Abstract
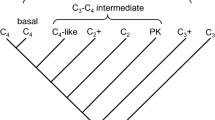
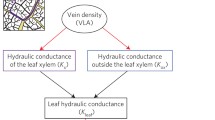
The conversion of carbon dioxide (CO2) and water into glucose and oxygen (O2) by photosynthesis has been a central component of the atmosphere and climate system over Earth history. The diffusive uptake of CO2 and its biochemical assimilation have in turn been strongly affected by atmospheric composition. Here, we illustrate how declining [CO2] and rising [O2] have exerted selective pressures to reduce the uptake of O2 (photorespiration) in favor of CO2 (photosynthesis) by the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco). In the last 10 Myr when [CO2] fell to less than 300 ppm, C3 photosynthesis became less efficient and mechanisms concentrating CO2 at the rubisco active site were favored leading to the expansion of C4 photosynthesis. The need to optimize carbon gain relative to water-loss has acted as a key selective pressure in the evolutionary development of stomatal function and epidermal patterning, to not only maximize diffusion of CO2 into the leaf but also regulate excessive transpirative water-loss. This stomatal control of photosynthesis generally allows angiosperms to sustain greater levels of stomatal conductance and CO2-uptake than species with more ancient evolutionary origins. This is particularly evident in the grasses, where dumb-bell stomata and the allocation of a higher percentage of the epidermis to gas exchange permit greater rates of stomatal conductance and photosynthesis than species with kidney-shaped stomata. The diffusive and biochemical components of photosynthesis have been strongly influenced by declining CO2:O2 over the past 100 Myr. However, current rising [CO2] may affect these selective pressures, having implications for future plant growth.
Similar content being viewed by others
References
Beerling DJ, Osborne CP, Chaloner WG (2001) Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the late Palaeozoic era. Nature 410(6826):352–354
Berner RA (2009) Phanerozoic atmospheric oxygen: new results using the Geocarbsulf model. Am J Sci 309(7):603–606. doi:10.2475/07.2009.03
de Boer HJ, Price CA, Wagner-Cremer F, Dekker SC, Franks PJ, Veneklaas EJ (2016) Optimal allocation of leaf epidermal area for gas exchange. New Phytol 210(4):1219–1228. doi:10.1111/nph.13929
Chater C, Gray JE, Beerling DJ (2013) Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr Opin Plant Biol 16(5):638–646. doi:10.1016/j.pbi.2013.06.013
Chater CC, Caine RS, Tomek M, Wallace S, Kamisugi Y, Cuming AC, Lang D, MacAlister CA, Casson S, Bergmann DC (2016) Origin and function of stomata in the moss Physcomitrella patens. Nat Plant 2:16179
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129(4):1773–1780. doi:10.1104/pp.002170
Duckett JG, Pressel S, P’ng KM, Renzaglia KS (2009) Exploding a myth: the capsule dehiscence mechanism and the function of pseudostomata in sphagnum. New Phytol 183(4):1053–1063. doi:10.1111/j.1469-8137.2009.02905.x
Edwards D, Kerp H, Hass H (1998) Stomata in early land plants: an anatomical and ecophysiological approach. J Exp Bot 49:255–278
Engineer CB, Hashimoto-Sugimoto M, Negi J, Israelsson-Nordström M, Azoulay-Shemer T, Rappel W-J, Iba K, Schroeder JI (2016) CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci 21(1):16–30. doi:10.1016/j.tplants.2015.08.014
Franks PJ, Beerling DJ (2009) Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci U S A 106(25):10343–10347. doi:10.1073/pnas.0904209106
Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143(1):78–87. doi:10.1104/pp.106.089367
Galmes J, Kapralov MV, Andralojc P, Conesa MÀ, Keys AJ, Parry MA, Flexas J (2014) Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant Cell Environ 37(9):1989–2001
Gowik U, Westhoff P (2011) The path from C3 to C4 photosynthesis. Plant Physiol 155(1):56–63
Hasper TB, Dusenge ME, Breuer F, Uwizeye FK, Wallin G, Uddling J (2017) Stomatal CO2 responsiveness and photosynthetic capacity of tropical woody species in relation to taxonomy and functional traits. Oecologia 184(1):43–57
Haworth M, Elliott-Kingston C, McElwain JC (2011) Stomatal control as a driver of plant evolution. J Exp Bot 62(8):2419–2423. doi:10.1093/jxb/err086
McAdam SAM, Brodribb TJ (2012) Stomatal innovation and the rise of seed plants. Ecol Lett 15(1):1–8. doi:10.1111/j.1461-0248.2011.01700.x
Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR (2009) Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol 23(5):922–930. doi:10.1111/j.1365-2435.2009.01577.x
Niklas KJ, Tiffney BH, Knoll AH (1983) Patterns in vascular land plant diversification. Nature 303(5918):614–616
Nisbet EG, Nisbet RER (2008) Methane, oxygen, photosynthesis, rubisco and the regulation of the air through time. Philos Trans R Soc B 363(1504):2745–2754
Osborne CP, Beerling DJ (2006) Nature’s green revolution: the remarkable evolutionary rise of C4 plants. Philos Trans R Soc B 361(1465):173–194
Raven JA (2014) Speedy small stomata? J Exp Bot 65(6):1415–1424. doi:10.1093/jxb/eru032
Robinson JM (1994) Speculations on carbon dioxide starvation, late tertiary evolution of stomatal regulation and floristic modernization. Plant Cell Environ 17(4):345–354
Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H (2001) Evolution and function of leaf venation architecture: a review. Ann Bot 87(5):553–566
Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63:19–47
Sperry JS, Hacke UG, Pittermann J (2006) Size and function in conifer tracheids and angiosperm vessels. Am J Bot 93(10):1490–1500. doi:10.3732/ajb.93.10.1490
Taylor SH, Ripley BS, Martin T, De-Wet LA, Woodward FI, Osborne CP (2014) Physiological advantages of C4 grasses in the field: a comparative experiment demonstrating the importance of drought. Glob Chang Biol 20(6):1992–2003
Tcherkez GG, Farquhar GD, Andrews TJ (2006) Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci 103(19):7246–7251
Tolbert NE, Benker C, Beck E (1995) The oxygen and carbon dioxide compensation points of C3 plants: possible role in regulating atmospheric oxygen. Proc Natl Acad Sci USA 92:11230–11233
Acknowledgments
We are grateful to funding from the EU FP7 project 3 to 4 (289582).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Haworth, M., Marino, G., Centritto, M. (2017). The Impact of Atmospheric Composition on the Evolutionary Development of Stomatal Control and Biochemistry of Photosynthesis over the Past 450 Ma. In: Nuno de la Rosa, L., Müller, G. (eds) Evolutionary Developmental Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-33038-9_171-1
Download citation
DOI: https://doi.org/10.1007/978-3-319-33038-9_171-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33038-9
Online ISBN: 978-3-319-33038-9
eBook Packages: Springer Reference Biomedicine and Life SciencesReference Module Biomedical and Life Sciences