Abstract
Revascularization of splanchnic and renal arteries is one of the critical steps in open repair of thoracoabdominal aortic aneurysm (TAAA). Several techniques are currently employed, but the most represented is the inclusion technique proposed by Stanley Crawford in the early 1970s: it consists in a side-to-end anastomosis aimed at reimplanting, on a side opening in the synthetic Dacron graft, an island of aortic wall from which the visceral arteries arise [1].
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Revascularization of splanchnic and renal arteries is one of the critical steps in open repair of thoracoabdominal aortic aneurysm (TAAA). Several techniques are currently employed, but the most represented is the inclusion technique proposed by Stanley Crawford in the early 1970s: it consists in a side-to-end anastomosis aimed at reimplanting, on a side opening in the synthetic Dacron graft, an island of aortic wall from which the visceral arteries arise [1].
The main drawback of this technique is that the retained portion of the diseased aorta might be prone to further dilatation, thus giving rise to a visceral aortic patch (VAP) aneurysm. The prevalence of this late complication is currently underreported, although the rate of VAP aneurysm (>5 cm) ranges from 1.1% to 7.7% after a median time interval from the index procedure ranging from 7 months to 16 years [2,3,4,5].
Different authors [2, 3, 6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] published small series regarding both redo-open or hybrid TAAA repair with high mortality and morbidity rate, and only two authors proposed a fenestrated or branched approach for two single cases [22, 23].
The real prevalence and incidence of VAP aneurysm are hard to define because long-term follow-up protocols are often lacking and a relevant proportion of TAAA patients have brief postoperative survival due to associated morbidities. However, everlasting follow-up imaging is warranted. Two clear risk factors have been associated with VAP aneurysm formation: the size of the aortic patch used to include the reimplanted visceral vessels and connective tissue disorders [24, 25].
Reimplantation of visceral vessels with the standard inclusion technique in open TAAA can be performed either with side-to-end anastomosis of an aortic patch including all vessels or including only three out of four vessels with separate reimplantation on the graft of the remaining vessel, usually the left renal artery. Obviously, the size of a four-vessel patch is going to be larger than that of a three-vessel patch, with a consequent increase in the surface of the diseased aortic wall kept exposed to the stress of systemic blood pressure. Notably, no VAP dilatation or aneurysm of a three-vessel patch was recorded in an old San Raffaele series despite the fact that in 23% of the patients of the cohort, an inclusion technique was employed to reattach the celiac trunk, the superior mesenteric, and the right renal artery [3]. Nowadays, the 3 + 1 configuration is preferred for splanchnic and renal revascularization [26].
Interestingly, the data emerging from Johns Hopkins series demonstrated that the incidence of VAP aneurysm was much higher in patients affected by collagenopathy 17.6%, with respect to atherosclerotic patients 5.6% (p = 0.034) [2]. For those reasons, in order to ensure a durable repair, in young or connective disease patients, we employ a presewn four-branch Dacron graft to revascularize the visceral vessels during open TAAA repair, thus minimizing the amount of residual diseased aorta, thereby preventing future development of VAP aneurysms [15]. The branched graft also provides an alternate repair option in patients whose visceral and renal arteries are too far apart to obtain a reasonably sized aortic patch [27] (Fig. 24.1).
Different types of revascularization of splanchnic and renal arteries during open repair. (a) Three-vessel visceral aortic patch (VAP) with separate surgical bypass to the left renal artery. (b) Three-vessel patch with sutureless reimplantation of the left renal artery. (c) Separate reimplantation of renal and splanchnic arteries with quadrifurcated branched presewn Dacron graft
2 Surgical Management
Surgical management consists in an open repair with re-inclusion technique via a thoraco-phreno-laparotomy access. All the visceral vessels were separately reimplanted with quadrifurcated branched Dacron graft (Vascutek Ltd., Renfrewshire, Scotland, UK) in the most recent seven cases (50%), and in the other cases, a combination of undersized VAP and separated bypasses was used [12]. No extracorporeal circulation was used and an intraoperative hypothermic selective perfusion through 9F Pruitt-Inahara occlusion/perfusion catheters (LeMaitre Vascular, Inc., Burlington, MA) of the splanchnic vessels with cold crystalloid (lactate ringer at 4 °C) and of the renal arteries with cold enriched crystalloid solution (Custodiol 4 °C; Dr. Franz Kohler Chemie 95 GmbH, Bensheim, Germany) [28] was performed (Table 24.1, 96 Fig. 24.2).
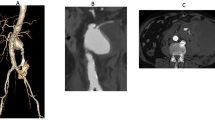
(a) Preoperative three-dimensional reconstruction of a visceral aortic patch (VAP). (b) Treatment was by means of separate reimplantation of renal and splanchnic arteries with quadrifurcated branched presewn Dacron graft (Vascutek Ltd., Renfrewshire, Scotland, UK). (c) The postoperative computed tomography scan demonstrates patency of all vessels reimplanted
3 Hybrid Management
Hybrid management, instead, consists in a single-staged repair with retrograde splanchnic and renal debranching with Dacron graft (6–8 mm in diameter) from the previous infrarenal graft or from the common iliac artery and VAP aneurysm exclusion using commercially available endograft [12]. The abdominal aorta, common iliac arteries, and the first 2 cm from the origin of the common hepatic artery, superior mesenteric artery, and renal arteries were exposed through a transperitoneal midline approach with the patient in a supine position. Separate bypass should be used for each recipient vessel, customized “Y” grafts, customized trifurcated grafts, or single bypass with sequential graft technique related to the specific anatomy of every case. For celiac trunk revascularization, the graft was routed in front of the renal vein behind the pancreas, an arteriotomy was made in the common hepatic artery, and an end-to-side anastomosis was made. An end-to-end anastomosis was usually preferred for the superior mesenteric artery, and this type of anastomosis was always used for the renal arteries. A radiopaque marker was placed at the origin of the more proximal visceral graft when originating from native or grafted abdominal aorta. The stent graft is inserted from the common femoral artery surgically exposed, and the ballooning was performed according to the instructions for use of the device (Table 24.1, Fig. 24.3).
(a) Preoperative three-dimensional reconstruction of a visceral aortic patch (VAP). (b) Treatment with a hybrid repair. A retrograde visceral and renal bypass from the right iliac artery to revascularize the right renal artery, superior mesenteric artery, and celiac trunk was performed, followed by endovascular exclusion of the aneurysm. The left renal artery revascularization was unsuccessful because of intraoperative adhesions. (c) The postoperative computed tomography scan demonstrates complete exclusion of the aneurysm and patency of target vessels
4 Endovascular Management
The experience on visceral aortic patch aneurysm management published in the literature highlights morbidity and mortality rates unfavorable with both redo-open TAAA repair and with hybrid approach [2, 3, 6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. These results are the rationale for an attempt of total endovascular repair of VAP aneurysms. Adam et al. reported the first endovascular treatment of a VAP aneurysm with a fenestrated custom-made device in 2007 [22]. Worldwide, three other single cases were treated by means of endovascular approach with octopus graft, surgeon-modified graft, or fenestrated graft [22,23,24,25].
The latter was used in elective cases and the first two options in emergent or symptomatic cases.
The advantages of this endovascular approach compared to either open or hybrid surgery are evident: it avoids a redo thoracotomy and/or laparotomy, thus reducing the risk of blood loss, respiratory complications, and organ injuries due to severe perivisceral adhesions, leading to prolonged ICU and in-hospital stay. Moreover, the visceral reconstruction might be jeopardized by such adhesions as well as by concomitant steno-occlusive disease of target vessels with the subsequent increase in risk of end-organ ischemia and related deaths (Table 24.1, Figs. 24.4 and 24.5) [6].
Schematic drawing (a) and preoperative three-dimensional reconstruction (b) demonstrate a visceral aortic patch (VAP) aneurysm; the celiac trunk and the renal arteries were patent. The superior mesenteric artery was occluded at the origin, with distal revascularization from anastomotic collaterals arising from both the celiac trunk and a patent inferior mesenteric artery (a). A two-fenestration and one-branch custom-made device was designed to seal the aneurysm: the branch was targeted to the left renal artery (LRA) with an indwelling catheter (c). The postoperative scan (d) demonstrates successful exclusion of the aneurysm with patency of all target vessels
Schematic drawing (a) and preoperative three-dimensional reconstruction (b) demonstrate a visceral aortic patch (VAP) aneurysm with all the visceral vessel patents. However, the celiac trunk presented with a tight stenosis near the patch suture line, and the left renal artery common trunk was shortened by the previous reimplantation. There was caliber mismatch between the native aorta and the surgical graft, so the planned strategy was a two-step repair. The first step was aimed at fixing the dilation at the level of the proximal anastomosis with a custom-made short-tapered thoracic component to create a proximal fixation site for the four fenestrations of the custom-made endograft (c) to be deployed in the second step. (d) Postoperative scan confirms complete exclusion of the VAP aneurysm with patency of all target vessels
5 Discussion
According to our experience, endovascular treatment of VAP aneurysm with custom-made devices brings along several technical challenges.
5.1 Graft Design
Firstly, the graft should be carefully designed, taking into consideration the reduced compliance of the Dacron graft (maximum 4 mm of oversizing instead of 20%), the limited room for guidewires and catheters maneuverability, and the alteration in visceral vessel anatomy induced by the aneurysmal degeneration of the patch. The presence of the previous surgical graft, as well as the reported [29, 30] access limitations, might worsen the navigation of the endograft and the ability to orientate the fenestrations in the desired position. For these reasons, it is of vital importance that the initial careful and correct opening of the custom-made device with double-diameter-reducing wires is of paramount importance.
5.2 Branch or Fenestration?
Secondly, when choosing graft designs, some adjustments are necessary in the treatment of this pathology: branches, despite being easier to manage in a more complex anatomy, are not an option for celiac trunk (CT) revascularization as its ostium is too close to the apex of suture line of the VAP, and no room for branch expansion within the previous surgical graft is left. Moreover, the splanchnic vessel course is distorted by the aneurysm itself, and, as the VAP dilates, both the CT and the superior mesenteric artery (SMA) tend to take a more and more upward direction. This typical upward direction mandates again a fenestrated design to facilitate target vessel catheterization and avoid kinking of bridging stents. The right renal artery (RRA)-bearing component is typically designed with a fenestration because of its proximity to the bottom of VAP suture line which brings it close to the surgical graft wall. Due to the likelihood of surgical modifications during initial open TAAA repair, the CT and the left renal artery (LRA) are the most complex vessels. When they are selectively anastomosed to the graft, the remaining vessel portion without branches is usually shortened, thus providing a decreased distal sealing zone for the bridging stent; while if the initial revascularization of the left renal artery had instead required graft interposition or direct stenting of the ostium, target vessel catheterization could be more difficult. In these cases, a branch design with a preloaded indwelling catheter can be considered to deal with these challenging vessel characteristics. Furthermore, in cases in which we anticipated the possible failure of targeting a vessel, the branch design allows, in the event of missed cannulation, the option of plugging it, in a second stage, after having performed revascularization of the missing vessel with an extra-anatomic bypass (i.e., iliac to renal bypass) [29].
5.3 Spinal Cord Ischemia
The last critical point to consider in the management of this pathology is the prevention of spinal cord ischemia. The eventual presence of intercostal artery patches or selective reimplantations on the surgical graft at the level of the proximal sealing zone should be evaluated, and while planning the custom-made graft, any attempt of not covering the intercostal patch with either the fabric or the proximal bare stent must be made. If sparing of this area is unachievable, a careful evaluation of risks/benefits needs to be carried on, staging should be considered, and preoperative cerebrospinal fluid drainage is strongly recommended. Such staging can be performed with an initial thoracic stent grafting in order to create a proximal sealing zone with covering the intercostal patch, if present, or a portion of the native thoracic aorta proximal to the open repair. Even if no adjunctive native aorta coverage is required, other factors might determine spinal cord ischemia such as hypotension, anemia, and intraoperative temporary hypogastric artery occlusion by the delivery system. Indeed, the whole planning procedure should be aimed at early removal of large femoral sheaths in order to reduce the pelvic and lower limb ischemia time [30]. However, in our experience, the procedural time was longer than expected because of increased difficulties during catheterization, and, in these patients with a “compulsory” fenestrated design, two possible solutions have been adopted to obtain early pelvic reperfusion: keeping the main large introducer below the hypogastric artery during the transfemoral bridging maneuvers and targeting at least one vessel from above through a brachial or axillary access so as to limit the size of the required contralateral femoral sheath.
6 Conclusion
In the last two decades, we managed different VAP aneurysms by means of open and hybrid repairs [3, 12], and although the results were acceptable for such complex operations, the mortality and morbidity rates were not negligible.
It is the authors’ opinion that endovascular option, whenever applicable, should be strongly recommended in patients unfit for open surgery, while hybrid surgery should be limited to single-vessel revascularization in the cases of endovascular failure of bridging stent placement or in very selected cases due to the poorer outcomes reported in our experience. Although concerns remain for young patients or patients with connective tissue disease, in whom the long-term patency and sealing of target vessels still need to be proven, a proximal and distal aortic neck within a previous surgical graft seems a safe landing zone even in cases with connective tissue disorders. In the future, in order to answer these questions, we would need an analysis of a larger series collected from a retrospective registry gathering the few cases treated in large-volume centers dealing with endovascular TAAA treatment.
References
Crawford ES. Thoraco-abdominal and abdominal aortic aneurysms involving renal, superior mesenteric, celiac arteries. Ann Surg. 1974;179:763–72.
Dardik A, Perler BA, Roseborough GS, Williams GM. Aneurysmal expansion of the visceral patch after thoracoabdominal aortic replacement: an argument for limiting patch size? J Vasc Surg. 2001;34:405–9.
Tshomba Y, Melissano G, Civilini E, Setacci F, Chiesa R. Fate of the visceral aortic patch after thoracoabdominal aortic repair. Eur J Vasc Endovasc Surg. 2005;29:383–9.
Schepens MA, Kelder JC, Morshuis WJ, Heijmen RH, van Dongen EP, ter Beek HT. Long-term follow-up after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2007;83:S851–5.
Coselli JS, LeMaire SA, Preventza O, de la Cruz KI, Cooley DA, Price MD, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151:1323–37.
Quiñones-Baldrich WJ, Nene SM, Gelabert HA, Moore WS. Rupture of the perivisceral aorta: atherosclerotic versus mycotic aneurysm. Ann Vasc Surg. 1997;11:331–41.
Carrel TP, Signer C. Separate revascularization of the visceral arteries in thoracoabdominal aneurysm repair. Ann Thorac Surg. 1999;68:573–5.
Gasparis AP, Da Silva MS, Semel L. Visceral patch rupture after repair of thoracoabdominal aortic aneurysm—a case report. Vasc Surg. 2001;35:491–4.
Clouse WD, Marone LK, Davison JK, Dorer DJ, Brewster DC, LaMuraglia GM, et al. Late aortic and graft-related events after thoracoabdominal aneurysm repair. J Vasc Surg. 2003;37:254–61.
Dias RR, Coselli JS, Stolf NA, Dias AR, Mady C, Oliveira SA. Aneurysmal dilation of the reimplant segment of the visceral vessels after thoracoabdominal aneurysm correction. Arq Bras Cardiol. 2003;81:273–8.
Lombardi JV, Carpenter JP, Pochettino A, Sonnad SS, Bavaria JE. Thoracoabdominal aortic aneurysm repair after prior aortic surgery. J Vasc Surg. 2003;38:1185–90.
Tshomba Y, Bertoglio L, Marone EM, Melissano G, Chiesa R. Visceral aortic patch aneurysm after thoracoabdominal aortic repair: conventional vs hybrid treatment. J Vasc Surg. 2008;48:1083–91.
Suzuki K, Kazui T, Ohno T, Sugiki K, Doi H, Ohkawa Y. Re-reconstruction of visceral arteries with thoracoabdominal aortic replacement using a branched graft. Jpn J Thorac Cardiovasc Surg. 2005;53:217–9.
LeMaire SA, Carter SA, Volguina IV, Laux AT, Milewicz DM, Borsato GW, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg. 2006;81:2063–78.
Cochennec F, Kobeiter H, Gohel M, Leopardi M, Raux M, Majewski M, et al. Early results of physician modified fenestrated stent grafts for the treatment of thoraco-abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;50:583–92.
van de Mortel RH, Vahl AC, Balm R, Buth J, Hamming JF, Schurink GW, et al. Collective experience with hybrid procedures for suprarenal and thoracoabdominal aneurysms. Vascular. 2008;16:140–6.
Kasirajan K. Branched grafts for thoracoabdominal aneurysms: off-label use of FDA-approved devices. J Endovasc Ther. 2011;18:471–6.
Juvonen T, Biancari F, Ylönen K, Perälä J, Rimpiläinen J, Lepojärvi M. Combined surgical and endovascular treatment of pseudoaneurysms of the visceral arteries and of the left iliac arteries after thoracoabdominal aortic surgery. Eur J Vasc Endovasc Surg. 2001;22:275–7.
Flye MW, Choi ET, Sanchez LA, Curci JA, Thompson RW, Rubin BG, et al. Retrograde visceral vessel revascularization followed by endovascular aneurysm exclusion as an alternative to open surgical repair of thoracoabdominal aortic aneurysm. J Vasc Surg. 2004;39:454–8.
Bakken AM, Protack CD, Waldman DL, Davies MG. Hybrid debranching-endovascular repair of visceral patch aneurysm after thoracoabdominal aneurysm repair. Vasc Endovasc Surg. 2007;41:249–53.
O’Connor DJ, Vouyouka A, Ellozy SH, Sundick SA, Lemasters P, Marin ML, et al. Stent graft repair of paraanastomotic aneurysms after open descending thoracic and thoracoabdominal aortic aneurysm repair. Ann Vasc Surg. 2013;27:693–8.
Adam DJ, Berce M, Hartley DE, Robinson DA, Anderson JL. Repair of recurrent visceral aortic patch aneurysm after thoracoabdominal aortic aneurysm repair with a branched endovascular stent graft. J Vasc Surg. 2007;45:183–5.
Gargiulo M, Gallitto E, Freyrie A, Stella A. Fenestrated endograft for recurrent paravisceral aortic pseudoaneurysm after thoracoabdominal aortic aneurysm open repair. J Vasc Surg. 2013;58:790–3.
Bertoglio L, Mascia D, Cambiaghi T, Kahlberg A, Melissano G, Chiesa R. Fenestrated and branched endovascular treatment of recurrent visceral aortic patch aneurysm after open thoracoabdominal repair. J Vasc Interv Radiol. 2018;29:72–7.
Bertoglio L, Mascia D, Cambiaghi T, Kahlberg A, Tshomba Y, Gomez JS, et al. Management of visceral aortic patch aneurysms after thoracoabdominal repair: open, hybrid and endovascular approach. J Vasc Surg. 2018;67:1358–69.
Chiesa R, Kahlberg A, Mascia D, Tshomba Y, Civilini E, Melissano G. Use of a novel hybrid vascular graft for sutureless revascularization of the renal arteries during open thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2014;60:622–30.
de la Cruz KI, LeMaire SA, Weldon SA, Coselli JS. Thoracoabdominal aortic aneurysm repair with a branched graft. Ann Cardiothorac Surg. 2012;1:381–93.
Tshomba Y, Kahlberg A, Melissano G, Coppi G, Marone E, Ferrari D, et al. Comparison of renal perfusion solutions during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2014;59:623–33.
Verhoeven EL, Katsargyris A, Bekkema F, Oikonomou K, Zeebregts CJ, Ritter W, et al. Ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg. 2015;49:524–31.
Maurel B, Delclaux N, Sobocinski J, Hertault A, Martin-Gonzalez T, Moussa M, et al. The impact of early pelvic and lower limb reperfusion and attentive peri-operative management on the incidence of spinal cord ischemia during thoracoabdominal aortic aneurysm endovascular repair. Eur J Vasc Endovasc Surg. 2015;49:248–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bertoglio, L., Grandi, A., Cambiaghi, T., Melloni, A., Salvati, S. (2019). Recurrent Thoracoabdominal Aortic Aneurysm with Visceral Artery Involvement: Treatment Options. In: Tshomba, Y., Baccellieri, D., Chiesa, R. (eds) Visceral Vessels and Aortic Repair. Springer, Cham. https://doi.org/10.1007/978-3-319-94761-7_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-94761-7_24
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94760-0
Online ISBN: 978-3-319-94761-7
eBook Packages: MedicineMedicine (R0)