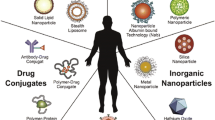
Abstract
A critical review is attempted to assess the status of nanomedicine entry onto the market.
The emergence of new potential therapeutic entities such as DNA and RNA fragments requires that these new “drugs” will need to be delivered in a cell-and organelle-specific manner. Although efforts have been made over the last 50 years or so to develop such delivery technology, no effective and above all clinically approved protocol for cell-specific drug delivery in humans exists as yet. Various particles, macromolecules, liposomes and most recently “nanomaterials” have been said to “show promise” but none of these promises have so far been “reduced” to human clinical practice.
The focus of this volume is on cancer indication since the majority of published research relates to this application; within that, we focus on solid tumors (solid malignancies). Our aim is to critically evaluate whether nanomaterials, both non-targeted and targeted to specific cells, could be of therapeutic benefit in clinical practice. The emphasis of this volume will be on pharmacokinetics (PK) and pharmacodynamics (PD) in animal and human studies.
Apart from the case of exquisitely specific antibody-based drugs, the development of target-specific drug–carrier delivery systems has not yet been broadly successful at the clinical level. It can be argued that drugs generated using the conventional means of drug development (i.e., relying on facile biodistribution and activity after (preferably) oral administration) are not suitable for a target-specific delivery and would not benefit from such delivery even when a seemingly perfect delivery system is available. Therefore, successful development of site-selective drug delivery systems will need to include not only the development of suitable carriers, but also the development of drug entities that meet the required PK/PD profile.
In general, human clinical studies are approved only after the expected benefits of targeting have been shown in pre-clinical, in vivo animal studies first. Therefore, quantitative data on biodistribution of targeted and non-targeted nanoparticles should be generated as the first step. This should be followed by determining whether an increased presence of nanoparticles in tumors also results in increased concentration of the free drug within the tumor space. Any “promise” for reproducing similar data in human clinical studies should be supported by relevant scaling from the animal model used to humans.
For too long now, the same or similar approaches have been used by researchers without success. We believe that new fundamentally different approaches are needed to make cell-specific drug delivery clinical reality. In this volume we want to focus on (a) how nanoparticles could be redesigned from the material-science point of view (for example, redesigning nanoparticles for long-circulating properties, passive (EPR) and active targeting concept); and (b) on the design and properties of drugs that would benefit from cell-specific targeting (examining why active targeting of drug carrier does not necessarily result in drug accumulation in tumor). Further, we will draw attention to (c) the manner pre-clinical animal data should be translated to humans using appropriate scaling, in particular with reference to the differences between mice and men in terms of differing vascular morphology and immunological background.
Successful development of cell-specific drug-delivery systems requires that reliable quantitative pharmacokinetic/pharmacodynamic (PK/PD) data are collected both in animal and human studies. This volume will include (d) information on improved body imaging technologies and on enabling quantitative tools available.
Finally, we address (e) the issue of diminishing academic funding of animal studies and of (f) the current dismal market and proprietary situation in the area of site-specific drug delivery.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- μCT:
-
microcomputed tomography
- ACA:
-
anticancer agent (functionalized oligomer with attached targeting motif)
- Ad-p53:
-
Human Adenovirus Type5 (dE1/E3) expressing Tumor Protein P53 (P53) under a CMV promoter
- ADMET:
-
absorption, distribution, metabolism, and excretion – toxicity in pharmacokinetics
- AuNC-CS-TPP:
-
Chitosan-coated gold nanocluster – triphenylphosphonium
- AuNP-TPP:
-
Triphenylphosphonium gold nanoparticles
- BITES:
-
bispecific T-cell engagers
- CAFs:
-
cancer associated fibroblasts
- CAGR:
-
compound annual growth rate
- CBER:
-
Center for Biologics Evaluation and Research
- CD3ɛ:
-
anti-human scFv monoclonal antibody
- CT:
-
computed tomography
- CTC:
-
circulating tumor cells
- DCE-CT:
-
dynamic contrast enhanced computed tomography
- DDD:
-
drug discovery and development
- DOX:
-
doxorubicin
- ECM:
-
extracellular cell matrix
- EPR:
-
enhanced permeability retention effect R –endoplasmic reticulum
- FA:
-
Folic acid
- FMT 3D:
-
fluorescence molecular tomography
- FRET:
-
Fluorescence Resonance Energy Transfer
- GFP:
-
Green Fluorescence Protein
- HA:
-
hyaluronic acid
- HPMA:
-
N-(2-hydroxypropyl) methacrylamide
- HTS:
-
high throughput screening
- HYAL:
-
hyaluronidase
- IFP:
-
interstitial fluid pressure
- mRNA:
-
messenger RNA
- MALDI-IMS:
-
Matrix-assisted laser desorption imaging – ionization mass spectrometry
- MHC I:
-
Multihistocompatibility complex I
- MHC II:
-
Multihistocompatibility complex II
- MRI:
-
magnetic resonance imaging
- MSP:
-
mononuclear phagocyte system
- NP:
-
nanoparticle
- NIRF:
-
near infrared fluorophore
- OI:
-
optical imaging
- OMICS:
-
a field of study in biology ending in -omics, such as genomics, proteomics or metabolomics
- PD:
-
pharmacodynamics
- PE-PEG-TPP:
-
phosphatidylethanolamine polyethylene glycol triphenyl phosphonium
- PL-TPP:
-
phospholipid triphenyl phosphonium
- PEG:
-
polyethylene glycol
- PEI-TPP:
-
polyethylene imine triphenyl phosphonium
- PET:
-
positron emission tomography
- PLGA-PEG-TPP:
-
poly(lactic-co-glycolic acid)- block – polyethylene glycol)triphenylphosphonium
- PIT:
-
photo-immunotherapy
- PK:
-
pharmacokinetics
- PMN:
- RES:
-
reticuloendothelial system
- SB:
-
systems biology
- SiNP:
-
silica based nanoparticle
- TPGS1000-TPP:
-
tocopherol polyethylene glycol 1000 succinate triphenylphosphonium
- STPP:
-
stearyl triphenyl phosphonium
- SUPR:
-
super enhanced permeability effect
- QSAR:
-
quantitative structure activity relationship
- T (see Fig. 1) or Tox:
-
toxicology
- TAMs:
-
tumor-associated macrophages
- TPP:
-
triphenylphosphonium
- TSAS:
-
tumor-specific antigen
- VW:
-
Volkmar Weissig
Web Citations and References
Web Citations
a.https://clinicaltrials.gov/. Accessed 23 Nov 2015
b.www.wiley.com/egacy/willeychi/genmed/clinical/. Accessed 15 Dec 2015.
c.http://www.researchgate.net/publication/237620188_Report_and_Recommendations_of_thPanel_to_Assess_the_NIH_Investment_in_Research_on_Gene_Therapy. Accessed 5 Dec 2015
d.http://www.researchgate.net/publication/237620188_Report_and_Recommendations_of_the_Panel_to_Assess_the_NIH_Investment_in_Research_on_Gene_Therapy. Accessed 5 Dec 2015
e.http://nexus.od.nih.gov/all/2013/09/24/one-nation-in-support-of-biomedical-research/
References
Anton N, Vandamme TF (2014) Nanotechnology for computed tomography: a real potential recently disclosed. Pharm Res 31:20–34
Barenholz Y (2012) Doxil(R) – the first FDA-approved nano-drug: lessons learned. J Control Release 160:117–134
Barua S, Mitragotri S (2014) Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today 9:223–243
Bawa R (2007) Patents and nanomedicine. Nanomedicine 2(3):351–374
BCC Report: Nanotechnology in medical applications. The global market, Jan 19, 2012, 187 pages. BCC Research LLC, Wellesley
Benien P, Solomon MA, Nguyen P, Sheehan EM, Mehanna AS, D’Souza GG (2015) Hydrophobized triphenyl phosphonium derivatives for the preparation of mitochondriotropic liposomes: choice of hydrophobic anchor influences cytotoxicity but not mitochondriotropic effect. J Liposome Res 26:1–7, March
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC (2014) Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Advan Drug Deliv Rev 66(1):2–26
Bielski ER, Zhong Q, Brown M, da Rocha SR (2015) Effect of the conjugation density of triphenylphosphonium cation on the mitochondrial targeting of poly(amidoamine) dendrimers. Mol Pharm 12:3043–3053
Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP (2012a) Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J Control Release 159:393–402
Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP (2012b) Surface conjugation of triphenylphosphonium to target poly (amidoamine) dendrimers to mitochondria. Biomaterials 33:4773–4782
Blanco E, Shen H, Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33(9):941–951
Blume G, Cevc G (1993) Molecular mechanism of the lipid vesicle longevity in vivo. Biochim Biophys Acta 1146:157–168
Boddapati SV, Tongcharoensirikul P, Hanson RN, D’Souza GG, Torchilin VP, Weissig V (2005) Mitochondriotropic liposomes. J Liposome Res 15:49–58
Boddapati SV, D’Souza GG, Erdogan S, Torchilin VP, Weissig V (2008) Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett 8:2559–2563
Callahan J, Kopecek J (2006) Semitelechelic HPMA copolymers functionalized with triphenylphosphonium as drug carriers for membrane transduction and mitochondrial localization. Biomacromolecules 7:2347–2356
Cavallo F, Calogero RA, Forni G (2007) Are oncoantigens suitable targets for anti-tumour therapy? Nat Rev Cancer 7(9):707–713
Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK (2011) Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu Rev Chem Biomol Eng 2:281–298
Cheng SM, Pabba S, Torchilin VP, Fowle W, Kimpfler A, Schubert R, Weissig V (2005) Towards mitochondria-specific delivery of apoptosis-inducing agents: DQAsomal incorporated paclitaxel. J Drug Delivery Sci Technol 15:81–86
Crommelin DJ, Florence AT (2013) Towards more effective advanced drug delivery systems. Int J Pharm 454(1):496–511
Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y, Xiang J, Yan S, Direito de Dawidczyk CM, Russell LM, Searson PC (2014) Nanomedicines or cancer therapy: state-of-the-art and limitations to pre-clinical studies that hinder future development. Front Chem 2:69, 25 Aug. eCollection
Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y, Xiang J, Yan S, de Morais Guerreiro MD, Veomett N, Dubrovsky L, Curcio M, Doubrovina E, Ponomarev V, Liu C, O’Reilly RJ, Scheinberg DA (2015) Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol 33(10):1079–1086
D’Souza GG, Weissig V (2009) Subcellular targeting: a new frontier for drug-loaded pharmaceutical nanocarriers and the concept of the magic bullet. Expert Opin Drug Deliv 6:1135–1148
Dawidczyk CM, Russell LM, Searson PC (2014) Nanomedicines for cancer therapy: state-of-the-art and limitations to pre-clinical studies that hinder future developments. Front Chem 2:69
Eaton MAW, Levy L, Fontaine OMA (2015) Delivering nanomedicines to patients: a practical guide. Nanomed Nanotechnol Biol Med 11:983–992
Etrych T, Chytil P, Koziolova E, Širova M, Hoffman S, Muller T, Mader K, Řihova B, Ulbrich K (2014) Polymer-drug conjugates for targeted tumor therapy. Anticancer Res 34:5761–6258 (extended abstract)
European Science Foundation (2004) Nanomedicine – an ESF–European Medical Research Councils (EMRC) forward look report. France ESF, Strasbourg
Faltas B (2012) Cornering metastases: therapeutic targeting of circulating tumor cells and stem cells. Front Oncol 3(2):68, Epub 3 Jul 2012
Friedmann T, Roblin R (1972) Gene therapy for human genetic disease? Science 175:949–955
Gregoriadis G, Ryman BE (1971) Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem J 124:58P
Groenink L, Folkerts G, Henk-Jan Schuurman H-J (2015) European Journal of Pharmacology, Special issue on translational value of animal models: Introduction. Eur J Pharmacol 759:1–2
Grubb JH, Vogler C, Sly WS (2010) New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Res 13:229–236
Guzman-Villanueva D, Mendiola MM, Nguyen HX, Weissig V (2015) Influence of triphenylphosphonium (TPP) cation hydrophobization with phospholipids on cellualr toxicity and mitochondrial selectivity. SOJ Pharm Pharm Sci 2:1–9
Haddish-Berhane N, Rickus JL, Haghighi K (2007) The role of multiscale computational approaches for rational design of conventional and nanoparticle oral drug delivery systems. Int J Nanomed 2(3):315–331
Hillaireau H, Couvreur P (2009) Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci 66(17):2873–2896
Hong CW, Zeng Q (2013) Tapping the treasure of intracellular oncotargets with munotherapy. FEBS Lett 588(2014):350–355
Horobin RW (2001) Uptake, distribution, and accumulation of dyes and fluorescent probes within living cells. A structure-activity modelling approach. Adv Colour Sci Technol 4:101–107
Horobin RW (2010) Can QSAR models describing small-molecule xenobiotics give useful tips for predicting uptake and localization of nanoparticles in living cells? And if not, why not? In: Weissig V, D’Souza GGM (eds) Organelle-specific pharmaceutical nanotechnology, vol 2010. Wiley, Hoboken, pp 193–206
Horobin RW, Trapp S, Weissig V (2007) Mitochondriotropics: a review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J Control Release 121:125–136
Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L (2011) Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A 108:10980–10985
Imming P, Sinning C, Meyer A (2006) Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov 5(10):821–834 Erratum in Nat Rev Drug Discov 6(2):126 (2007)
Jain RK (2013) Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 31(17):2205–2208
Jain R, Stylianopoulos T, Economides EA, Baish JW, Fukumura D, Jain RK (2015) Towards optimal design of cancer nanomedicines: multi-stage nanoparticles for the treatment of solid tumors. Ann Biomed Eng 11 Feb 2015. [Epub ahead of print]
Jones HM, Rowland-Yeo K (2013) Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. Pharmacometrics Syst Pharmacol 2:e63
Khawar IA, Kim JH, Kuh H-J (2015) Improving drug delivery to solid tumors: priming the tumor microenvironment. J control Release 201:78–89
Kinnaird A, Michelakis ED (2015) Metabolic modulation of cancer: a new frontier with great translational potential. J Mol Med 93:127–142
Klibanov AL, Maruyama K, Torchilin VP, Huang L (1990) Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268:235–237
Kobayashi H, Watanabe R, Choyke PL (2014) Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 4(1):81–89
Krauss M, Schaller S, Borchers S, Findeisen R, Lippert J, Kuepfer L (2012) Integrating cellular metabolism into a multiscale whole-body model. PLOS Comput Biol 8(10):e1002750, 1 Oct 2012
Kunjachan S, Gremse F, Thee B, Koczera P, Pola R, Pechar M, Etrych T, Ulbrich K, Storm G, Kiessling F, Lammers T (2013) Noninvasive optical imaging of nanomedicine biodistribution. ACS Nano 7(1):252–262
Lammers T, Storm G, Kiessling F (2010) Nanomedicine formulations for combination therapies. Nano Rev 1:5705–5708
Lehner R, Wang X, Marsch S, Hunziker P (2015) Intelligent nanomaterials for medicine: carrier platforms and targeting strategies in the context of clinical application. Nanomed: Nanotechnol Biol Med 9:742–757
Lim CS (2007) Organelle-specific targeting in drug delivery and design. Adv Drug Deliv Rev 59:697
Liu L, Hitchens TK, Ye Q, Wu Y, Barbe B, Prior DE, Li WF, Yeh F-C, Foley LM, Bain DJ, Ho C (2013) Decreased reticuloendothelial system clearance and increased blood half-life and immune cell labeling for nano- and micron-sized superparamagnetic iron-oxide particles upon pre-treatment with Intralipid. Biochim Biophys Acta 1830(6):3447–3453
Lucas AT, Madden AJ, Zamboni WC (2016) Challenges in preclinical to clinical translation for anticancer carrier-mediated agents. WIREs Nanomed Nanobiotechnol 8:642–653. doi:10.1002/wnan.1394
Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS (2000) Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem 275:1625–1629
Lyrawati D, Trounson A, Cram D (2011) Expression of GFP in the mitochondrial compartment using DQAsome-mediated delivery of an artificial mini-mitochondrial genome. Pharm Res 28:2848–2862
Maeda H (2015) Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev 91:3–6
Malhi SS, Budhiraja A, Arora S, Chaudhari KR, Nepali K, Kumar R, Sohi H, Murthy RS (2012) Intracellular delivery of redox cycler-doxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes. Int J Pharm 432:63–74
Marrache S, Dhar S (2012) Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A 109:16288–16293
Marrache S, Dhar S (2013) Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc Natl Acad Sci U S A 110:9445–9450
Matsumura Y (2014) The drug discovery by nanomedicine and its clinical experience. Jpn J Clin Oncol 44(6):515–525
Mechler K, Mountford WK, Hoffmann GF, Ries M (2015) Pressure for drug development in lysosomal storage disorders – a quantitative analysis thirty years beyond the US orphan drug act. Orphanet J Rare Dis 10:46
Merril CR, Geier MR, Petricciani JC (1971) Bacterial virus gene expression in human cells. Nature 233:398–400
Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH, Yang KS, Laughney AM, Wojtkiewicz G, Kamaly N, Bhonagiri S, Pittet MJ, Farokhzad C, Weissleder R (2015) Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci Transl Med 7(314):314ra183. 18 Nov 2015
Munyon W, Kraiselburd E, Davis D, Mann J (1971) Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol 7:813–820
Needham D, McIntosh TJ, Lasic DD (1992) Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochim Biophys Acta 1108:40–48
Nichols JW, Bae YH (2014) EPR: evidence and fallacy. J Control Release 28(190):451–464
Nikulnikov PI (2015) Experience of application of the gene inductor of vascular endothelium growth factor in treatment of patients, suffering atherosclerotic Ischemia of the lower extremities tissues. Klin Khir 2015:41–43
Nishihara H (2014) Human pathological basis of blood vessels and stromal tissue for nanotechnology. Adv Drug Deliv Rev 74:19–27
Osada K, Christie RJ, Kataoka K (2009) Polymeric micelles from poly(ethyleneglycol)–poly(amino acid) block copolymer for drug and gene delivery. J R Soc Interface 6:S325–S339
Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E (2013) Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol 8:61–68
Patel NR, Hatziantoniou S, Georgopoulos A, Demetzos C, Torchilin VP, Weissig V, D’Souza GG (2010) Mitochondria-targeted liposomes improve the apoptotic and cytotoxic action of Sclareol. J Liposome Res 20:244–249
Pearson S, Jia H, Kandachi K (2004) China approves first gene therapy. Nat Biotechnol 22:3–4
Petrák K (2005) Essential properties of drug-targeting delivery systems. Drug Discov Today 10(23/24):1667–1673
Petrák K (2012) Targeted drug delivery – Quo vadis? Drug Dev Res 73(2):59–65
Petrak K, Hasirci V (2006) Targeting drug-delivery systems: promises, promises, and more promises…Let’s change the paradigm. J Biomater Sci Polym Ed 17(11):1207–1208
Pilari S, Huisinga W (2010) Lumping of physiologically-based pharmacokinetic models and a mechanistic derivation of classical compartmental models. J Pharmacokinet Pharmacodyn 37:365–405
Prokop A, Michelson S (2012) Systems biology in biotech and pharma. A changing paradigm, Springer briefs in pharmaceutical science and drug development. Springer, Dordrecht, p 127
Qiao J, Liu Z, Tian Y, Wu M, Niu Z (2015) Multifunctional self-assembled polymeric nanoprobes for FRET-based ratiometric detection of mitochondrial H2O2 in living cells. Chem Commun (Camb) 51:3641–3644
Rivera Gil P, Hühn D, del Mercato LL, Sasse D, Parak WJ (2010) Nanophar¬macy: inorganic nanoscale devices as vectors and active compounds. Pharmacol Res 62(2):115–125
Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DF (2013) Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 339:971–975
Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, Karson EM, Lotze MT, Yang JC, Topalian SL et al (1990) Gene transfer into humans – immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med 323:570–578
Sakhrani NM, Padh H (2013) Organelle targeting: third level of drug targeting. Drug Des Devel Ther 7:585–599
Savic R, Luo L, Eisenberg A, Maysinger D (2003) Micellar nanocontainers distribute to defined cytoplasmic organelles. Science 300:615–618
Scodeller P, Catalano PN, Salguero N, Duran H, Wolosiuk A, Soler-Illia GJ (2013) Hyaluronan degrading silica nanoparticles for skin cancer therapy. Nanoscale 5(20):9690–9698
Scodeller P (2014) Hyaluronidase and other extracellular matrix degrading enzymes for cancer therapy: new uses and nano-formulations. J Carcinog Mutagen 5:4–8
Seksek O, Biwersi J, Verkman AS (1997) Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J Cell Biol 138:131–142
Sharma A, Soliman GM, Al-Hajaj N, Sharma R, Maysinger D, Kakkar A (2012) Design and evaluation of multifunctional nanocarriers for selective delivery of coenzyme Q10 to mitochondria. Biomacromolecules 13:239–252
Sosale NG, Spinler KR, Alvey C, Discher DE (2015) Macrophage engulfment of a cell or nanoparticle is regulated by unavoidable opsonization, a species-specific ‘marker of self’ CD47, and target physical properties. Curr Opin Immunol 35:107–112
Stapleton S, Milosevic MD, Tannock IF, Allen C, Jaffray DA (2015) The intra-tumoral relationship between microcirculation, interstitial fluid pressure and liposome accumulation. J Control Release 211:163–170
Stylianopoulos T, Jain RK (2015) Design considerations for nanotherapeutics in oncology. Nanomed 11(8):1893–1907
Theodossiou TA, Sideratou Z, Katsarou ME, Tsiourvas D (2013) Mitochondrial delivery of doxorubicin by triphenylphosphonium-functionalized hyperbranched nanocarriers results in rapid and severe cytotoxicity. Pharm Res 30:2832–2842
Thiel C, Schneckener M, Krauss M, Ghallab A, Hoffmann U, Kanacher T, Zellmer S, Gebhard R, Hengstler JG, Kuepfer L (2015) A Systematic evaluation of the use of physiologically based pharmacokinetic modeling for cross-species extrapolation. J Pharm Sci 104:191–206
van der Meel R, Vehmeijer LJC, Kok RJ, Storm G, van Gaal EVB (2013) Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Advan Drug Deliv Rev 65(10):1284–1298
Wang M, Thanou M (2010) Targeting nanoparticles to cancer. Pharmacol Res 62:90–99
Weiss MJ, Wong JR, Ha CS, Bleday R, Salem RR, Steele GD Jr, Chen LB (1987) Dequalinium, a topical antimicrobial agent, displays anticarcinoma activity based on selective mitochondrial accumulation. Proc Natl Acad Sci U S A 84:5444–5448
Weissig V (2005) Targeted drug delivery to mammalian mitochondria in living cells. Expert Opin Drug Deliv 2:89–102
Weissig V (2011a) From serendipity to mitochondria-targeted nanocarriers. Pharm Res 28:2657–2668
Weissig V (2011b) Mitochondrial delivery of biologically active molecules. Pharm Res 28:2633–2638
Weissig V (2015) DQAsomes as the prototype of mitochondria-targeted pharmaceutical nanocarriers: preparation, characterization, and use. Methods Mol Biol 265:1–11
Weissig V, Guzman-Villanueva D (2015) Nanopharmaceuticals (part 2): products in the pipeline. Int J Nanomed 10:1245–1257
Weissig V, Lasch J, Erdos G, Meyer HW, Rowe TC, Hughes J (1998a) DQAsomes: a novel potential drug and gene delivery system made from Dequalinium. Pharm Res 15:334–337
Weissig V, Moegel HJ, Wahab M, Lasch J (1998b) Computer simulations of DQAsomes. Proc Int Symp Control Release Bioact Mater 25:312
Weissig V, Hughes J, Rowe T, Lasch J (2000) Materials and methods for intracellular delivery of biologically active molecules, US Patent No. 6,090,619
Weissig V, Hughes, J, Rowe T, Lasch J (2001) Materials and methods for intracellular delivery of biologically active molecules, US Patent No. 6,171,863 B1
Weissig V, Hughes J, Rowe T, Lasch J (2003) Materials and methods for intracellular delivery of biologically active molecules, US Patent No. 6,627,618 B2
Weissig V, Pettinger TK, Murdock N (2014) Nanopharmaceuticals (part 1): products on the market. Int J Nanomedicine 9:4357–4373
Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD (2011) Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov 1:291–296
Wikswo JP, Prokop A, Baudenbacher FJ, Cliffel D, Csukas B, Velkovsky M (2006) The engineering challenges of BioMEMS: the integration of microfluidics, micro- and nano-devices, models, and external control for systems biology. IEE Proc Nanobiotechnol 153(4):81–101
Wong AD, Ye M, Ulmschneider MB, Searson PC (2015) Quantitative analysis of the enhanced permeation and retention (EPR) Effect. PLoS ONE 10(5):e0123461. doi:10.1371/journal
Yang Y, Gao N, Hu Y, Jia C, Chou T, Du H, Wang H (2015) Gold nanoparticle-enhanced photodynamic therapy: effects of surface charge and mitochondrial targeting. Ther Deliv 6:307–321
Yildirim MA, K-Il G, Cusick ME, Barabasi A-L, Vidal M (2007) Drug target network. Nat Biotechnol 25:1119–1126
Yla-Herttuala S (2012) Endgame: glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol Ther 20:1831–1832
Zhou J, Zhao WY, Ma X, Ju RJ, Li XY, Li N, Sun MG, Shi JF, Zhang CX, Lu WL (2013) The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials 34:3626–3638
Zhuang Q, Jia H, Du L, Li Y, Chen Z, Huang S, Liu Y (2014) Targeted surface-functionalized gold nanoclusters for mitochondrial imaging. Biosens Bioelectron 55:76–82
Zschaler J, Schlorke D, Arnhold J (2014) Differences in innate immune response between man and mouse. Crit Rev Immunol 34(5):433–454
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Prokop, A., Weissig, V. (2016). Overview of Present Problems Facing Commercialization of Nanomedicines. In: Prokop, A., Weissig, V. (eds) Intracellular Delivery III. Fundamental Biomedical Technologies. Springer, Cham. https://doi.org/10.1007/978-3-319-43525-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-43525-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43523-7
Online ISBN: 978-3-319-43525-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)