Abstract
Functional near-infrared spectroscopy (fNIRS) is an increasingly common neuromonitoring technique used to observe evoked haemodynamic changes in the brain in response to a stimulus. The measurement is typically in terms of concentration changes of oxy- (∆HbO2) and deoxy- (∆HHb) haemoglobin. However, noise from systemic fluctuations in the concentration of these chromophores can contaminate stimulus-evoked haemodynamic responses, leading to misinterpretation of results. Short-separation channels can be used to regress out extracerebral haemodynamics to better reveal cerebral changes, significantly improving the reliability of fNIRS. Broadband NIRS can be used to additionally monitor concentration changes of the oxidation state of cytochrome-c-oxidase (∆oxCCO). Recent studies have shown ∆oxCCO to be a depth-dependent and hence brain-specific signal. This study aims to investigate whether ∆oxCCO can produce a more robust marker of functional activation. Continuous frontal lobe NIRS measurements were collected from 17 healthy adult volunteers. Short 1 cm source-detector separation channels were regressed from longer separation channels in order to minimise the extracerebral contribution to standard fNIRS channels. Significant changes in ∆HbO2 and ∆HHb were seen at 1 cm channels but were not observed in ∆oxCCO. An improvement in the haemodynamic signals was achieved with regression of the 1 cm channel. Broadband NIRS-measured concentration changes of the oxidation state of cytochrome-c-oxidase has the potential to be an alternative and more brain-specific marker of functional activation.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Near-infrared spectroscopy
- Functional activation
- Cytochrome-c-oxidase
- Haemodynamics
- Short-separation regression
1 Introduction
Functional near-infrared spectroscopy is commonly used to monitor stimulus-evoked cerebral haemodynamic responses due to neurovascular coupling, via measured regional concentration changes of oxygenated and deoxygenated haemoglobin . A typical functional activation haemodynamic response function consists of an increase in ∆HbO2 and a concurrent, co-located decrease in ∆HHb. However, one of the main problems with fNIRS is its susceptibility to noise. This can arise from two main sources : task-related systemic activity and systemic-driven changes in the extracerebral layers [1]. The latter is a significant source of interference due to the nature of fNIRS measurements , where light propagates through the superficial layers upon emission and detection in reflectance mode, and hence is highly sensitive to changes in these superficial layers [2].
An increasingly common method to reduce contributions from extracerebral layers is with the use of short source-detector separation NIRS channels to sample only those layers, and use of the resulting signals as regressors in a linear model of the longer fNIRS channels [3]. It has been shown that a short source-detector separation of ~0.8 cm can effectively sample the extracerebral layer [2]. Hence, short channels can facilitate removal of superficial contamination from standard fNIRS channels, significantly improving the reliability of fNIRS measurements.
Using broadband NIRS we can additionally monitor oxidation changes of cytochrome-c-oxidase (CCO) . CCO is the terminal electron acceptor in the electron transport chain in the mitochondria and is responsible for more than 95% of oxygen metabolism. Measurements of ∆oxCCO therefore provides information about oxygen utilisation at a cellular level [4]. The concentration of CCO is less than 10% in vivo than that of haemoglobin, hence a broadband NIRS system is required to accurately separate changes in attenuation due to this chromophore [5].
Recently, it has been shown using a broadband NIRS system with multiple source-detector separations that ∆oxCCO signals display a depth-dependence not seen in haemodynamic signals during oxygen delivery challenges in the healthy adult head [6]. The ∆oxCCO response was only seen at longer source-detector separations (3.5 cm) compared to ∆HbO2 and ∆HHb, which showed a response at all source-detector separations (2, 2.5, 3 and 3.5 cm) interrogating both scalp and brain. This depth-dependence suggests CCO is brain-specific; this is likely due to the higher mitochondrial density in tissue with higher metabolic rates such as the brain compared to tissue with lower metabolic rates [6]. The ∆oxCCO signal therefore offers an alternative cerebral optical signal to haemodynamic signals.
This study aims to investigate the use of ∆oxCCO as an alternative and more robust marker of functional activation due to its brain-specificity compared to haemoglobin signals. The extracerebral contribution to the haemodynamic and metabolic signals is investigated with the use of a multi-distance broadband NIRS system, with short separation of 1 cm to probe mostly the skin, scalp and skull.
2 Methods
Healthy adult volunteers were recruited for this study and written informed consent obtained. The study was approved by the University College London (UCL) Ethics Committee . Data were collected from 17 healthy adult volunteers (13 male; age range 22–34 years) during functional activation. A working memory challenge in the form of either a Stroop task or anagram-solving task was used in order to induce a frontal haemodynamic response. Data were collected continuously for a 30 s baseline period, followed by 30 s of activation and 30 s of rest, alternated 4 times. The total acquisition time was therefore 270 s.
Data were collected using a broadband NIRS system called CYRIL (CYtochrome Research Instrument and appLication) , previously described [7]. It is an 8-channel system with two light emission fibres; optode holders were placed on the right and left sides of the forehead of each subject as shown in Fig. 19.1. Intensity data from the 4 cm channels displayed poor signal-to-noise ratio so were excluded from further analysis.
CYRIL is comprised of a white light source and a lens-based spectrometer with the capacity to collect light intensity data for 136 wavelengths between 771–906 nm at 1 Hz sampling rate. Measured changes in light attenuation were then converted to concentration changes of ∆HbO2, ∆HHb and ∆oxCCO using the UCLn algorithm [5]. As the differential pathlength factor is likely to vary across different source-detector separations, no pathlength was used. Instead, concentration data are presented in terms of μM.cm.
Data analysis was carried out in MATLAB (Mathworks, USA) and using modified versions of functions from the HOMER2 NIRS processing package (http://www.homer-fnirs.org). Concentration data were zero-meaned and bandpass-filtered in the range [0.005 0.3] Hz, using a 5th order Butterworth filter, to remove physiological noise. The haemodynamic response was then extracted using one of two methods: the first was a block-average across all the events for all the subjects to isolate stimulus-related activations from uncorrelated haemodynamic trends (‘block average method ’); the second involved regression of the 1 cm channel by building a simple general linear model of each chromophore signal from the 2 and 3 cm channels (‘short-separation regression method ’). This general linear model uses a series of Gaussian basis functions convolved with a vector defining the time of the stimulus presentation to model the haemodynamic response function [8].
All statistical analysis was carried out in SPSS (IBM, USA). A baseline period was defined as the 10 s window immediately prior to the onset of activation, and 10 s activation period was chosen around the maximum change in the middle of the stimulus period. The response for each subject was defined as the difference between activation and baseline . Student’s unpaired, one-sided t-test was used to compare the response of the activation compared to zero (i.e. no response) and values of p < 0.05 were considered significant. Results are presented as mean ± standard error.
3 Results
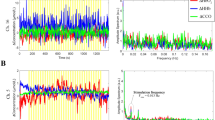
The block average and short-separation regressed results for the 1, 2 and 3 cm channels are shown in Fig. 19.2.
Results of the block-average method for the right side show an increase in ∆HbO2 at all source-detector separations, with a mean increase of 1.2 ± 0.5 μM.cm at 1 cm, 4.5 ± 1.6 μM.cm at 2 cm and 3.6 ± 1.6 μM.cm at 3 cm. No response is seen in ∆HHb at any of the channels. The ∆oxCCO shows no significant response at 1 cm and 2 cm but a large response is seen at 3 cm of 1.3 ± 0.8 μM.cm. Results for the short-separation regression method show a significant increase in ∆HbO2 at only 3 cm of 0.9 ± 0.4 μM.cm, whilst ∆HHb now displays a significant decrease at 3 cm of −0.6 ± 0.5 μM.cm. The ∆oxCCO shows similar results as before, with the mean response of 1.3 ± 0.4 μM.cm at 3 cm.
Results for the block-average method for the left side show an increase in ∆HbO2 at all separations, with mean values of 1.1 ± 0.4 μM.cm at 1 cm, 3.3 ± 1.3 μM.cm at 2 cm and 3.2 ± 1.5 μM.cm at 3 cm. There is no significant change in ∆HHb at any of the separations. ∆oxCCO shows a significant increase at only the 3 cm channel of 0.9 ± 0.8 μM.cm. After short-separation regression, no significant changes are seen in either of the haemodynamic variables. ∆oxCCO displays a similar response as to the block average, with a significant increase of 0.8 ± 0.3 μM.cm at 3 cm.
4 Discussion
We observed a response in ∆oxCCO in only the longer source-detector separation of 3 cm but not in the shorter separations of 1 cm and 2 cm. The regression of the 1 cm channel made no difference to the ∆oxCCO response.
There was a response in ∆HbO2 at all source-detector separations, whereas ∆HHb showed no significant changes in any of the channels. After short-separation regression, the 2 cm channel did not show any significant response in the ∆HbO2 and ∆HHb signals for either side, whilst at 3 cm there was a significant increase in ∆HbO2 and decrease in ∆HHb for the right side and a similar but not significant trend was seen for the left side.
A scalp response was seen in both the right and left sides in the 1 cm channel. Results for the right side block-average at 3 cm show a false negative; no functional activation observed from the haemodynamic signals due to the absence of a significant decrease in ∆HHb. However, ∆oxCCO shows a strong increase that is indicative of functional activation. When the 1 cm short separation is regressed from the longer channels, we see a functional response in the haemoglobins not previously seen, with a significant increase in ∆HbO2 and now significant decrease in ∆HHb. If only the changes in ∆HbO2 were reported, then at 1 cm and 2 cm one would observe functional activation; however, taking into account ∆oxCCO and the short-separation regressed ∆HHb signals, we observe no functional activation in these channels. In this case, the ∆oxCCO and regressed ∆HHb signals have the capacity to rule out false positives.
Results for the left hemisphere show no functional activation from the haemoglobin signals at 3 cm even after regression. If we rely on the significant increase in ∆oxCCO as an indicator of functional activation , the haemodynamic result is a false negative. The 1 cm short-separation regression in this case did not have the capacity to recover the false negative in the haemodynamic signals, which may be due to the scalp response not being fully removed. A false negative could also be caused by systemic changes such as blood pressure producing a significant effect on the brain physiology that masked activation. We did not measure systemic variables so cannot comment on the cause. However, previous studies have found systemic task-related changes to influence NIRS signals [9].
Note that the amplitude of the haemoglobin signals is on a similar scale to that of the ∆oxCCO signals after regression. This could be due to the fundamental characteristics of the regression method. The least-squares static estimator regression approach employed here can result in an underestimation of the haemodynamic response: either because the short-separation measurement has a non-negligible sensitivity to cortical tissue or because the superficial short-separation signal during the functional response mimics the true cerebral response. This effect could likely be improved through the use of a dynamic estimation approach, such as the Kalman filter methodology employed by Gagnon et al. [10].
This study demonstrates that inclusion of the ∆oxCCO signal during functional activation studies with fNIRS can provide an additional robust marker of brain activation allowing better identification of false positives and negatives.
References
Tachtsidis I, Scholkmann F (2016) False positives and false negatives in functional near-infrared spectroscopy : issues, challenges, and the way forward. Neurophotonics 3:030401–1 – 030401–6
Brigadoi S, Cooper RJ (2015) How short is short? Optimum source–detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics 2:025005–1–02005–9
Saager RB, Telleri NL, Berger AJ (2011) Two-detector corrected near infrared spectroscopy (C-NIRS) detects hemodynamic activation responses more robustly than single-detector NIRS. NeuroImage 55:1679–1685
Bainbridge A, Tachtsidis I, Faulkner SD et al (2013) Brain mitochondrial oxidative metabolism during and after cerebral hypoxia-ischemia studied by simultaneous phosphorus magnetic-resonance and broadband near-infrared spectroscopy. NeuroImage 102:173–183
Bale G, Elwell CE, Tachtsidis I (2016) From Jöbsis to the present day: a review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J Biomed Opt 21:091307–1–091307–18
Kolyva C, Ghosh A, Tachtsidis I et al (2014) Cytochrome c oxidase response to changes in cerebral oxygen delivery in the adult brain shows higher brain-specificity than haemoglobin. NeuroImage 85:234–244
Bale G, Mitra S, Meek J et al (2014) A new broadband near-infrared spectroscopy system for in-vivo measurements of cerebral cytochrome-c-oxidase changes in neonatal brain injury. Biomed Opt Express 5:663–676
Gagnon L, Cooper RJ, Yücel M et al (2012) Short separation channel location impacts the performance of short channel regression in NIRS. NeuroImage 59:2518–2528
Tachtsidis I, Leung TS, Devoto L et al (2008) Measurement of frontal lobe functional activation and related systemic effects: a near infrared spectroscopy investigation. Adv Exp Med Biol 614:397–403
Gagnon L, Perdue K, Greve DN et al (2011) Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling. NeuroImage 56:1362–1371
Acknowledgments
The authors would like to thank the Centre for Doctoral Training Integrated Photonic and Electronic Systems (EP/L015455/1) and The Wellcome Trust (104580/Z/14/Z) for the financial support of this work. RJC is funded by EPRSC fellowship EP/N025946/1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
de Roever, I., Bale, G., Cooper, R.J., Tachtsidis, I. (2017). Functional NIRS Measurement of Cytochrome-C-Oxidase Demonstrates a More Brain-Specific Marker of Frontal Lobe Activation Compared to the Haemoglobins. In: Halpern, H., LaManna, J., Harrison, D., Epel, B. (eds) Oxygen Transport to Tissue XXXIX. Advances in Experimental Medicine and Biology, vol 977. Springer, Cham. https://doi.org/10.1007/978-3-319-55231-6_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-55231-6_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55229-3
Online ISBN: 978-3-319-55231-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)