus causes fatal infection of an ostensible reservoir host, the Jamaican fruit bat. J Virol 86:5791–5799
Colpitts TM, Moore AC, Kolokoltsov AA, Davey RA (2007) Venezuelan equine encephalitis virus infection of mosquito cells requires acidification as well as mosquito homologs of the endocytic proteins Rab5 and Rab7. Virology 369:78–91
Constantine DG (1970) Bats in relation to the health, welfare and economy of man. In: Winsatt WE (ed) Biology of bats. Academic, New York, pp 319–449
Cook S, Holmes EC (2006) A multigene analysis of the phylogenetic relationships among the flaviviruses (Family: Flaviviridae) and the evolution of vector transmission. Arch Virol 151:309–325
Coura JR, Junqueira ACV, Fernandes O, Valente SAS, Miles MA (2002) Emerging Chagas disease in Amazonian Brazil. Trends Parasitol 18:171–176
Cupp EW, Klingler K, Hassan HK, Viguers LM, Unnasch TR (2003) Transmission of eastern equine encephalomyelitis virus in central Alabama. Am J Trop Med Hyg 68:495–500
Cusi MG, Savellini GG, Zanelli G (2010) Toscana virus epidemiology: from Italy to beyond. Open Virol J 4:22–28
da Silva Azevedo RS, Barros VLRS, Martins LC, Ribeiro Cruz AC, Rodrigues SG, da Costa Vasconcelos PF (2010) Pathogenesis of the Ilheus virus in golden hamsters (Mesocricetus auratus). Rev Pan-Amaz Saude 1:73–80
Daniels JB, Stuart G, Wheeler RE, Gifford C, Ahearn JP, Philbrook R, Hayes RO, MacCready RA (1960) A search for encephalitis and rabies in bats of eastern Massachusetts. N Engl J Med 263:516–520
Darai G, Handermann M, Sonntag HG, Zöller L (2011) Lexikon der Infektionskrankheiten des Menschen: Erreger, Symptome, Diagnose. Springer, Berlin, 130 pp
Daubney R, Hudson JR (1931) Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep, cattle and man from East Africa. J Pathol 34:545–579
Dauphin G, Zientara S, Zeller H, Murgue B (2004) West Nile: worldwide current situation in animals and humans. Comp Immunol Microbiol Infect Dis 27:343–355
Davis A, Bunning M, Gordy P, Panella N, Blitvivh B, Bowen R (2005) Experimental and natural infection of North American bats with West Nile virus. Am J Trop Med Hyg 73:467–469
de Thoisy B, Lacoste V, Germain A, Muñoz-Jordán J, Colón C, Mauffrey J-F, Delaval M, Catzeflis F, Kazanji M, Matheus S, Dussart P, Morvan J, Setién AA, Deparis X, Lavergne A (2009) Dengue infection in neotropical mammals. Vector Borne Zoonotic Dis 9:157–169
Delaunay P, Blanc V, Del Giudice P, Levy-Bencheton A, Chosidow O, Marty P, Brouqui P (2011) Bedbugs and infectious diseases. Clin Infect Dis 52:200–210
Depaquit J, Grandadam M, Fouque F, Andry PE, Peyrefitte C (2010) Arthropod-borne viruses transmitted by phlebotomine sandflies in Europe: a review. Euro Surveill 15:19507
Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA (1997) Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 336:1867–1874
Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D (1999) Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg 60:281–286
Diaz LA, Ré V, Almirón WR, Farías A, Vázquez A, Sanchez-Seco MP, Aguilar J, Spinsanti L, Konigheim B, Visintin A, García J, Morales MA, Tenorio A, Contigiani M (2006) Genotype III Saint Louis encephalitis virus outbreak, Argentina, 2005. Emerg Infect Dis 12:1752–1754
Diaz LA, Llinás GA, Vázquez A, Tenorio A, Contigiani MS (2012) Silent circulation of St. Louis encephalitis virus prior to an encephalitis outbreak in Cordoba, Argentina (2005). PLoS Negl Trop Dis 6:e1489
Dick GW (1952) Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 46:521–534
Dick GW, Kitchen SF, Haddow AJ (1952) Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520
Dobson AP (2005) What links bats to emerging infectious diseases? Science 310:628–629
Doherty RL, Gorman BM, Whitehead RH, Carley JG (1966) Studies of arthropod-borne virus infections in Queensland. V. Survey of antibodies to group A arboviruses in man and other animals. Aust J Exp Biol Med Sci 44:365–377
Dohm DJ, Rowton ED, Lawyer PG, O’Guinn M, Turell MJ (2000) Laboratory transmission of Rift Valley fever virus by Phlebotomus duboscqi, Phlebotomus papatasi, Phlebotomus sergenti and Sergentomyia schwetzi (Diptera: Psychodidae). J Med Entomol 37:435–438
Dorn PL, Melgar S, Rouzier V, Gutierrez A, Combe C, Rosales R, Rodas A, Kott S, Slavia D, Monroy CM (2003) The Chagas vector, Triatoma dimidiata (Hemiptera: Reduviidae), is panmictic within and among adjacent villages in Guatemala. J Med Entomol 40:436–440
Downs WG, Anderson CR, Spence L, Aitken THG, Greenhall AH (1963) Tacaribe virus, a new agent isolated from Artibeus bats and mosquitos in Trinidad, West Indies. Am J Trop Med Hyg 12:640–646
Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB (2009) Zika virus outbreak on Yap Island, federated states of Micronesia. N Engl J Med 360:2536–2543
Duval L, Robert V, Csorba G, Hassanin A, Randrianarivelojosia M, Walston J, Nhim T, Goodman SM, Ariey F (2007) Multiple host-switching of Haemosporidia parasites in bats. Malar J 29:157
Duval L, Mejean C, Maganga GD, Makanga BK, Mangama Koumba LB, Peirce MA, Ariey F, Bourgarel M (2012) The chiropteran haemosporidian Polychromophilus melanipherus: a worldwide species complex restricted to the family Miniopteridae. Infect Genet Evol 12:1558–1566
Eklund CM (1954) Mosquito-transmitted encephalitis viruses: a review of their insect and vertebrate hosts and the mechanisms for survival and dispersion. Exp Parasitol 3:285–305
Elliott RM (1997) Emerging viruses: the Bunyaviridae. Mol Med 3:572–577
Elliott RM, Blakqori G (2011) Molecular biology of orthobunyaviruses. In: Plyusnin A, Elliott RM (eds) Bunyaviridae, molecular and cell biology. Caister Academic, Norwich, pp 1–39
Ellis BR, Barrett DT (2008) The enigma of yellow fever in East Africa. Rev Med Virol 18:331–346
Ferro C, Boshell J, Moncayo AC, Gonzalez M, Ahumada ML, Kang W, Weaver SC (2003) Natural enzootic vectors of venezuelan equine encephalitis virus, Magdalena Valley, Colombia. Emerg Infect Dis 9:49–54
Fine DL, Roberts BA, Teehee ML, Terpening SJ, Kelly CLH, Raetz JL, Baker DC, Powers AM, Bowen RA (2007) Venezuelan equine encephalitis virus vaccine candidate (V3526) safety, immunogenicity and efficacy in horses. Vaccine 25:1868–1876
Flores FS, Diaz LA, Batallán GP, Almirón WR, Contigiani MS (2010) Vertical transmission of St. Louis encephalitis virus in Culex quinquefasciatus (Diptera: Culicidae) in Córdoba, Argentina. Vector Borne Zoonotic Dis 10:999–1002
Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG (1998) New vectors of Rift Valley fever in West Africa. Emerg Infect Dis 4:289–293
Fothergill LD, Dingle JH, Farber S, Connerly ML (1938) Human encephalitis caused by the virus of the eastern variety of equine encephalomyelitis. N Engl J Med 219:411
García L, Ortiz S, Osorio G, Torrico MC, Torrico F, Solari A (2012) Phylogenetic analysis of Bolivian bat trypanosomes of the subgenus Schizotrypanum based on cytochrome b sequence and minicircle analyses. PLoS One 7:e36578
Gardener RA, Molyneux DH (1988) Polychromophilus murinus: a malaria parasite of bats: life history and ultrastructural studies. Parasitology 96:591–605
Garmendia AE, Van Kruiningen HJ, French RA (2001) The West Nile virus: its recent emergence in North America. Microbes Infect 3:223–229
George S, Gourie-Devi M, Rao JA, Prasad SR, Pavri KM (1984) Isolation of West Nile virus from the brains of children who had died of encephalitis. Bull World Health Organ 62:879–882
Getting VA (1941) Equine encephalomyelitis in Massachusetts: an analysis of the 1938 outbreak. N Eng J Med 224:999–1006
Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa south of Sahara. Publ South Afr Inst Med Res 55:1–143
Goddard J, deShazo R (2009) Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 301:1358–1366
Gonzales JP, Georges AJ (1988) Bunyaviral fecers: Bunyamwera. Ilesha, Germiston, Bwamba, and Tataguine. In: Monath TP (ed) The arboviruses:epidemiology and Ecology, vol II. CRC, Boca Raton, FL, pp 87–98
Grady GF, Maxfield HK, Hildreth SW, Timperi RJ Jr, Gilfillan RF, Rosenau BJ, Francy DB, Calisher CH, Marcus LC, Madoff MA (1978) Eastern equine encephalitis in Massachusetts, 1957–1976. Am J Epidemiol 107:170–178
Grayson MA, Galindo P (1968) Epidemiologic studies of Venezuelan equine encephalitis virus in Almirante, Panama. Am J Epidemiol 88:80–96
Griffin DE (2001) Alphaviruses. In: Knipe DM, Howley PM (eds) Field virology. Lippincott Williams and Wilkins, Philadelphia, PA, pp 917–962
Gubler DJ (2007) The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis 45:1039–1046
Haddow AJ, Williams MC, Woodall JP, Simpson DIH, Goma LKH (1964) Twelve isolations of Zika virus from Aedes (Stegomyia) Africanus (Theobald) taken in and above a Uganda forest. Bull World Health Organ 31:57–69
Halpin K, Young PL, Field HE, Mackenzie JS (2000) Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol 81:1927–1932
Hamilton PB, Teixeira MGM, Stevens JR (2012a) The evolution of Trypanosoma cruzi: the ‘bat seeding’ hypothesis. Trends Parasitol 28:136–141
Hamilton PB, Cruickshank C, Stevens JR, Teixeira MMG, Mathews F (2012b) Parasites reveal movement of bats between the New and Old Worlds. Mol Phylogenet Evol 63:521–526
Hanson RP (1957) An epizootic of equine encephalomyelitis that occurred in Massachusetts in 1831. Am J Trop Med Hyg 6:858–862
Hardy JL, Apperson G, Asman SM, Reeves WC (1978) Selection of a strain of Culex tarsalis highly resistant to infection following ingestion of western equine encephalomyelitis virus. Am J Trop Med Hyg 27:313–321
Harley D, Ritchie S, Phillips D, van den Hurk A (2000) Mosquito isolates of Ross River virus from Cairns, Queensland, Australia. Am J Trop Med Hyg 62:561–565
Harley D, Sleigh A, Ritchie S (2001) Ross River virus transmission, infection and disease: a cross-disciplinary review. Clin Microbiol Rev 14:909–932
Hart KL, Keen D, Belle EA (1964) An outbreak of eastern equine encephalitis in Jamaica West Indies. I. Description of human cases. Am J Trop Med Hyg 13:331–341
Hayes CG (2006) West Nile virus: Uganda, 1937, to New York City, 1999. Ann N Y Acad Sci 951:25–37
Hayes EB (2009) Zika virus outside Africa. Emerg Infect Dis 15:1347–1350
Hayes RO, Daniels JB, Maxfield HK, Wheeler RE (1964) Field and laboratory studies on eastern encephalitis in warm- and coldblooded vertebrates. Am J Trop Med Hyg 13:595–606
Hemmersbach-Miller M, Parola P, Charrel RN, Durand JP, Brouqui P (2004) Sandfly fever due to Toscana virus: an emerging infection in southern France. Eur J Intern Med 15:316–317
Higgs S (2006) The 2005–2006 Chikungunya epidemic in the Indian Ocean. Vector Borne Zoonotic Dis 6:115–117
Hoshino K, Isawa H, Tsuda Y, Sawabe K, Kovbayashi M (2009) Isolation and characterization of a new insect Flavivirus from Aedes albopictus and Aedes flavopictus mosquitoes in Japan. Virology 391:119–129
Hu W, Tong S, Mengersen K, Oldenburg B, Dale P (2006) Mosquito species (Diptera: Culicidae) and the transmission of Ross River virus in Brisbane, Australia. J Med Entomol 43:375–381
Hubálek Z (2008) Mosquito-borne viruses in Europe. Parasitol Res 103:29–43
Hubálek Z, Halouzka J (1999) West Nile fever – a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis 5:643–650
Hutson AM, Mickleburgh SP, Racey PA (2001) Microchiropteran bats: global status survey and conservation active plan. IUCN/SSC Chiroptera Specialist Group. IUCN, Gland, Switzerland. 258 pp
Ikegami T (2012) Molecular biology and genetic diversity of Rift Valley fever virus. Antiviral Res 95:293–310
Ikegami T, Makino S (2011) The pathogenesis of Rift valley fever. Viruses 3:493–519
IUCN red list (2012) Information about different bat species. http://www.iucnredlist.org/
Iversson LB, Silva RAMS, Travassos da Rosa APA, Barros VLRS (1993) Circulation of eastern equine encephalitis, western equine encephalitis, Ilheus, Maguari and Tacaiuma viruses in equines of the Brazilian Pantanal, South America. Rev Inst Med Trop Sao Paulo 35:355–359
Jacob BG, Burkett-Cadena ND, Luvall JC, Parcak SH, McClure CJ, Estep LK, Hill GE, Cupp EW, Novak RJ, Unnasch TR (2010) Developing GIS-based eastern equine encephalitis vector-host models in Tuskegee, Alabama. Int J Health Geogr 9:12
Johnson HN (1964) Diseases derived from Wildlife. Proceedings of the 2nd vertebrate pest control conference, pp 138–142
Johnson KM, Martin DH (1974) Venezuelan equine encephalitis. Adv Vet Sci Comp Med 18:79–116
Johnson KM, Shelekov A, Peralta PH, Dammin GJ, Young NA (1968) Recovery of Venezuelan equine encephalitis virus in Panama. A fatal case in man. Am J Trop Med Hyg 17:432–440
Johnson BW, Cruz C, Felices V, Espinoza WR, Manock SR, Guevara C, Olson JG, Kochel TJ (2007) Ilheus virus isolate from a human, Ecuador. Emerg Infect Dis 13:956–958
Jones KE, Purvis A, MacLarnon A, Bininda-Emonds OR, Simmons NB (2002) A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biol Rev Camb Philos Soc 77:223–259
Jöst H, Bialonski A, Storch V, Günther S, Becker N, Schmidt-Chanasit J (2010) Isolation and phylogenetic analysis of Sindbis viruses from mosquitoes in Germany. J Clin Microbiol 48:1900–1903
Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA (1997) Early clinical and laboratory indicators of acute Dengue illness. J Infect Dis 176:313–321
Karabatsos, N. (ed) (1985). International catalogue of Arboviruses including certain other virus of vertebrates. 3rd ed., San Antonio, Texas. Am. Soc. Trop. Med. Hyg
Karstad LH, Hanson RP (1958) Infections in wildlife with the viruses of vesicular stomatitis and eastern equine encephalomyelitis. Trans N Am Wildl Conf 23:175–186
Kelser RA (1933) Mosquitoes as vectors of the virus of equine encephalomyelitis. J Am Vet Med Assoc 82:767
Kelser RA (1937) Transmission of the virus of equine encephalomyelitis by Aedes taeniorhynchus. Science 85:178
Kelser RA (1938) Transmission of the virus of equine encephalomyelitis by Aedes taeniorhynchus. J Am Vet Med Assoc 92:195
Knowlton GF, Rowe JA (1935) Handling mosquitoes on equine encephalomyelitis investigation. J Econ Entomol 28:824–829
Kramer LD, Scherer WF (1976) Vector competence of mosquitoes as a marker to distinguish Central American and Mexican epizootic from enzootic strains of Venezuelan encephalitis virus. Am J Trop Med Hyg 25:336–346
Krenn HW, Aspöck H (2010) Bau, Funktion und Evolution der Mundwerkzeuge blutsaugender Arthopoden. In: Aspöck H (ed) Krank durch Arthropoden. Denisia 30. Land Oberösterreich. Biologiezentum/Oberösterreichisches Landesmuseum, Linz, pp 81–108
Krenn HW, Aspöck H (2012) Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Struct Dev 41:101–118
Krishna MR, Reddy MK, Reddy SR (2006) Chikungunya outbreaks in Andhra Pradesh, South India. Curr Sci 91:570–571
Kumm HW (1932) Yellow fever transmission experiments with South American bats. Ann Trop Med Parasitol 26:207–213
Kurkela S, Manni T, Myllynen J, Vaheri A, Vapalahti O (2005) Clinical and laboratory manifestations of Sindbis virus infection: prospective study, Finland, 2002–2003. J Infect Dis 191:1820–1829
Kurkela S, Rätti O, Huhtamo E, Uzcátegui NY, Nuorti JP, Laakkonen J, Manni T, Helle P, Vaheri A, Vapalahti O (2008) Sindbis virus infection in resident birds, migratory birds, and humans, Finland. Emerg Infect Dis 14:41–47
Laemmert HW, Hughes T (1947) The virus of Ilheus encephalitis: isolation, serological specificity and transmission. J Immunol 55:61–67
Laine M, Luukkainen R, Toivanen A (2004) Sindbis viruses and other alphaviruses as cause of human arthritic disease. J Intern Med 256:457–471
Lambert AJ, Lanciotti RS (2008) Molecular characterization of medically important viruses of the genus Orthobunyavirus. J Gen Virol 89:2580–2585
Landau I, Rosin G, Miltgen F (1980) The genus Polychromophilus (Haemoproteidae, parasite of Microchiroptera). Ann Parasitol Hum Comp 55:13–32
Landau I, Chavatte JM, Karadjian G, Chabaud A, Beveridge I (2012) The haemosporidian parasites of bats with description of Sprattiella alecto gen. nov., sp. nov. Parasite 19:137–146
Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH, Wong SS, Leung SY, Chan KH, Yuen KY (2005) Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA 102:14040–14045
LeDuc JW, Kahlon SS (2012) Bunyaviridae. In: Warrell D, Cox T, Firth J, Török E (eds) Oxford textbook of medicine: infection. Oxford University Press, Oxford, pp 182–185
Lee VH, Moore DL (1972) Vectors of the 1969 yellow fever epidemic on the Jos Plateau, Nigeria. Bull World Health Organ 46:669–673
Lee VH, Monath TP, Tomori O, Fagbami A, Wilson DC (1974) Arbovirus studies in Nupeko Forest, a possible natural focus of yellow fever in Nigeria. II. Entomological investigations and viruses isolated. Trans R Soc Trop Med Hyg 68:39–43
Lehane M (2005) The biology of blood-sucking in insects. Cambridge University Press, Cambridge, MA, 321 pp
Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Delicat A, Paweska JT, Gonzalez JP, Swanepoel R (2005) Fruit bats as reservoirs of Ebola virus. Nature 438:575–576
Letson GW, Bailey RE, Pearson J, Tsai TF (1993) Eastern equine encephalitis (EEE): a description of the 1989 outbreak, recent epidemiologic trends, and the association of rainfall with EEE occurrence. Am J Trop Med Hyg 49:677–685
Lewis DJ (1974) Biology of Phlebotomidae in relation to leishmaniasis. Annu Rev Entomol 19:363–384
Li L, Victoria JG, Wang C, Jones M, Fellers GM, Kunz TH, Delwart E (2010) Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J Virol 14:6955–6965
Löscher T, Burchard GD (2008) Tropenmedizin in Klinik und Praxis : mit Reise– und Migrationsmedizin. Georg Thieme Verlag, Stuttgart, 248 pp
Lundström JO, Pfeffer M (2010) Phylogeographic structure and evolutionary history of Sindbis virus. Vector Borne Zoonotic Dis 10:889–907
Lutwama JJ, Rwaguma EB, Nawanga PL, Mukuye A (2002) Isolations of Bwamba virus from south central Uganda and north eastern Tanzania. Afr Health Sci 2:24–28
Mackenzie JS, Williams DT (2008) The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australiasia: the potential for emergent viruses. Zoonoses Public Health 56:338–356
Mackenzie JS, Lindsay MD, Coelen RJ, Broom AK, Hall RA, Smith DW (1994) Arboviruses causing human disease in the Australasian zoogeographic region. Arch Virol 136:447–467
Mackenzie JS, Gubler DJ, Petersen LR (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and Dengue viruses. Nat Med 10:98–109
Mackenzie JS, Williams DT, Smith DW (2006) Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. In: Tabor E (ed) Emerging viruses in human populations. Elsevier, Amsterdam, pp 201–268
Madsen DE, Knowlton GF (1935) Mosquito transmission of equine encephalomyelitis. J Am Vet Med Assoc 86:662
Madsen DE, Knowlton GF, Rowe JA (1936) Further studies on the transmission of equine encephalomyelitis by mosquitoes. J Am Vet Med Assoc 89:187
Maeda FY, Alves RM, Cortez C, Lima FM, Yoshida N (2011) Characterization of the infective properties of a new genetic group of Trypanosoma cruzi associated with bats. Acta Trop 120:231–237
Mahmood F, Crans WJ (1998) Effect of temperature on the development of Culiseta melanura (Diptera: Culicidae) and its impact on the amplification of eastern equine encephalitis virus in birds. J Med Entomol 35:1007–1012
Main AJ (1979a) Virologic and serologic survey for eastern equine encephalomyelitis and certain other viruses in colonial bats of New England. J Wildl Dis 15:455–464
Main AJ (1979b) Eastern equine encephalomyelitis virus in experimentally infected bats. J Wildl Dis 15:467–477
Marchette NJ, Garcia R, Rudnick A (1969) Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg 18:411–415
Marcili A, Lima L, Cavazzana MR, Junqueira ACV, Veludo HH, Maia da Silva F, Campaner M, Paiva F, Nunes VLB, Teixeira MMG (2009) A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology 136:641–655
Marinkelle CJ, Grose ES (1972) A review of bats as carriers of organisms which are capable of infecting man or domestic animals. Mitt Inst Colombo Aleman Invest Cient 6:31–51
Maroli M, Bigliocchi F, Khoury C (1994) Sandflies in Italy: observation on their distribution and methods for control. Parasitologia 36:251–264
McLean RG, Trevino HA, Sather GE (1979) Prevalence of selected zoonotic diseases in vertebrates from Haiti, 1972. J Wildl Dis 15:327–330
McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST (2012) A new Phlebovirus associated with severe febrile illness in Missouri. N Engl J Med 367:834–841
McMurray DN, Stroud J, Murphy JJ, Carlomagno MA, Greer DL (1982) Role of immunoglobulin classes in experimental histoplasmosis in bats. Dev Comp Immunol 6:557–567
Megali A, Yannic G, Christe P (2011) Disease in the dark: molecular characterization of Polychromophilus murinus in temperate zone bats revealed a worldwide distribution of this malaria-like disease. Mol Ecol 20:1039–1048
Mehlhorn H (ed) (2001) Encyclopedic reference of parasitology. Springer, Berlin, 676 pp
Merrill MH, Ten Broeck C (1935) The transmission of equine encephalomyelitis virus by Aedes aegypti. J Exp Med 62:687–695
Merrill MH, Lacaillade CW, Ten Broeck C (1934) Mosquito transmission of equine encephalitis. Science 80:251–252
Messenger SL, Rupprecht CE, Smith JS (2003) Bats, emerging virus infections, and the rabies paradigm. In: Kunz TH, Fenton MB (eds) Bat ecology. The University of Chicago Press, Chicago, pp 622–679
Meyer CG (2007) Tropenmedizin: Infektionskrankheiten. Hüthig Jehle Rehm GmbH, ecomed Medizin, 51 pp
Miller BR, Godsey MS, Crabtree MB, Savage HM, Al-Mazrao Y, Al-Jeffri MH, Abdoon A-M M, Al-Seghayer SM, Al-Shahrani AM, Ksiazek TG (2002) Isolation and genetic characterization of Rift Valley fever virus from Aedes vexans arabiensis, Kingdom of Saudi Arabia. Emerg Infect Dis 8:1492–1494
Modlmaier M, Kuhn R, Kaaden O-R, Pfeffer M (2002) Transmission studies of European Sindbis virus in the floodwater mosquito Aedes vexans (Diptera: Culicidae). Int J Med Microbiol 291:164–170
Mohrig W (1969) Die Culiciden Deutschlands. Parasitologische Schriftenreihe 18, Gustav Fischer Verlagm, Jena, 260 pp
Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, David-West TS, Kemp GE (1975) Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol 69:49–64
Morse RP, Bennish ML, Darras BT (1992) Eastern equine encephalitis presenting with a focal brain lesion. Pediatr Neurol 8:473–475
Murray KO, Mertens E, Desprès P (2010) West Nile virus and its emergence in the United States of America. Vet Res 41:67
Nabeshima T, Loan HTK, Inoue S, Sumiyoshi M, Haruta Y, Nga PT, Huoung VTQ, del Carmen PM, Hasebe F, Morita K (2009) Evidence of frequent introductions of Japanese encephalitis virus from south-east Asia and continental east Asia to Japan. J Gen Virol 90:827–832
Nalca A, Fellows PF, Whitehouse CA (2003) Vaccines and animal models for arboviral encephalitides. Antiviral Res 60:153–174
Netolitzky DJ, Schmaltz FL, Parker MD, Rayner GA, Fisher GR, Trent DW, Bader DE, Nagata LP (2000) Complete genomic RNA sequence of western equine encephalitis virus and expression of the structural genes. J Gen Virol 81:151–159
Norder H, Lundström JO, Kozuch O, Magnius LO (1996) Genetic relatedness of Sindbis virus strains from Europe, Middle East, and Africa. Virology 222:440–445
Novotny V, Basset Y, Miller SE, Weiblen GD, Bremer B, Cizek L, Drozd P (2002) Low host specificity of herbivorous insects in a tropical forest. Nature 416:841–844
Oelofsen MJ, van der Ryst E (1999) Could bats act as reservoir hosts for Rift Valley fever virus? Onderstepoort J Vet Res 66:51–54
Omatsu T, Watanabe S, Akashi H, Yoshikawa Y (2007) Biological characters of bats in relation to natural reservoir of emerging viruses. Comp Immunol Microbiol Infect Dis 30:357–374
Osborne JC, Rupprecht CE, Olson JG, Ksiazek TG, Rollin PE, Niezgoda M, Goldsmith CS, An US, Nichol ST (2003) Isolation of Kaeng Khoi virus from dead Chaerephon plicata bats in Cambodia. J Gen Virol 84:2685–2689
PAHO (2011) Preparedness and response for Chikungunya virus introduction in the Americas. http://new.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=16984&Itemid=
Paul SD, Rajagopalan PK, Screenivasan MA (1970) Isolation of the West Nile virus from the frugivorous bat, Rousettus leschenaultia. Indian J Med Res 58:1–3
Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J (2010) Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res 41:61
Pilipski JD, Pilipski LM, Risley LS (2004) West Nile virus antibodies in bats from New Jersey and New York. J Wildl Dis 40:335–337
Pires DA, Gleiser RM (2010) Mosquito fauna inhabiting water bodies in the urban environment of Córdoba city, Argentina, following a St. Louis encephalitis outbreak. J Vector Ecol 35:401–409
Pittman PR, Makuch RS, Mangiafico JA, Cannon TL, Gibbs PH, Peters CJ (1996) Log-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine 14:337–343
Platt KB, Mangiafico JA, Rocha OJ, Zaldivar ME, Mora J, Trueba G, Rowley WA (2000) Detection of Dengue virus neutralizing antibodies in bats from Costa Rica and Ecuador. J Med Entomol 37:965–967
Powers AM, Logue CH (2007) Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 88:2363–2377
Price JL (1978) Serological evidence of infection of Tacaribe virus and arboviruses in Trinidadian bats. Am J Trop Med Hyg 27:162–167
Rappole JH, Derrickson SR, Hubálek Z (2000) Migratory birds and spread of West Nile virus in the Western hemisphere. Emerg Infect Dis 6:319–328
Ravanini P, Huhtamo E, Ilaria V, Crobu MG, Nicosia AM, Servino L, Rivasi F, Allegrini S, Miglio U, Magri A, Minisini R, Vapalahti O, Boldorini R (2012) Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Euro Surveill 17(28), pii = 20221
Ready P (2013) Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol 58:227–250
Reagan RL, Rumbaugh H, Nelson H, Brueckner AL (1955) Effect of Zika virus and Bwamba virus in the cave bat (Myotis lucifugus). Trans Am Microsc Soc 74:77–79
Reinert JF, Harbach RE, Kitching IJ (2004) Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool J Linn Soc 142:289–368
Reisen W (2003) Epidemiology of St. Louis encephalitis virus. Adv Virus Res 61:139–183
Reiskind MH, Wund MA (2009) Experimental assessment of the impacts of northern long-eared bats on ovipositing Culex (Diptera: Culicidae) Mosquitoes. J Med Entomol 49:1037–1043
Reiskind MH, Wund MA (2010) Bats & mosquitoes. Testing conventional wisdom. Bats 28:6–7
Reisler RB, Gibbs PH, Danner DK, Boudreau EF (2012) Immune interference in the setting of same-day administration of two similar inactivated alphavirus vaccines: eastern equine and western equine encephalitis. Vaccine 30:7271–7277
Reiter P, Fontenille D, Paupy C (2006) Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect Dis 6:463–464
Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A (2007) Infection with Chikungunya virus in Italy: an outbreak in a temperature region. Lancet 370:1840–1846
Rivas F, Diaz LA, Cardenas VM, Daza E, Bruzon L, Alcala A, De la Hoz O, Caceres FM, Aristizabal G, Martinez JW, Revelo D, De la Hoz F, Boshell J, Camacho T, Calderon L, Olano VA, Villarreal LI, Roselli D, Alvarez G, Ludwig G, Tsai T (1997) Epidemic Venezuelan equine encephalitis in La Guajira, Colombia, 1995. J Infect Dis 175:828–832
Rodrigues SG, Nunes MRT, Casseb SMM, Prazeres ASC, Rodrigues DSG, Silva MO, Cruz ACR, Tavares-Neto JC, Vasconcelos PFC (2010) Molecular epidemiology of Saint Louis encephalitis virus in the Brazilian Amazon: genetic divergence and dispersal. J Gen Virol 91:2420–2427
Rolle M, Mayr A (2007) Medizinische Mikrobiologie, Infektions- und Seuchenlehre: Für Tierärzte. Enke Verlag, Stuttgart, 627 pp
Romero JR, Newland JG (2003) Viral meningitis and encephalitis: traditional and emerging viral agents. Semin Pediatr Infect Dis 14:72–82
Rossi C, Rey O, Jenik P, Franze-Fernandez MT (1996) Immunological identification of Tacaribe virus proteins. Res Virol 147:203–211
Russell PK (1999) Vaccines in civilian defense against bioterrorism. Emerg Infect Dis 5:531–533
Russell RC (2002) Ross River virus: ecology and distribution. Annu Rev Entomol 47:1–31
Ryan PA, Martin L, Mackenzie JS, Kay BH (1997) Investigation of gray-headed flying foxes (Pteropus poliochephalus) (Megachiroptera: Pteropodidae) and mosquitoes in the ecology of Ross River virus in Australia. Am J Trop Med Hyg 57:476–482
Rydell J, Parker McNeill D, Eklöf J (2002) Capture success of little brown bats (Myotis lucifugus) feeding on mosquitoes. J Zool 256:379–381
Sailer R (1952) The bedbug. An old bedfellow that’s still with us. Pest Control 10:22–24, 70, 72
Sallum MAM, Schultz TR, Wilkerson RC (2000) Phylogeny of Anophelinae (Diptera Culicidae) based on morphological characters. Ann Entomol Soc Am 93:745–775
Sam I-C, Path MRC, AbuBakar S (2006) Chikungunya virus infection. Med J Malaysia 61:264–270
Sambri V, Cavrini F, Rossini G, Pierro A, Landini MP (2008) The 2007 epidemic outbreak of Chikungunya virus infection in the Romagna region of Italy: a new perspective for the possible diffusion of tropical diseases in temperate areas. New Microbiol 31:303–304
Sanbonmatsu-Gámez S, Pérez-Ruiz M, Collao X, Sánchez-Seco MP, Morillas-Márquez F, De la Roas-Fraile M, Navarro-Marí JM, Tenorio A (2005) Toscana virus in Spain. Emerg Infect Dis 11:1701–1707
Sarkar SK, Chakravarty AK (1991) Analysis of immunocompetent cells in the bat, Pteropus giganteus: isolation and scanning electron microscopic characterization. Dev Comp Immunol 15:423–430
Schäfer M, Storch V, Kaiser A, Beck M, Becker N (1997) Dispersal behavior of adult snow melt mosquitoes in the Upper Rhine Valley, Germany. J Vector Ecol 22:1–5
Scherer WF, Campillo-Sainz C, de Mucha-Macias J, Dickerman RW, Chia CW, Zarate ML (1972) Ecologic studies of venezuelan encephalitis virus in southeastern Mexico. VII. Infection of man. Am J Trop Med Hyg 21:79–85
Schwartz O, Albert ML (2010) Biology and pathogenesis of Chikungunya virus. Nat Rev Microbiol 8:491–500
Scott TW (2001) Are bats really involved in Dengue virus transmission? J Med Entomol 38:771–772
Scott TW, Weaver SC (1989) Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res 37:277–328
Service MW (1993) Mosquitoes (Culicidae). In: Lane RP, Grosskey RW (eds) Medical insects and arachnids. Chapman & Hall, London, pp 120–240
Seymour C, Dickerman R, Martin MS (1978a) Venezuelan encephalitis virus infection in neotropical bats. I. Natural infection in a Guatemalan enzootic focus. Am J Trop Med Hyg 27:290–296
Seymour C, Dickerman R, Martin MS (1978b) Venezuelan encephalitis virus infection in neotropical bats. II. Experimental infections. Am J Trop Med Hyg 27:297–306
Simmons JS, Reynolds FHK, Cornell VH (1936) Transmission of the virus of equine encephalomyelitis through Aedes albopictus. Am J Trop Med Hyg 16:289–302
Simpson DI (1964) Zika virus infection in man. Trans R Soc Trop Med Hyg 58:335–338
Simpson DIH, O’Sullivan JP (1968) Studies on arbovirus on bats (Chiroptera) in East Africa. I.-Experimental infection of bats and virus transmission attempts in Aedes (Stegomyia) aegypti (Linnaeus). Ann Trop Med Parasitol 62:422–431
Smith DR, Adams AP, Kenney JL, Wang E, Weaver SC (2008) Venezuelan equine encephalitis virus in the mosquito vector Aedes taeniorhynchus: infection initiated by a small number of susceptible epithelial cells and a population bottleneck. Virology 372:176–186
Smith DW, Speers DJ, Mackenzie JS (2011) The viruses of Australia and the risk to tourists. Travel. Med Infect Dis 9:113–125
Smithburn KC, Mahaffy AF, Paul JH (1941) Bwamba fever and its causative agent. Am J Trop Med 21:75–90
Snodgrass RE (1959) The anatomical life of the mosquito. Smithsonian Misc Collect 139:1–85
Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI (2005) The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214–217
Soldan SS, González-Scarano F (2005) Emerging infectious diseases: the Bunyaviridae. J Neurovirol 11:412–423
Solomon T, Ni H, Beasley DWC, Ekkelenkamp M, Cardosa MJ, Barrett ADT (2003) Origin and evolution of Japanese encephalitis virus in Southeast Asia. J Virol 77:3091–3098
Spence L, Anderson CR, Downs WG (1962) Isolation of Ilheus virus from human beings in Trinidad, West Indies. Trans R Soc Trop Med Hyg 56:504–509
Spinsanti L, Basquiera AL, Bulacio S, Somale V, Kim SCH, Ré V, Rabbat D, Zárate A, Zlocowski JC, Mayor CR, Contigiani M, Palacio S (2003) St. Louis encephalitis in Argentina: the first case reported in the last seventeen years. Emerg Infect Dis 9:271–273
Srihongse S, Johnson CM (1967) Isolation of Ilheus from man in Panama. Am J Trop Med Hyg 16:516–518
Strauss JH, Strauss EG (1994) The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 58:491–562
Sudia WD, Newhouse VF, Henderson BE (1971) Experimental infection of horses with three strains of Venezuelan equine encephalomyelitis virus II. Experimental vector studies. Am J Epidemiol 93:206–211
Sulkin SE, Allen R, Miura T, Toyokawa K (1970) Studies of arthropod-borne virus infections in Chiroptera. VI Isolation of Japanese B encephalitis virus from naturally infected bats. Am J Trop Med Hyg 19:77–87
Tajima S, Takasaki T, Matsuno S, Nakayama M, Kurane I (2005) Genetic characterization of Yokose virus, a Flavivirus isolated from the bat in Japan. Virology 332:38–44
Teeling EC, Springer MS, Madsen O, Bates P, O’Brien SJ, Murphy WJ (2005) A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307:580–584
Tesh RB (1982) Arthritides caused by mosquito-borne viruses. Annu Rev Med 33:31–40
Tesh RB, Jahrling PB, Salas R, Shope RE (1994) Description of Guanarito virus (Arenaviridae: Arenavirus), the etiologic agent of Venezuelan hemorrhagic fever. Am J Trop Med Hyg 50:452–459
Thomas SM, Fischer D, Fleischmann S, Bittner T, Beierkuhnlein C (2011) Risk assessment of Dengue virus amplification in Europe based on spatio-temporal high resolution climate change projectations. Erdkunde 65:137–150
Tiawsirisup S (2011) Chikungunya virus in Thailand; an update. Thai J Vet Med 41:133–134
Tiawsisirsup S, Junpee A, Nuchprayoon S (2012) Mosquito distribution and Japanese encephalitis virus infection in a bat cave and its surrounding area in Lopburi province, Central Thailand. Thai J Vet Med 42:43–49
Tikasingh ES, Ardoin P, Williams MC (1974) First isolation of Catú virus from a human in Trinidad. Trop Geogr Med 26:414–416
Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S (2007) A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3:e201
Ubico SR, McLean RG (1995) Serologic survey of neotropical bats in Guatemala for virus antibodies. J Wildl Dis 31:1–9
Usinger RL (1966) Monograph of Cimicidae. Entomological Society of America, Washington, DC, 585 pp
Valassina M, Cusi MG, Valensin PE (2003) A Mediterranean arbovirus: the Toscana virus. J Neurovirol 9:577–583
Van der Hurk AF, Ritchie SA, Mackenzie JS (2009a) Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol 54:17–35
Van der Hurk AF, Smith CS, Field HE, Smith IL, Northill JA, Taylor CT, Jansen CC, Smith GA, Mackenzie JS (2009b) Transmission of Japanese encephalitis virus from the black flying fox, Pteropus alecto, to Culex annulirostris mosquitoes, despite the absence of detectable viremia. Am J Trop Med Hyg 81:457–462
Venegas EA, Aguilar PV, Cruz C, Guevara C, Kochel TJ, Vargas J, Halsey ES (2012) Ilheus virus infection in human, Bolivia. Emerg Infect Dis 18:516–518
Wang J-L, Pan X-L, Zhang H-L, Fu S-H, Wang H-Y, Tang Q, Wang L-F, Liang G-D (2009) Japanes encephalitis viruses from bats in Yunnan, China. Emerg Infect Dis 15:939–942
Weaver SC, Bellew LA, Rico-Hesse R (1992) Phylogenetic analysis of alphaviruses in the Venezuelan equine encephailitis complex and identification of the source of epizootic viruses. Virology 191:282–290
Weaver SC, Ferro C, Barrera R, Boshell J, Navarro JC (2004) Venezuelan equine encephalitis. Annu Rev Entomol 49:141–174
Weidmann M, Rudaz V, Nunes MRT, Vasconcelos PFC, Hufert FT (2003) Rapid detection of human pathogenic orthobunyaviruses. J Clin Microbiol 41:3299–3305
Weinstein P, Judge D, Carver S (2011) Biological and cultural coevolution and emerging infection disease: Ross River virus in Australia. Med Hypotheses 76:893–896
Whitley RJ, Gnann JW (2002) Viral encephalitis: familiar infections and emerging pathogens. Lancet 359:507–513
WHO (2000) Report on global surveillance of epidemic-prone infectious diseases. WHO/CDS/CSR/ISR/20001, pp 1–15
WHO (2004) The vector-borne human infections of Europe – their distribution and burden on public health. http://www.euro.who.int/__data/assets/pdf_file/0008/98765/e82481.pdf
WHO (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. http://www.who.int/rpc/guidelines/9789241547871/en/
WHO (2011) West Nile virus, fact sheet N° 354. http://www.who.int/mediacentre/factsheets/fs354/en/index.html
WHO (2012) Global alert and response (GAR), Rift Valley fever in Mauretania. http://www.who.int/csr/don/2012_11_01/en/index.html
WHO (2013a) Global alert and response (GAR), Rift Valley fever. http://www.who.int/csr/disease/riftvalleyfev/en/
WHO (2013b) Rift Valley fever, fact sheet N° 207. http://www.who.int/mediacentre/factsheets/fs207/en/
Williams MC, Simpson DIH, Shepherd RC (1964) Bats and arbovirus in east Africa. Nature 203:670
Williams JE, Imlarp S, Top FH Jr, Cavanaugh DC, Russell PK (1976) Kaeng Khoi virus from naturally infected bedbugs (Cimicidae) and immature free-tailed bats. Bull World Health Organ 53:365–369
Witsenburg F, Salamin N, Christe P (2012) The evolutionary host switches of Polychromophilus: a multi-gene phylogeny of the bat malaria genus suggests a second invasion of mammals by a haemosporidian parasite. Malar J 11:53
Wong S, Lau S, Woo P, K-Ym Y (2007) Bat as a continuing source of emerging infections in humans. Rev Med Virol 17:67–91
Woods CW, Karpati AM, Grein T, McCarthy N, Gaturuku P, Muchiri E, Dunster L, Henderson A, Khan AS, Swanepoel R, Bonmarin I, Martin L, Mann P, Smoak BL, Ryan M, Ksiazek TG, Arthur RR, Ndikuyeze A, Agata NN, Peters CJ (2002) An outbreak of Rift Valley fever in northeastern Kenya, 1997–98. Emerg Infect Dis 8:138–144
Zacks MA, Paessler S (2010) Encephalitic alphaviruses. Vet Microbiol 140:281–286
Zeledón R, Rabinovich JE (1981) Chagas’ disease: an ecological appraisal with special emphasis on its insect vectors. Annu Rev Entomol 26:101–133
Zeller HG, Schuffenecker I (2004) West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur J Clin Microbiol Infect Dis 23:147–156
Acknowledgements
The present study was financially supported by the research funding programme “LOEWE—Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of Hesse’s Ministry of Higher Education, Research, and the Arts and by the SAW (Senate Competition Committee) grant (SAW-2011-BNI-3) of the Leibniz Association.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Melaun, C., Werblow, A., Busch, M.W., Liston, A., Klimpel, S. (2014). Bats as Potential Reservoir Hosts for Vector-Borne Diseases. In: Klimpel, S., Mehlhorn, H. (eds) Bats (Chiroptera) as Vectors of Diseases and Parasites. Parasitology Research Monographs, vol 5. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39333-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-642-39333-4_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39332-7
Online ISBN: 978-3-642-39333-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)