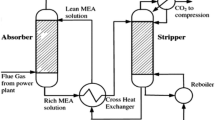
Abstract
In the development of energy efficient solvents for advanced CO2 capture processes, it has often been the case that potentially excellent solvents are overlooked or set aside because of perceived process difficulties or extra costs due to the physical, chemical or thermodynamic properties of the solvent. This chapter considers the process implications of solvent selection with the objective of selecting and designing the process to suit the solvent rather than forcing the solvent into an existing process. The chapter discusses the design, modelling and costing of conventional amine processes insofar as advanced energy efficient solvents still rely on this process. Individual solvent properties, including reaction kinetics, thermodynamics and physical properties are outlined and their impact on design are discussed. Aspects such as degradation products, corrosivity and environmental considerations will impact the selection of process equipment and materials of construction. The impact of these properties of energy efficient solvents on individual unit operations are also discussed in some detail.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- FDG:
-
Flue gas desulphurization
- HPLC:
-
High pressure liquid chromatography
- HSS:
-
Heat stable salts
- HTU:
-
Height of a transfer unit
- IC:
-
Ion chromatography
- LP:
-
Low pressure
- MDEA:
-
Methyldiethanolamine
- MEA:
-
Monoethanolamine
- MNPZ:
-
Mono-nitrosopiperazine
- MP:
-
Medium pressure
- PCC:
-
Post-combustion capture
- PZ:
-
Piperazine
- SCR:
-
Selective catalytic reduction
- VLE:
-
Vapour-liquid-equilibrium
- Cp :
-
Heat capacity (constant pressure)
- E:
-
Efficiency; capture efficiency; Energy
- G:
-
Mass flowrate of gas
- H:
-
Height (of packing)
- \(\Delta H_{{CO_{2} }}^{vap}\) :
-
Desorption enthalpy for CO2
- I:
-
Interest
- L:
-
Mass flowrate of liquid
- m:
-
Mass flowrate
- MW:
-
Molecular weight
- P:
-
Pressure, Plant capacity
- Q:
-
Heat, Energy
- T:
-
Temperature
- v :
-
Volumetric fraction of a component in a gas
- w:
-
Weight fraction
- α:
-
CO2 loading (moles CO2/moles active solvent component)
- Δ:
-
Difference
- 0:
-
Unloaded solvent
- am:
-
Amine or other active component of the capture solvent
- cap:
-
Capital (cost)
- CO2 :
-
Value for CO2 or its fraction of a stream
- cond:
-
Condenser, Condensate
- cw:
-
Cooling water
- DES:
-
Desorber (stripper)
- FG:
-
Flue Gas
- in:
-
Inlet flow
- L:
-
Lean (stripped) solvent
- out:
-
Outlet flow
- R:
-
Rich (containing captured CO2) solvent
- sens:
-
Sensible heat
- tot:
-
Total
References
Kohl AL, Nielsen R (1997) Gas purification, 5th edn. Gulf Publishing
Sander MT, Mariz CL (1992) The Fluor Daniel Econamine FG process—past experience and present-day focus. In: 1st International Conference on Carbon Dioxide Removal (ICCDR), Amsterdam, Netherlands, 04–06 Mar 1992, pp 341–348
Budzianowski WM (2015) Single solvents, solvent blends, and advanced solvent systems in CO2 capture by absorption: a review. Int J Glob Warm 7(2):184–225. Doi:10.1504/ijgw.2015.067749
Ramezan M, Skone TJ, Nsakala NY, Liljedahl GN (2007) Carbon dioxide capture from existing coal-fired power plants. National Energy Technology Laboratory
Cottrell AJ, McGregor JM, Jansen J, Artanto Y, Dave N, Morgan S, Pearson P, Attalla MI, Wardhaugh L, Yu H, Allport A, Feron PHM (2009) Post-combustion capture R&D and pilot plant operation in Australia. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia. pp 1003–1010. Doi:10.1016/j.egypro.2009.01.133
Cousins A, Wardhaugh LT, Feron PHM (2011) A survey of process flow sheet modifications for energy efficient CO2 capture from flue gases using chemical absorption. Int J Greenhouse Gas Control 5:605–619. Doi:10.1016/j.ijggc.2011.01.002
Cousins A, Cottrell A, Lawson A, Huang S, Feron PHM (2012) Model verification and evaluation of the rich-split process modification at an Australian-based post combustion CO2 capture pilot plant. Greenh Gases 2(5):329–345
Puxty G, Rowland R, Allport A, Yang Q, Bown M, Burns R, Maeder M, Attalla M (2009) Carbon dioxide postcombustion capture: a novel screening study of the carbon dioxide absorption performance of 76 amines. Environ Sci Technol 43(16):6427–6433
Plaza JM, Van Wagener D, Rochelle GT (2009) Modeling CO2 capture with aqueous monoethanolamine. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia. pp 1171–1178. Doi:10.1016/j.egypro.2009.01.154
Zhang Y, Chen H, Chen CC, Plaza JM, Dugas R, Rochelle GT (2009) Rate-based process modeling study of CO2 capture with aqueous monoethanolamine solution. Ind Eng Chem Res 48(20):9233–9246. Doi:10.1021/ie900068k
Freguia S, Rochelle GT (2003) Modeling of CO2 capture by aqueous monoethanolamine. AIChE J 49(7):1676–1686
von Harbou I, Imle M, Hasse H (2014) Modeling and simulation of reactive absorption of CO2 with MEA: results for four different packings on two different scales. Chem Eng Sci 105:179–190. Doi:10.1016/j.ces.2013.11.005
Razi N, Svendsen HF, Bolland O (2014) Assessment of mass transfer correlations in rate-based modeling of a large-scale CO2 capture with MEA. Int J Greenhouse Gas Control 26:93–108. Doi:10.1016/j.figgc.2014.04.019
Dugas R, Alix P, Lemaire E, Broutin P, Rochelle G (2009) Absorber model for CO2 capture by monoethanolamine; application to CASTOR pilot results. Energy Procedia 1(1):103–107
Saimpert M, Puxty G, Qureshi S, Wardhaugh L, Cousins A (2013) A new rate based absorber and desorber modelling tool. Chem Eng Sci 96:10–25. Doi:10.1016/j.ces.2013.03.013
Yu YS, Lu HF, Wang GX, Zhang ZX, Rudolph V (2013) Characterizing the transport properties of multiamine solutions for CO2 capture by molecular dynamics simulation. J Chem Eng Data 58(6):1429–1439. Doi:10.1021/je3005547
Rubin ES, Short C, Booras G, Davison J, Ekstrom C, Matuszewski M, McCoy S (2013) A proposed methodology for CO2 capture and storage cost estimates. Int J Greenhouse Gas Control 17:488–503. Doi:10.1016/j.ijggc.2013.06.004
Dave N, Do T, Palfreyman D, Feron PHM, Xu S, Gao S, Liu L (2011) Post-combustion capture of CO2 from coal-fired power plants in China and Australia: an experience based cost comparison. In: Gale J, Hendriks C, Turkenberg W (eds) 10th international conference on Greenhouse Gas Control Technologies, vol 4. Energy Procedia, pp 1869–1877. Doi:10.1016/j.egypro.2011.02.065
Peters MS, Timmerhaus KD, West RE (2003) Plant design and economics for chemical engineers, 5th edn. McGraw-Hill, Boston
Kierzkowska-Pawlak H, Chacuk A, Siemieniec M (2014) Reaction kinetics of CO2 in aqueous 2-(2-aminoethylamino)ethanol solutions using a stirred cell reactor. Int J Greenhouse Gas Control 24:106–114. Doi:10.1016/j.ijggc.2014.03.004
Conway W, Beyad Y, Maeder M, Burns R, Feron P, Puxty G (2014) CO2 absorption into aqueous solutions containing 3-piperidinemethanol: CO2 mass transfer, stopped-flow kinetics, H-1/C-13 NMR, and vapor-liquid equilibrium investigations. Ind Eng Chem Res 53(43):16715–16724. Doi:10.1021/ie503195x
Kunze A-K, Dojchinov G, Haritos VS, Lutze P (2015) Reactive absorption of CO2 into enzyme accelerated solvents: from laboratory to pilot scale. Appl Energy 156:676–685. Doi:10.1016/j.apenergy.2015.07.033
Bishnoi S, Rochelle GT (2000) Absorption of carbon dioxide into aqueous piperazine: reaction kinetics, mass transfer and solubility. Chem Eng Sci 55(22):5531–5543
Dang HY, Rochelle GT (2003) CO2 absorption rate and solubility in monoethanolamine/piperazine/water. Sep Sci Technol 38(2):337–357. Doi:10.1081/ss-12016678
Jou FY, Otto FD, Mather AE (1994) Vapor-liquid-equilibrium of carbon-dioxide in aqueous mixtures of monoethanolamine and methyldiethanolamine. Ind Eng Chem Res 33(8):2002–2005
Kumar PS, Hogendoorn JA, Feron PHM, Versteeg GF (2003) Equilibrium solubility of CO2 in aqueous potassium taurate solutions: Part 1. Crystallization in carbon dioxide loaded aqueous salt solutions of amino acids. Ind Eng Chem Res 42(12):2832–2840. Doi:10.1021/ie0206002
Fan GJ, Wee AGH, Idem R, Tontiwachwuthikul P (2009) NMR studies of amine species in MEA-CO2-H2O system: modification of the model of vapor-liquid equilibrium (VLE). Ind Eng Chem Res 48(5):2717–2720. Doi:10.1021/ie8015895
Park SJ, Shin HY, Min BM, Cho A, Lee JS (2009) Vapor-liquid equilibria of water plus monoethanolamine system. Korean J Chem Eng 26(1):189–192
Puxty G, Rowland R (2011) Modeling CO2 mass transfer in amine mixtures: PZ-AMP and PZ-MDEA. Environ Sci Technol 45(6):2398–2405. Doi:10.1021/es1022784
Li H, Le Moullec Y, Lu JH, Chen J, Marcos JCV, Chen GF, Chopin F (2015) CO2 solubility measurement and thermodynamic modeling for 1-methylpiperazine/water/CO2. Fluid Phase Equilib 394:118–128. Doi:10.1016/j.fluid.2015.03.021
Freeman SA, Dugas R, Van Wagener D, Nguyen T, Rochelle GT (2009) Carbon dioxide capture with concentrated, aqueous piperazine. 10.1016/j.egypro.2009.01.195. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia, pp 1489–1496
Cousins A, Nielsen P, Huang S, Cottrell A, Chen E, Rochelle GT, Feron PHM (2015) Pilot-scale evaluation of concentrated piperazine for CO2 capture at an Australian coal-fired power station: duration experiments. Greenhouse Gases 5(4):363–373. Doi:10.1002/ghg.1507
Moser P, Schmidt S, Stahl K, Vorberg G, Lozano GA, Stoffregen T, Roesler F (2014) Demonstrating emission reduction—results from the post-combustion capture pilot plant at Niederaussem. In: Dixon T, Herzog H, Twinning S (eds) 12th international Conference on Greenhouse Gas Control Technologies, Ghgt-12, vol 63. Energy Procedia. pp 902–910. Doi:10.1016/j.egypro.2014.11.100
Pinto DDD, Knuutila H, Fytianos G, Haugen G, Mejdell T, Svendsen HF (2014) CO2 post combustion capture with a phase change solvent. Pilot plant campaign. Int J Greenhouse Gas Control 31:153–164. Doi:10.1016/j.ijggc.2014.10.007
Song D, Seibert AF, Rochelle GT (2014) Effect of liquid viscosity on the liquid phase mass transfer coefficient of packing. In: Dixon T, Herzog H, Twinning S (eds) 12th international conference on Greenhouse Gas Control Technologies, Ghgt-12, vol 63. Energy Procedia. Elsevier Science Bv, Amsterdam, pp 1268–1286. Doi:10.1016/j.egypro.2014.11.136
Freeman SA, Rochelle GT (2011) Density and viscosity of aqueous (piperazine plus carbon dioxide) solutions. J Chem Eng Data 56(3):574–581. Doi:10.1021/je1012263
Amundsen TG, Oi LE, Eimer DA (2009) Density and viscosity of monoethanolamine plus water plus carbon dioxide from (25 to 80) degrees C. J Chem Eng Data 54(11):3096–3100. Doi:10.1021/je900188m
Kumar PS, Hogendoorn JA, Feron PHM, Versteeg GF (2001) Density, viscosity, solubility, and diffusivity of N2O in aqueous amino acid salt solutions. J Chem Eng Data 46(6):1357–1361
Shokouhi M, Jalili AH, Samani F, Hosseini-Jenab M (2015) Experimental investigation of the density and viscosity of CO2-loaded aqueous alkanolamine solutions. Fluid Phase Equilib 404:96–108. Doi:10.1016/j.fluid.2015.06.034
Weiland RH, Dingman JC, Cronin DB, Browning GJ (1998) Density and viscosity of some partially carbonated aqueous alkanolamine solutions and their blends. J Chem Eng Data 43(3):378–382
Chang R (1998) Chemistry, 6th edn. McGraw Hill, New York
Tsai RE, Schultheiss P, Kettner A, Lewis JC, Seibert AF, Eldridge RB, Rochelle GT (2008) Influence of surface tension on effective packing area. Ind Eng Chem Res 47(4):1253–1260. Doi:10.1021/ie0707801
Sedelies R, Steiff A, Weinspach P-M (1987) Mass transfer area in different gas-liquid reactors as a function of liquid properties. Chem Eng Technol 10:1–15
Radgen P, Rode H, Reddy S, Yonkoski J (2014) Lessons learned from the operation of a 70 tonne per day post combustion pilot plant at the coal fired power plant in Wilhelmshaven, Germany. In: Dixon T, Herzog H, Twinning S (eds) 12th international conference on Greenhouse Gas Control Technologies, Ghgt-12, vol 63. Energy Procedia. Elsevier Science Bv, Amsterdam, pp 1585–1594. Doi:10.1016/j.egypro.2014.11.168
Reitenbach G (2015) SaskPower’s Boundary Dam Carbon Capture Project Wins POWER’s Highest Award. Power 159(8):24
Jackson P, Attalla MI (2010) N-Nitrosopiperazines form at high pH in post-combustion capture solutions containing piperazine: a low-energy collisional behaviour study. Rapid Commun Mass Spectrom 24(24):3567–3577. Doi:10.1002/rcm.4815
Knuutila H, Svendsen HF, Asif N (2014) Decomposition of nitrosamines in aqueous monoethanolamine (MEA) and diethanolamine (DEA) solutions with UV-radiation. Int J Greenhouse Gas Control 31:182–191. Doi:10.1016/j.ijggc.2014.10.002
Fine NA, Rochelle GT (2013) Thermal decomposition of n-nitrosopiperazine. In: Dixon T, Yamaji K (eds) Ghgt-11, vol 37. Energy Procedia. Elsevier Science Bv, Amsterdam, pp 1678–1686. Doi:10.1016/j.egypro.2013.06.043
Pearson P, Cousins A (2015) Assessment of corrosion in amine-based post-combustion capture of carbon dioxide systems. In: Feron P (ed) Absorption-based post-combustion capture of Carbon Dioxide, in press edn. Elsevier (in press)
GPSA (2004) GPSA engineering data book. Tulsa, Oklahoma
Dupart MS, Bacon TR, Edwards DJ (1993) Understanding corrosion in alkanolamine gas treating plants. 1. Proper mechanism diagnosis optimizes amine operations. Hydrocarbon Process 72(4):75–80
Dupart MS, Bacon TR, Edwards DJ (1993) Understanding corrosion in alkanolamine gas treating plants. Hydrocarbon Process 72(5):89–94
Johnson DW, Reddy S, Brown JH (2009) Commercially available CO2 capture technology. Power 153(8):58–60
Kittel J, Gonzalez S (2014) Corrosion in CO2 post-combustion capture with alkanolamines—a review. Oil Gas Sci Technol 69(5):915–929. Doi:10.2516/ogst/2013161
Bello A, Idem RO (2006) Comprehensive study of the kinetics of the oxidative degradation of CO2 loaded and concentrated aqueous monoethanolamine (MEA) with and without sodium metavanadate during CO2 absorption from flue gases. Ind Eng Chem Res 45(8):2569–2579. Doi:10.1021/ie050562x
de Koeijer G, Enge Y, Sanden K, Graff OF, Falk-Pedersen O, Amundsen T, Overa S (2011) CO2 Technology Centre Mongstad—design, functionality and emissions of the amine plant. In: Gale J, Hendriks C, Turkenberg W (eds) 10th international conference on Greenhouse Gas Control Technologies, vol 4. Energy Procedia, pp 1207–1213. Doi:10.1016/j.egypro.2011.01.175
Moser P, Schmidt S, Uerlings R, Sieder G, Titz J-T, Hahn A, Stoffregen T (2011) Material testing for future commercial post-combustion capture plants—results of the testing programme conducted at the Niederaussem pilot plant. In: Gale J, Hendriks C, Turkenberg W (eds) 10th international conference on Greenhouse Gas Control Technologies, vol 4. Energy Procedia, pp 1317–1322. Doi:10.1016/j.egypro.2011.01.189
Cousins A, Ilyushechkin A, Pearson P, Cottrell A, Huang S, Feron PHM (2013) Corrosion coupon evaluation under pilot-scale CO2 capture conditions at an Australian coal-fired power station. Greenh Gases 3(3):169–184. Doi:10.1002/ghg.1341
Kittel J, Idem R, Gelowitz D, Tontiwachwuthikul P, Parrain G, Bonneau A (2009) Corrosion in MEA units for CO(2) capture: pilot plant studies. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia, vol 1, pp 791–797. Doi:10.1016/j.egypro.2009.01.105
Monea M (2013) Boundary Dam—progress and status. Paper presented at the 7th Trondheim CCS conference, Trondheim 4–6 June 2013
Weiland RH, Dingman JC, Cronin DB (1997) Heat capacity of aqueous monoethanolamine, diethanolamine, N-methyldiethanolamine, and N-methyldiethanolamine-based blends with carbon dioxide. J Chem Eng Data 42(5):1004–1006
Shaikh MS, Shariff AM, Bustam MA, Murshid G (2014) Physicochemical properties of aqueous solutions of sodium l-prolinate as an absorbent for CO2 removal. J Chem Eng Data 59(2):362–368. Doi:10.1021/je400830w
Aroonwilas A, Veawab A (2007) Heat recovery gas absorption process
Leites IL, Sama DA, Lior N (2003) The theory and practice of energy saving in the chemical industry: some methods for reducing thermodynamic irreversibility in chemical technology processes. Energy 28(1):55–97
Cousins A, Wardhaugh LT, Feron PHM (2011) Preliminary analysis of process flow sheet modifications for energy efficient CO2 capture from flue gases using chemical absorption. Chem Eng Res Des 89(8A):1237–1251. Doi:10.1016/j.cherd.2011.02.008
Oyenekan BA, Rochelle GT (2007) Alternative stripper configurations for CO2 capture by aqueous amines. AIChE J 53(12):3144–3154. Doi:10.1002/aic.11316
Freeman SA, Dugas R, Van Wagener DH, Nguyen T, Rochelle GT (2010) Carbon dioxide capture with concentrated, aqueous piperazine. Int J Greenhouse Gas Control 4(2):119–124. Doi:10.1016/j.ijggc.2009.10.008
Cousins A, Huang S, Cottrell A, Feron PHM, Chen E, Rochelle GT (2015) Pilot-scale parametric evaluation of concentrated piperazine for CO2 capture at an Australian coal-fired power station. Greenhouse Gases 5(1):7–16. Doi:10.1002/ghg.1462
Kuntz J, Aroonwilas A (2008) Performance of spray column for CO2 capture application. Ind Eng Chem Res 47(1):145–153. Doi:10.1021/ie0617021
Seyboth O, Zimmermann S, Heidel B, Scheffknecht G (2014) Development of a spray scrubbing process for post combustion CO2 capture with amine based solvents. In: Dixon T, Herzog H, Twinning S (eds) 12th international conference on Greenhouse Gas Control Technologies, Ghgt-12, vol 63. Energy Procedia. Elsevier Science Bv, Amsterdam, pp 1667–1677. Doi:10.1016/j.egypro.2014.11.176
Kavoshi L, Rahimi A, Hatamipour MS (2015) CFD modeling and experimental study of carbon dioxide removal in a lab-scale spray dryer. Chem Eng Res Des 98:157–167. Doi:10.1016/j.cherd.2015.04.023
Awtry AR, Brasseur JK, Henshaw TL, Hobbs KR, McDermott WE, Miller NJ, Neumann DK, Nizamov BR, Tobias JA, Anderson JL, Courtright JL (2009) Gas liquid contactor module, useful for e.g. removing gas phase molecules, comprises liquid inlet, gas inlet, gas outlet, array of nozzles in communication with the liquid inlet and gas inlet, gas liquid separator and liquid outlet. CN102217152-B
Awtry A (2013) Status of the Carbon Dioxide Absorber Retrofit Equipment (CARE) program. Paper presented at the 2013 CO2 Capture Technical Meeting
Wardhaugh L (2010) Toward significant capital cost reduction in post combustion capture plants. Paper presented at the Chemeca 2010, Adelaide, South Australia, 26–29 Sept 2010
Ramshaw C (1983) HIGEE Distillation—an example of process intensification. Chem Eng Lond 389:13–14
Cheng HH, Tan CS (2009) Carbon dioxide capture by blended alkanolamines in rotating packed bed. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia, pp 925–932. Doi:10.1016/j.egypro.2009.01.123
Yu CH, Wu TW, Tan CS (2013) CO2 capture by piperazine mixed with non-aqueous solvent diethylene glycol in a rotating packed bed. Int J Greenhouse Gas Control 19:503–509. Doi:10.1016/j.ijggc.2013.10.014
Jassim MS, Rochelle G, Eimer D, Ramshaw C (2007) Carbon dioxide absorption and desorption in aqueous monoethanolamine solutions in a rotating packed bed. Ind Eng Chem Res 46(9):2823–2833. Doi:10.1021/ie051104r
Zhang L-L, Wang J-X, Liu Z-P, Lu Y, Chu G-W, Wang W-C, Chen J-F (2013) Efficient capture of carbon dioxide with novel mass-transfer intensification device using ionic liquids. AIChE J 59(8):2957–2965. Doi:10.1002/aic.14072
Makarytchev SV, Langrish TAG, Fletcher DF (2005) Exploration of spinning cone column capacity and mass transfer performance using CFD. Chem Eng Res Des 83(A12):1372–1380. Doi:10.1205/cherd.03413
Wang YD, Fei WY, Sun JH, Wan YK (2002) Hydrodynamics and mass transfer performance of a modified rotating disc contactor (MRDC). Chem Eng Res Des 80(4):392–400
Wardhaugh L, Allport A, Garland E, Solnordal C (2013) A novel gas-liquid contactor for PCC based on a rotating liquid sheet. Paper presented at the The 7th Trondheim CCS Conference (TCCS-7): CO2 Capture, Transport and Storage, Trondheim, Norway, 4–6 June 2013
Knuutila H, Aronu UE, Kvamsdal HM, Chikukwa A (2011) Post combustion CO2 capture with an amino acid salt. In: Gale J, Hendriks C, Turkenberg W (eds) 10th international conference on Greenhouse Gas Control Technologies, vol 4. Energy Procedia, pp 1550–1557. Doi:10.1016/j.egypro.2011.02.024
Eide-Haugmo I, Brakstad OG, Hoff KA, Sorheim KR, da Silva EF, Svendsen HF (2009) Environmental impact of amines. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia, vol 1, pp 1297–1304. Doi:10.1016/j.egypro.2009.01.170
Cousins A, Nielsen PT, Huang S, Rowland R, Edwards B, Cottrell A, Chen E, Rochelle GT, Feron PHM (2015) Pilot-scale evaluation of concentrated piperazine for CO2 capture at an Australian coal-fired power station: Nitrosamine measurements. Int J Greenhouse Gas Control 37:256–263. Doi:10.1016/j.ijggc.2015.03.007
Dai N, Shah AD, Hu L, Plewa MJ, McKague B, Mitch WA (2012) Measurement of nitrosamine and nitramine formation from NO reactions with amines during amine-based carbon dioxide capture for postcombustion carbon sequestration. Environ Sci Technol 46(17):9793–9801. Doi:10.1021/es301867b
Azzi M, Tibbett A, Halliburton B, Element A, Artanto Y, Meuleman E, Feron P (2014) Assessing atmospheric emissions from amine-based CO2 post-combustion capture processes and their impacts on the environment—a case study, vol 1. Measurement of emissions from a monoethanolamine-based post-combustion CO2 capture pilot plant. 1
Azzi M, Angove D, Campbell I, Cope M, Emmerson K, Feron P, Patterson M, Tibbett A, White S (2014) Assessing atmospheric emissions from an amine-based CO2 post-combustion capture processes and their impacts on the environment—a case study, vol 2. Atmospheric chemistry of MEA and 3D air quality modelling of emissions from the Loy Yang PCC plant, Final report. Global Carbon Capture and Storage Institute
Nurrokhmah L, Mezher T, Abu-Zahra MRM (2013) Evaluation of handling and reuse approaches for the waste generated from MEA-based CO2 capture with the consideration of regulations in the UAE. Environ Sci Technol 47(23):13644–13651. Doi:10.1021/es4027198
Sexton A, Dombrowski K, Nielsen P, Rochelle G, Fisher K, Youngerman J, Chen E, Singh P, Davison J (2014) Evaluation of reclaimer sludge disposal from post-combustion CO2 capture. In: Dixon T, Herzog H, Twinning S (eds) 12th international conference on Greenhouse Gas Control Technologies, Ghgt-12, vol 63. Energy Procedia, pp 926–939. Doi:10.1016/j.egypro.2014.11.102
Rochelle G, Chen E, Freeman S, Van Wagener D, Xu Q, Voice A (2011) Aqueous piperazine as the new standard for CO2 capture technology. Chem Eng J 171(3):725–733. Doi:10.1016/j.cej.2011.02.011
Yang N, Yu H, Li LC, Xu DY, Han WF, Feron P (2014) Aqueous ammonia (NH3) based post combustion CO2 capture: a review. Oil Gas Sci Technol 69(5):931–945. Doi:10.2516/ogst/2013160
Darde V, Thomsen K, van Well WJM, Stenby EH (2009) Chilled ammonia process for CO2 capture. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia, pp 1035–1042. Doi:10.1016/j.egypro.2009.01.137
Telikapalli V, Kozak F, Francois J, Sherrick B, Black J, Muraskin D, Cage M, Hammond M, Spitznogle G (2011) CCS with the Alstom chilled ammonia process development program—field pilot results. In: Gale J, Hendriks C, Turkenberg W (eds) 10th international conference on Greenhouse Gas Control Technologies, vol 4. Energy Procedia, Elsevier Science Bv, Amsterdam, pp 273–281. Doi:10.1016/j.egypro.2011.01.052
Yu H, Qi G, Xiang Q, Wang S, Fang M, Yang Q, Wardhaugh L, Feron P (2013) Aqueous ammonia based post combustion capture: results from pilot plant operation, challenges and further opportunities. Paper presented at the the 11th Greenhouse Gas Control Technologies (GHGT) conference, Kyoto International Conference Center, Japan, 18–22 Nov 2012
Aleixo M, Prigent M, Gibert A, Porcheron F, Mokbel I, Jose J, Jacquin M (2011) Physical and chemical properties of DMX (TM) solvents. In: Gale J, Hendriks C, Turkenberg W (eds) 10th international conference on Greenhouse Gas Control Technologies, vol 4. Energy Procedia. Elsevier Science Bv, Amsterdam, pp 148–155. Doi:10.1016/j.egypro.2011.01.035
Raynal L, Alix P, Bouillon PA, Gomez A, de Nailly ML, Jacquin M, Kittel J, di Lella A, Mougin P, Trapy J (2011) The DMX (TM) process: an original solution for lowering the cost of post-combustion carbon capture. In: Gale J, Hendriks C, Turkenberg W (eds) 10th international conference on Greenhouse Gas Control Technologies, vol 4. Energy Procedia. Elsevier Science Bv, Amsterdam, pp 779–786. Doi:10.1016/j.egypro.2011.01.119
Misiak K, Sanchez CS, van Os P, Goetheer E (2013) Next generation post-combustion capture: Combined CO2 and SO2 removal. In: Dixon T, Yamaji K (eds) Ghgt-11, vol 37. Energy Procedia. Elsevier Science Bv, Amsterdam, pp 1150–1159. Doi:10.1016/j.egypro.2013.05.212
Cousins A, Artanto Y, Meuleman E, Pearson P, Puxty G, Jansen J, Conway W, Slater N, Curtis E, Monch A, Feron P, Verheyen V, Misiak K, Huizinga A, Sanchez Sanchez C, van Os P, Goetheer E, Castelow P (2014) Combined low-cost pre-treatment of flue gas and capture of CO2 from brown coal-fired power stations. Paper presented at the Low Rank Coal, Third International Industry Symposium, Melbourne, Australia, 28 Apr–1 May 2014
Yu H, Qi G, Wang S, Morgan S, Allport A, Cottrell A, Do T, McGregor J, Wardhaugh L, Feron P (2012) Results from trialling aqueous ammonia-based post-combustion capture in a pilot plant at Munmorah Power Station: gas purity and solid precipitation in the stripper. Int J Greenhouse Gas Control 10:15–25. Doi:10.1016/j.ijggc.2012.04.014
Puxty G, Wei SC-C, Feron P, Meuleman E, Beyad Y, Burns R, Maeder M (2014) A novel process concept for the capture of CO2 and SO2 using a single solvent and column. In: Dixon T, Herzog H, Twinning S (eds) 12th international conference on Greenhouse Gas Control Technologies, Ghgt-12, vol 63. Energy Procedia, pp 703–714. Doi:10.1016/j.egypro.2014.11.078
McLarnon CR, Duncan JL (2009) Testing of ammonia based CO(2) capture with multi-pollutant control technology. In: Gale J, Herzog H, Braitsch J (eds) Greenhouse Gas Control Technologies 9, vol 1. Energy Procedia, vol 1, pp 1027–1034. Doi:10.1016/j.egypro.2009.01.136
Manning FS, Thompson RE (1991) Oilfield processing of petroleum, vol 1: Natural gas, vol 1. Penwell Publishing Company, Tulsa
Campbell KLS, Lapidot T, Williams DR (2015) Foaming of CO2-loaded amine solvents degraded thermally under stripper conditions. Ind Eng Chem Res 54(31):7751–7755. Doi:10.1021/acs.iecr.5b01935
Chen X, Freeman SA, Rochelle GT (2010) Foaming of aqueous piperazine and monoethanolamine for CO2 capture. Int J Greenhouse Gas Control 5(2):381–386. Doi:10.1016/j.ijggc.2010.09.006
Richner G, Puxty G (2012) Assessing the chemical speciation during CO2 absorption by aqueous amines using in situ FTIR. Ind Eng Chem Res 51(44):14317–14324. Doi:10.1021/ie302056f
Artanto Y, Jansen J, Pearson P, Do T, Cottrell A, Meuleman E, Feron P (2012) Performance of MEA and amine-blends in the CSIRO PCC pilot plant at Loy Yang Power in Australia. Fuel 101:264–275. Doi:10.1016/j.fuel.2012.02.023
Azzi M, Day S, French S, Halliburton B, Jackson P, Lavrencic S, Riley K, Tibbett A (2010) CO2 Capture Mongstadt—Project A—Establishing sampling and analytical procedures for potentially harmful components from post-combustion amine based CO2 capture. Task 2: Procedures for manual sampling. EP 105456, CSIRO, Australia
Gassnova (2015) http://www.gassnova.no/en/ccs-projects/full-scale-mongstad. Accessed July 2015
Bui M, Gunawan I, Verheyen TV, Meuleman E, Feron P (2014) Dynamic operation of post-combustion CO2 capture in Australian coal-fired power plants. In: Dixon T, Herzog H, Twinning S (eds) 12th international conference on Greenhouse Gas Control Technologies, Ghgt-12, vol 63. Energy Procedia, pp 1368–1375. Doi:10.1016/j.egypro.2014.11.146
Freeman SA (2011) Thermal degradation and oxidation of aqueous piperazine for carbon dioxide capture. Ph.D. thesis, University of Texas at Austin
Notz R, Mangalapally HP, Hasse H (2012) Post combustion CO2 capture by reactive absorption: Pilot plant description and results of systematic studies with MEA. Int J Greenhouse Gas Control 6:84–112. Doi:10.1016/j.ijggc.2011.11.004
Nielsen PT, Li L, Rochelle GT (2013) Piperazine degradation in pilot plants. In: Dixon T, Yamaji K (eds) Ghgt-11, vol 37. Energy Procedia, pp 1912–1923. Doi:10.1016/j.egypro.2013.06.072
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Wardhaugh, L.T., Cousins, A. (2017). Process Implications of CO2 Capture Solvent Selection. In: Budzianowski, W. (eds) Energy Efficient Solvents for CO2 Capture by Gas-Liquid Absorption. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-47262-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-47262-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47261-4
Online ISBN: 978-3-319-47262-1
eBook Packages: EnergyEnergy (R0)