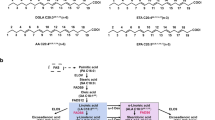
Nomenclature
EC number
1.14.19.5
Systematic name
acyl-CoA,hydrogen donor:oxygen Δ11-oxidoreductase
Recommended name
Δ11-fatty-acid desaturase
Synonyms
APTQ desaturase <13> [15]
Cro-Z/E11 <6> [8]
D11 desaturase <3,4,10> [6,12,13,16]
D11-(Z)-desaturase <1,2> [4]
D11-desaturase <5,6,8,9,14,15,16> (<5> several D11-desaturase systems: one
produces a large quantitiy of (Z)-11-hexadecenoic acid and another produces
(E)1-tetradecenoic acid [7]) [2,7,8,9,17,18]
D11-fatty-acid desaturase <17,18> [19,20]
D11-myristoyl-CoA desaturase <4> [1]
D11-palmitoyl-CoA-desaturase <4> [3]
D11-palmitoyl-coenzyme A desaturase <6> [8]
Dpu-D11-APSQ <17> [19]
Dpu-D11-LPAE <17> [19]
LATPG1 <18> [20]
Lca-KPVQ <14> [17]
OfuZ/E11 protein <15> [18]
OscZ/E11 protein <16> [18]
PDesat-TnD11 Z protein <7> [11]
Sls//E11 <10> [6]
TpDESN <9> [2]
Z/E11-desaturase <6> [8]
acyl-CoA <6> [8]
acyl-CoA D11-desaturase <7> [11]
acyl-CoA desaturase <14> [17]
bifunctional Δ11-desaturase <4> [12]
bifunctional Z-Δ11-desaturase <13> [15]
fatty acid Δ11-desaturase <6> [8]
sphingolipid long chain base Δ8 desaturase <8> [9]
CAS registry number
77000-04-5
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Pinilla, A.; Camps, F.; Fabrias, G.: Cryptoregiochemistry of the Δ11-myristoyl-CoA desaturase involved in the biosynthesis of Spodoptera littoralis sex pheromone. Biochemistry, 38, 15272-15277 (1999)
Tonon, T.; Harvey, D.; Qing, R.; Li, Y.; Larson, T.R.; Graham, I.A.: Identification of a fatty acid Δ11-desaturase from the microalga Thalassiosira pseudonana. FEBS Lett., 563, 28-34 (2004)
Rodriguez, F.; Hallahan, D.L.; Pickett, J.A.; Camps, F.: Characterization of the Δ11 -palmitoyl-CoA-desaturase from Spodoptera littoralis (Lepidoptera:-Noctuidae). Insect Biochem. Mol. Biol., 22, 143-148 (1992)
Svatos, A.; Kalinova, B.; Boland, W.: Stereochemistry of lepidopteran sex pheromone biosynthesis: a comparison of fatty acid-CoA Δ11-(Z)-desaturases in Bombyx mori and Manduca sexta female moths. Insect Biochem. Mol. Biol., 29, 225-232 (1999)
Liu, W.; Jiao, H.; O’Connor, M.; Roelofs, W.L.: Moth desaturase characterized that produces both Z and E isomers of Δ11-tetradecenoic acids. Insect Biochem. Mol. Biol., 32, 1489-1495 (2002)
Rodriguez, S.; Hao, G.; Liu, W.; Pina, B.; Rooney, A.P.; Camps, F.; Roelofs, W.L.; Fabrias, G.: Expression and evolution of Δ9 and Δ11 desaturase genes in the moth Spodoptera littoralis. Insect Biochem. Mol. Biol., 34, 1315-1328 (2004)
Wolf, W.A.; Roelofs, W.L.: Reinvestigation confirms action of Δ11-desaturases in spruce budworm moth sex pheromone biosynthesis. J. Chem. Ecol., 13, 1019-1027 (1987)
Hao, G.; O’Connor, M.; Liu, W.; Roelofs, W.L.: Characterization of Z/E11- and Z9-desaturases from the obliquebanded leafroller moth, Choristoneura rosaceana. J. Insect Sci., 2, 26 (2002)
Abad, J.L.; Villorbina, G.; Fabrias, G.; Camps, F.: Synthesis and use of stereospecifically deuterated analogues of palmitic acid to investigate the stereochemical course of the Δ11 desaturase of the processionary moth. J. Org. Chem., 69, 7108-7113 (2004)
Abad, J.L.; Villorbina, G.; Fabrias, G.; Camps, F.: Synthesis of fluorinated analogs of myristic acid as potential inhibitors of Egyptian armyworm (Spodoptera littoralis) DELA11 desaturase. Lipids, 38, 865-871 (2003)
Knipple, D.C.; Rosenfield, C.L.; Miller, S.J.; Liu, W.; Tang, J.; Ma, P.W.; Roelofs, W.L.: Cloning and functional expression of a cDNA encoding a pheromone gland-specific acyl-CoA Δ11-desaturase of the cabbage looper moth,Trichoplusia ni. Proc. Natl. Acad. Sci. USA, 95, 15287-15292 (1998)
Serra, M.; Pina, B.; Bujons, J.; Camps, F.; Fabrias, G.: Biosynthesis of 10,12-dienoic fatty acids by a bifunctional Δ11 desaturase in Spodoptera littoralis. Insect Biochem. Mol. Biol., 36, 634-641 (2006)
Serra, M.; Gauthier, L.T.; Fabrias, G.; Buist, P.H.: Δ11 desaturases of Trichoplusia ni and Spodoptera littoralis exhibit dual catalytic behaviour. Insect Biochem. Mol. Biol., 36, 822-825 (2006)
Fukuzawa, M.; Fu, X.; Tatsuki, S.; Ishikawa, Y.: cDNA cloning and in situ hybridization of Δ11-desaturase, a key enzyme of pheromone biosynthesis in Ostrinia scapulalis (Lepidoptera: Crambidae). J. Insect Physiol., 52, 430-435 (2006)
Matouskova, P.; Pichova, I.; Svatos, A.: Functional characterization of a desaturase from the tobacco hornworm moth (Manduca sexta) with bifunctional Z11- and 10,12-desaturase activity. Insect Biochem. Mol. Biol., 37, 601-610 (2007)
Villorbina, G.; Roura, L.; Camps, F.; Joglar, J.; Fabrias, G.: Enzymatic desaturation of fatty acids: Δ11 desaturase activity on cyclopropane acid probes. J. Org. Chem., 68, 2820-2829 (2003)
Lienard, M.A.; Strandh, M.; Hedenstroem, E.; Johansson, T.; Loefstedt, C.: Key biosynthetic gene subfamily recruited for pheromone production prior to the extensive radiation of Lepidoptera. BMC Evol. Biol., 8, 270 (2008)
Sakai, R.; Fukuzawa, M.; Nakano, R.; Tatsuki, S.; Ishikawa, Y.: Alternative suppression of transcription from two desaturase genes is the key for species-specific sex pheromone biosynthesis in two Ostrinia moths. Insect Biochem. Mol. Biol., 39, 62-67 (2009)
Lienard, M.A.; Lassance, J.M.; Wang, H.L.; Zhao, C.H.; Piskur, J.; Johansson, T.; Loefstedt, C.: Elucidation of the sex-pheromone biosynthesis producing 5,7-dodecadienes in Dendrolimus punctatus (Lepidoptera: Lasiocampidae) reveals Δ11- and Δ9-desaturases with unusual catalytic properties. Insect Biochem. Mol. Biol., 40, 440-452 (2010)
Fujii, T.; Ito, K.; Tatematsu, M.; Shimada, T.; Katsuma, S.; Ishikawa, Y.: Sex pheromone desaturase functioning in a primitive Ostrinia moth is cryptically conserved in congeners genomes. Proc. Natl. Acad. Sci. USA, 108, 7102-7106 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Schomburg, D., Schomburg, I. (2013). Δ11-fatty-acid desaturase 1.14.19.5. In: Schomburg, D., Schomburg, I. (eds) Class 1 Oxidoreductases. Springer Handbook of Enzymes. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36265-1_96
Download citation
DOI: https://doi.org/10.1007/978-3-642-36265-1_96
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36264-4
Online ISBN: 978-3-642-36265-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)