Abstract
In addition to fluid haemostasis and lipid absorption, the lymphatic system and lymphoid tissues serve as the major host of immune cells where immune responses are evoked. Impaired function of the immune system might lead to serious diseases which are often treated by immunomodulators. This chapter briefly explores the physiology of an important part of the lymphatic system, the gut-associated lymphoid tissues (GALT). Currently used strategies for targeting GALT by immunomodulators for enhanced activity and/or decreased side effects are discussed. Strategies range from simple oral co-administration of immunomodulators with lipids to more advanced lipid-based formulations, polymer-based nanoparticle formulations and prodrugs. These targeting approaches successfully increase the concentration of immunomodulators achieved in the GALT and, more importantly, enhance immunomodulatory effects. Therefore, targeting immunomodulators to GALT represent a promising approach in the treatment of diseases where the immune system is actively involved.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- GALT
- Immunomodulators
- Targeting strategies
- Lipid-based drug delivery systems
- Nanoparticles
- Prodrugs
- Long-chain triglycerides
- Chylomicrons
- Cannabinoids
- Autoimmune disorders
Introduction
The current understanding of the lymphatic system and lymphoid tissues was developed over the centuries. In the fourth century B.C., components of the lymph system, particularly the auxiliary lymph nodes, were first described by Hippocrates as ‘vessels containing white blood’ [1]. However, it was not until the sixteenth century A.D. when the Italian physician Aselli succeeded to describe the lymphatic system in the gut of fed dogs. This discovery was made accidentally while trying to observe the diaphragm. He noticed a network of vessels containing milky white fluid that he later named ‘lacteal vessels’. However, Aselli suggested that these vessels deliver their contents to the liver. It took many decades until the French physician Pecquet identified the thoracic ducts and proved that these ducts receive flow from the lacteals discovered by Aselli [1–4]. More accurate anatomical descriptions were developed later, using wax injections to uncover parts of the system. This included the discovery of lymph nodules in the mucous membrane of the small intestine, Peyer’s patches (PP), which were named after their discoverer Johann Conrad Peyer [1, 3].
Accurate functional description of the discovered structures was not proposed until the publication of William Hunter’s research. It was suggested in this research that lymphatics and lacteals are structural units of one large system distributed in all remote parts of the body [1–3]. Indeed, the lymphatic system was thought to be merely a drainage system for fluids and proteins from interstitial space back to the blood [5]. Currently, however, the lymphatic system is considered to have a central role in the pathogenesis of several diseases such as cancers, viral infections, some parasitic infections and autoimmune disorders. In fact, it is the main pathway for the metastases of some epithelial origin solid tumours, such as those of the colon, breasts, lungs and prostate [6]. In addition, the lymphatic system is now recognised as a crucial part of the immune system. It is here where invader antigens are trapped, processed and presented to immune cells and consequently where immune responses are evoked [1]. These responses are important for the protection of the body from bacterial, viral, parasitic and fungal threats, as well as the growth of tumour cells [7]. Deficiencies in the immune responses, whether inherited or acquired, weaken the body’s defence mechanisms. On the other hand, over-reactive immune responses might cause life-threatening diseases, commonly called autoimmune diseases. Therefore, substances that can positively or negatively modify weak or over-reactive immune responses, respectively, provide a novel approach in the treatment of disorders where the immune system has a central role. These substances are collectively referred to as immunomodulators [8].
The last decade had witnessed the use of immunomodulators as promising therapeutic agents in the treatment of infectious diseases, autoimmune diseases and cancers and for prevention of organ transplant rejection. The therapeutic effects of immunomodulators can be achieved by either augmenting or suppressing the activity of immune cells [9, 10]. Since the lymphatic system is the major host of immune cells, the focus of this chapter is to highlight the current strategies of targeting immunomodulators, in particular cannabinoids, to the gut-associated lymphoid tissue (GALT).
Functions of the Lymphatic System
As mentioned above, a substantial interest was developed in the nineteenth century to elucidate the functions of the lymphatic system. These functions can be summarised as follows:
Fluid Recovery
Fluids continuously escape from blood capillaries to the surrounding tissues. However, a significant proportion of these fluids cannot be reabsorbed by venous capillaries. Indeed, up to four litres of fluids and half of all plasma proteins can extravasate each day. This in turn could lead to circulatory failure and increased tissue pressure if unrecovered. The lymphatic system, therefore, maintains the body’s fluid balance by reabsorption of the extravasated fluids and proteins back to the systemic circulation [2, 11, 12].
Lipid Absorption
The intestinal lymphatic system has an essential physiological role in the absorption of dietary lipids and lipid-soluble vitamins [2, 11]. The first step in the absorption of dietary lipids is their digestion and micellar solubilisation in the gastrointestinal lumen. This happens mainly by the action of pancreatic lipase/co-lipase complex and bile salts in the small intestine. Once digested, the products of lipid hydrolysis are then incorporated into mixed micelles, which promote the diffusion of digested lipids to the apical membrane of enterocytes [13]. Inside enterocytes, most of the long-chain triglycerides (LCT) are resynthesised from long-chain fatty acids and monoglycerides, mainly by the action of acyltransferases. LCT are then assembled with apolipoprotein B (Apo B), phospholipids, cholesterol and cholesterol esters to form large lipoproteins with a lipid core (chylomicrons, CM). Mature CM are then secreted by exocytosis through the basolateral membrane of enterocytes. Being large particles, CM cannot pass the walls of vascular capillaries but are absorbed to the lymph lacteals instead [14–17]. Because of the presence of lipids in the form of CM, lymph fluid following high-fat meal looks like a turbid emulsion which is commonly called ‘chyle’ [6].
Immunity
The immune system is not a definite organ system per se, but rather a population of cells distributed in all organs to defend the body against any potential invaders. The most important cells involved in immune responses are lymphocytes. Over 90 % of lymphocytes are localised in the lymphatic system [11, 18].
When collecting fluid and plasma proteins, the lymphatic system also picks up foreign bodies from tissues. These bodies are drained along the lymph to the regional lymph nodes where immune cells can initiate an immune response. Therefore, lymph nodes stand as checkpoints that examine lymph fluid before it is drained to the bloodstream [11].
Components of the Lymphatic System
Lymph
Lymph is usually a clear and colourless fluid which is drained from the interstitium. In addition to the recovered fluids and plasma proteins, lymph may also contain lipids, immune cells, hormones, bacteria, viruses, cellular debris or even cancer cells. Substantial differences in lymph composition arise from physiological and/or pathological conditions of the tissue from which lymph is drained, as well as its location along the lymphatic vessels [11, 19].
Lymphatic Vessels
The lymphatic system is the body’s second circulatory system. However, unlike the closed structure of the blood vessels, the lymphatic system consists of unidirectional, blind-ended and thin-walled capillary vessels where lymph is driven without a central pump [5, 20, 21]. Lymphatic capillaries drain in the afferent collecting vessels, which then pass through one or more gatherings of lymph nodes. Lymph fluid then passes through the efferent collecting vessels, larger trunks and finally the lymphatic ducts. Subsequently, ducts drain lymph to the systemic circulation [6, 22].
Lymphatic Organs
The lymphatic organs can be classified as primary or secondary. Primary lymphatic organs include the thymus gland and bone marrow, which produce mature lymphocytes (that can identify and respond to antigens). Secondary lymphatic organs include lymph nodes, spleen and mucosa-associated lymph tissues (MALT) [23–25]. It is within the secondary lymphatic organs that lymphocytes initiate immune responses. MALT are distributed throughout mucous membranes and provide a defence mechanism against a wide variety of inhaled or ingested antigens. MALT can be categorised according to their anatomical location to bronchus-associated lymphoid tissue (BALT), nasal-associated lymphoid tissue (NALT), salivary gland duct-associated lymphoid tissue (DALT), conjunctiva-associated lymphoid tissue (CALT), lacrimal duct-associated lymphoid tissue (LDALT) and gut-associated lymphoid tissue (GALT) [23, 26].
Gut-Associated Lymphoid Tissue (GALT)
GALT consists of effector and immune induction sites. The former is represented by lymphocytes distributed throughout the lamina propria (LP) and intestinal epithelium, while the latter involves organised tissues such as mesenteric lymph nodes (MLN), PP and smaller isolated lymphoid follicles (ILF) [27–30]. Some authors, however, define MLN as separate lymphatic organs rather than a part of GALT [31, 32]. In this chapter, MLN are included when referring to the GALT.
Mesenteric lymph nodes (MLN) are the largest gatherings of lymph nodes in the body, found in the base of the mesentery. The structure of MLN is similar to that of peripheral lymph nodes and can be divided into two regions: the medulla and cortex. The cortex is mainly composed of T-cell areas and B-cell follicles. It is within the T-cell area where circulating lymphocytes enter the lymph node and dendritic cells (DC) present antigens to T-cells [17, 33, 34]. Lymph (containing cells, antigens and chylomicrons) is collected from the intestinal mucosa and reaches MLN via the afferent lymphatics. Lymph fluid subsequently leaves MLN through efferent lymphatics to reach the thoracic duct that drains to the blood [27, 34].
Peyer’s patches (PP) are a collection of lymphoid nodules distributed in the mucosa and submucosa of the intestine. They consist of a sub-epithelial dome area and B-cell follicles dispersed in a T-cell area. A single layer of epithelial cells, called follicle-associated epithelium (FAE), separates lymphoid areas of PP from the intestinal lumen. FAE is permeated by specialised enterocytes called microfold (M) cells. These cells are considered as a gate for the transport of luminal antigens to PP [27, 30].
Isolated lymphoid follicles (ILF) are a combination of lymphoid cells in the intestinal LP. ILF are structurally similar to PP in the sense that they are composed of germinal centre covered by FAE containing M-cells. However, unlike PP, ILF lack a discrete T-cell area. Although its function is not completely understood, ILF is thought to be a complementary system to PP for the induction of intestinal immunity [32, 35].
It is noteworthy that GALT is the largest lymphatic organ in the human body and contains more than half of the body’s lymphocytes [36, 37]. GALT is also exposed to more antigens than any other part of the body, in the form of commensal bacteria and alimentary antigens, in addition to those from invasive pathogens. The intestinal immune system must therefore be able to distinguish antigens that require a protective immune response and to develop a state of immune hypo-responsiveness (oral tolerance) for those antigens that are harmless to the body [27, 30, 32]. The mechanism governing this process involves sampling of luminal antigens in the intestinal epithelium by DC. Antigens can cross the epithelium through M-cells that are found in the FAE of PP. The antigens can then interact with DC in the underlying sub-epithelial dome region. Antigens are then presented to local T-cells in PP by DC. DC can also migrate to the draining MLN where they present antigens to local lymphocytes [23, 27, 30, 38]. Alternative pathways for antigen transport across the intestinal epithelial cells involve receptor-mediated transport, as well as direct sampling from the lumen by DC’s projections. Antigen-loaded DC then migrate to the MLN through afferent lymphatics where they present antigens to T-cells. Subsequently, differentiated lymphocytes migrate from MLN through the thoracic duct and blood stream and eventually accumulate in the mucosa for an appropriate immune response (Fig. 14.1) [27, 39].
Schematic representation of the gut-associated lymphoid tissue (GALT). Dendritic cells (DC) can sample luminal antigens that (1) cross M-cells of Peyer’s patches (PP) and isolated lymphoid follicles (ILF) and (2) transported to lamina propria (LP) by receptor-mediated mechanisms. In addition, DC can use trans-epithelial projections to sample antigens directly from the lumen. DC then present antigens to local lymphocytes or migrate to mesenteric lymph nodes (MLN) for lymphocyte priming
Targeting GALT
In general, GALT could be a target (effective compartment) and/or a route through which therapeutic agents are delivered to the systemic circulation.
Advantages of Targeting GALT
-
Achieving high local concentration in the GALT could be of particular importance for pharmacological agents such as immunomodulators, for example, cannabinoids, some chemotherapeutic agents and anti-infective agents, thereby decreasing dose-related systemic side effects as well as systemic dilution [6, 18]. The lymphatic system is a main pathway of intestinal tumour metastases; therefore, targeting cytotoxic drugs to the intestinal lymphatics could provide advantage in the treatment of tumour metastases [40, 41]. Being the largest lymphatic organ, GALT provide a valid delivery target for antiviral agents, as some viruses spread and develop within the lymphatic system. Those of particular importance are human immunodeficiency virus (HIV) morbillivirus, canine distemper virus, severe acute respiratory syndrome (SARS)-associated coronavirus, hepatitis B and hepatitis C [42].
-
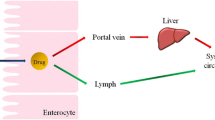
Increasing the bioavailability of lipophilic drugs when orally co-administered with lipid vehicles could be another advantage. This primarily occurs as a result of enhancing micellar solubilisation of the drug in the small intestine and drug-CM association in enterocytes [43]. One important reason is that intestinal lymphatic transport avoids hepatic first-pass metabolic loss by diverting the absorption of lipophilic drugs towards intestinal lymphatics rather than the portal vein, which is extremely important for drugs exhibiting significant first-pass metabolism [16].
-
Intestinal lymphatic transport of lipophilic drugs results in delivery of the drug to the systemic circulation in CM-associated form, which might attenuate the pharmacokinetic and/or pharmacodynamic properties [41, 44].
Miura et al. [34] have shown that oral administration of the LCT (olive oil) can enhance lymphocyte transport in mesenteric lymphatics of rats more than tenfold. Miura et al. also demonstrated that the enhancement of lymphocyte flux was selective to the administration of the long-chain but not the medium-chain fatty acids. This in turn was secondary to the assembly of CM by enterocytes to enhance the absorption of orally administered long-chain fatty acids, as the co-administration of Pluronic l-81 (an inhibitor of intracellular CM transport and secretion) significantly decreased lymphocyte transport (Fig. 14.2). Moreover, the effect of long-chain fatty acids (particularly the monounsaturated fatty acids) was not limited to the augmentation of lymphocyte flux, but also stimulated lymphocyte proliferation. The precise mechanism governing these effects is unclear. However, a mechanism that involves the utilisation of CM’s phospholipids and fatty acids has been suggested [37, 45]. This view is supported by the observations by Calder et al. [46] who found that lymphocytes have lipoprotein lipase activities and are able to release fatty acids from triglycerides (TG) present in CM and very low-density lipoproteins. In addition, the study also showed that TG rich in polyunsaturated fatty acids (PUFA) such as linoleic acid are potent inhibitors of lymphocyte proliferation in vitro, while this effect was not observed when using the monounsaturated oleic acid. This can in part explain the clinical benefits of daily administration of vegetable oils containing linoleic acid to patients suffering from inflammatory and autoimmune diseases, such as rheumatoid arthritis, psoriasis and multiple sclerosis [46, 47]. Therefore, it is reasonable to conclude that targeting of immunomodulators to GALT could present a valid treatment strategy for a wide range of serious diseases.
Lymphocyte flux of intestinal lymph (mean ± SEM, n = 6) after administration of oleic acid (○──○), octanoic acid (□──□), oleic acid with Pluronic l-81 (●──●) and control (sodium taurocholate, Δ----Δ) into the duodenum of lymph-fistulated rats. One-way ANOVA was used to assess statistical differences from the control values. ** p < 0.01; *** p < 0.001 (Reproduced with permission from Miura et al. [45])
Strategies for Targeting the GALT
Lipid-Based Drug Delivery Systems (LBDDS)
In a list of the top 200 marketed orally administered drugs, up to 40 % are poorly water-soluble, which is usually associated with poor absorption by the gut and low bioavailability [48]. Many biologically active drugs are also highly metabolised before they are able to exert their beneficial effect, which also reduces bioavailability. The combination of poor absorption and high first-pass metabolism has created the need for drug delivery systems that can improve the absorption of poorly water-soluble drugs and also protect them from degradation. One of the most promising strategies to address these issues is LBDDS [49–51].
Co-administration with Lipids
The simplest method of targeting drugs to the GALT is by co-administration of lipids with the drug. Oral administration of lipids can change the pharmacokinetic and pharmacodynamic profiles of drugs [52] by reducing the rate of gastric emptying and stimulating the release of bile (containing surfactants such as bile acids and phospholipids) from the gall bladder. Bile acids are hydrophilic on one end and hydrophobic on the other, whereas phospholipids generally have one hydrophilic tail and two hydrophobic tails (two fatty acids). These amphiphilic surfactants emulsify the lipids to form a fine emulsion, which prevents the droplets from aggregating back into larger particles. This emulsification vastly increases the surface area of the lipids and provides an interface for pancreatic lipase/co-lipase complex to digest them [53].
The digestion of TG by pancreatic lipase forms monoglycerides and fatty acids, as previously described in this chapter. TG, cholesterol and cholesterol esters are transported by CM, which typically vary from 75 to 1200 nm in diameter [54]. After leaving the enterocyte, CM are unable to enter the portal circulation due to their large size and enter the lymphatics instead, which allows passage of large particles [55]. Some drugs can exploit this transport pathway by associating with fatty acids and TG at any of the aforementioned steps, ending up inside the core of CM. Due to the hydrophobic nature of this core, highly lipophilic drugs are very good candidates to be transported via this pathway. Drug candidates for intestinal lymphatic transport have been classically described as having a water-to-octan-1-ol partition coefficient (log P) higher than 4.7 and TG solubility higher than 50 mg/mL [16, 56]. More recently other physicochemical properties have been included, most notably a drug’s distribution coefficient at pH 7.4 (log D7.4) [57].
An example of immunomodulatory drug targeted to the GALT by co-administration with lipids is JWH-015. This drug is an investigational lipophilic cannabinoid 2 (CB2) receptor agonist that has immunomodulatory effects [58] and therapeutic benefits in animal model of multiple sclerosis [59]. Cannabinoids in general are a group of chemical compounds that act on cannabinoid receptors and have been reported to have immunomodulatory effects [60]. In a study by Trevaskis et al. [18], the intestinal lymphatic transport and the recovery of JWH-015 in the collected lymph lymphocytes were assessed in mesenteric lymph duct-cannulated rats following intraduodenal infusion with oleic acid. In this study, JWH-015 was administered in lipid formulations containing either 4 or 40 mg oleic acid. The authors concluded that proportions of JWH-015 doses recovered in the mesenteric lymph and lymphocytes were significantly higher (53 and 176 fold, respectively) following the administration of 40 compared to 4 mg oleic acid formulations. Although lymphocyte flux into the mesenteric lymph was elicited by as low as 4 mg oleic acid, high lipid formulation (40 mg oleic acid) increased lymphocyte flux up to fivefold. Thus, in this study, co-administration of JWH-015 with long-chain fatty acids affected GALT’s lymphocytes by three mechanisms: enhancement of drug absorption from the intestinal lumen, stimulation of the intestinal lymphatic transport of the drug and increase in lymphocyte flux to the area. Furthermore, Trevaskis et al. compared the lymphatic transport of JWH-015 to that of other model lipophilic molecules, namely, dichlorodiphenyltrichloroethane (DDT), halofantrine, ciclosporin and diazepam which are insecticidal, antimalarial, immunosuppressant and central nervous system depressant drugs, respectively. The magnitude of the intestinal lymphatic transport correlated with the lipophilicity and TG solubility of these drugs. The results showed that the extent of intestinal lymphatic transport was enhanced when the drug was co-administered with 40 compared to 4 mg oleic acid for all drugs (Fig. 14.3).
Effect of drug lipophilicity and co-administration of 4 mg (filled bars) and 40 mg oleic acid (open bars) on the extent of intestinal lymphatic transport (mean ± SEM, n = 4 or 5) in mesenteric lymph duct-cannulated rats. One-way ANOVA with Tukey’s post hoc test was used for statistical analysis. * Significantly higher than 4 mg of lipid group. DZ diazepam, CYC ciclosporin, JWH JWH-015, HF halofantrine, DDT dichlorodiphenyltrichloroethane (Reproduced with permission from Trevaskis et al. [18])
Dexanabinol is another non-psychotropic synthetic cannabinoid that has been suggested to have therapeutic immunomodulatory effects in the treatment of experimental multiple sclerosis [61]. Gershkovich et al. [62] evaluated the lymphatic transport of dexanabinol following oral administration in LCT-based formulation in rats. The authors found that the concentration of dexanabinol recovered in the mesenteric lymph was around 80-fold higher than that in plasma. In the same study, another, more lipophilic cannabinoid (PRS-211,220) has been found to have more than 550-fold higher concentrations in the mesenteric lymph versus plasma. These findings suggest that the administration of lipophilic cannabinoids with LCT is a promising targeting strategy to GALT.
Emulsions
Emulsions are defined as mixtures of two or more immiscible liquids (Fig. 14.4a). For pharmaceutical applications, emulsions are generally made from three components: oil, surfactant and water. The hydrophile-lipophile balance of these components determines whether the resulting emulsion is oil droplets in water (oil-in-water), water droplets in oil (water-in-oil), micelles, oily dispersions or isotropic solutions that are emulsified upon contact with water. The last of these mixtures have been termed self-emulsifying drug delivery systems (SEDDS). By forming their own emulsion, drugs delivered this way are protected from degradative enzymes [63] and are not as reliant on endogenous surfactants to increase their surface area for absorption [64, 65], while the presence of lipid within the emulsion also stimulates lymphatic transport [66].
Drug delivery systems targeting the gut-associated lymphoid tissue (GALT) and their absorption in the intestine. (a) The structure of unilamellar liposomes, emulsions, polymeric nanoparticles (PLGA-NP) and solid lipid nanoparticles (SLN). (b) The two main pathways of the uptake of these drug delivery systems in the intestine
A well-known example where an immunomodulatory agent was orally delivered in a microemulsion is ciclosporin, a polypeptide drug widely used to prevent rejection of organs after transplantation by suppressing the activity of T-cells [67]. However, ciclosporin has very low solubility in water (23 μg/mL at 20 °C) and is also extensively metabolised by cytochrome P-450 enzymes [68–71]. Substantial research has been done about formulating ciclosporin into emulsions containing lipid microspheres [67] or milk fat globule membranes [65]. Since its approval for use, a number of different formulations of ciclosporin became commercially available, many of which are emulsions, the most common being Sandimmune® and its newer formulation Neoral® [65]. Ciclosporin is an important candidate for intestinal lymphatic transport, since it is highly metabolised in the liver into metabolites with lower immunosuppressive activity [69, 70]. Sandimmune®, the original formulation of ciclosporin, vastly improved its bioavailability but had high inter- and intra-patient variability. Therefore, an optimised formulation consisting of dl-α-tocopherol, corn oil derivatives and polyoxyl 40 hydrogenated castor oil, named Neoral®, was tested and resulted in more predictable pharmacokinetic profiles and more extensive drug absorption [65, 72, 73].
Another example of an immunomodulatory drug that was targeted to the GALT using emulsion-based formulation is in work done by Zhang et al. [74]. Morin, a xanthine oxidase inhibitor which has been shown to play a role in the treatment of gout was formulated into a self-nanoemulsifying drug delivery system (SNEDDS) to improve its oral absorption. SNEDDS are made from very similar components to other emulsions but are distinct in that they are not thermodynamically stable (but kinetically stable), which means that emulsification of SNEDDS is not as affected by temperature and dilution, but the emulsion will separate into different phases after prolonged storage [74–76]. In their work, Zhang et al. conjugated a phospholipid complex to morin in addition to incorporation into SNEDDS to increase its intestinal permeability and examined the intestinal absorption and lymphatic transport of their SNEDDS compared to conjugated drug and free drug. The group showed that SNEDDS were found in the GALT after oral administration, particularly from the segments closer to the ileum, due to the presence of PP and M-cells [74].
Liposomes
Liposomes are closed spherical structures consisting of at least one phospholipid bilayer, ranging from 100 to 5000 nm. Much like other phospholipid bilayers, they are capable of containing an aqueous phase within (see Fig. 14.4a). As mentioned earlier, the amphiphilic nature of phospholipids allows them to hold both hydrophilic (contained within the aqueous phase of the liposome) and hydrophobic (incorporated in the bilayer membrane) drugs [77]. Drugs that are incorporated in liposomes are also protected from degradation, which increases their therapeutic efficacy and reduces side effects [78].
Liposomes release their contents upon degradation in the lysosome or fusion with another lipid bilayer, such as those in the cell membrane, or phagocytes [79–81]. Liposome membranes are extremely modifiable and have been shown to deliver their encapsulated drugs by a wide number of stimuli, such as pH [82, 83], temperature [84], redox potential [85, 86], magnetism [84, 87], ultrasound [88, 89] and light [90]. Although liposomes have also been shown to successfully target different parts of the lymphatic system following various routes of administration, such as subcutaneous, pulmonary and intramuscular injection [77, 79, 81, 84, 86, 91], this section will focus on liposomes that target the GALT following oral administration. Liposomes are too large to enter the intestinal blood capillaries, which have a pore size between 60 and 80 nm [55, 92, 93], and therefore enter the lymphatics instead.
Liposomes have been used in the delivery of proteins [91–93] and DNA [94, 95]. Perrie et al. [94] incorporated plasmid DNA encoding a small region of hepatitis B surface antigen into liposomes and studied the immunisation conferred by oral administration in mice. Compared to naked DNA, mice receiving DNA delivered via liposomes showed a higher IgA response. The group went on to study gene expression in mice after oral administration of liposomes containing plasmid DNA encoding green fluorescent protein and found that liposomal delivery of plasmid DNA yielded much higher gene expression in the draining mesenteric lymph nodes than mice given naked plasmid DNA. The authors concluded that liposomes could be useful agents in lymphatic delivery of DNA [94].
Masuda et al. [95] incorporated ovalbumin as a model antigen into liposomes for oral delivery and examined their ability to induce oral tolerance in mice. Ovalbumin in liposomes of different compositions successfully suppressed proliferative responses of popliteal lymph node cells in mice, suggesting that liposomes were taken up by the lymphatics and induced tolerance to ovalbumin more effectively than ovalbumin administered in aqueous suspension [93]. Other research has focused on delivery of antigens encapsulated in liposomes to the lymphatics, but not via oral route [96].
Nanoparticles
Nanoparticles are particles smaller than 1000 nm in size and have been used in the delivery of drugs to the GALT, via uptake by ILF and PP, as mentioned previously in this chapter [97]. Although the exact mechanism of uptake is unclear, there have been many examples of nanoparticulate drug delivery systems that use this uptake pathway to access the GALT [98–102], some examples of which will be discussed in this section.
Lipid Nanoparticles
After the discovery and use of liposomes in the 1970s [103], a number of drawbacks were also discovered, such as drug leakage upon storage, physical instability, aggregation, presence of organic solvent residue, cytotoxicity and lack of cost-effective methods of high-quality production [104, 105]. Drug delivery systems based on naturally occurring lipids were then developed in order to overcome these problems. Lipid nanoparticles with a solid matrix had high drug loading, more controlled drug release profiles and better long-term stability and were more easily produced than emulsions or liposomes on a large scale [75, 104, 106]. One type of lipid-based nanoparticle that has been used to target the GALT is solid lipid nanoparticles.
Solid Lipid Nanoparticles (SLN)
SLN are usually made from biocompatible lipids and surfactants, such as tripalmitin [107, 108], tristearin, poloxamer 188 [109], dioleoylphosphatidylethanolamine, tricaprin, Tween 80 [110], glyceryl monostearate [111] and soya lecithin [112]. These components are in solid form at room temperature and can therefore be more stable and provide controlled release and more specific drug targeting compared to liposomes [113]. SLN are made of a solid lipid core and stabilised by surfactants. The loaded drug would then fit in the gaps between fatty acid chains of the lipid core (Fig. 14.4a). The size of particles in this type of formulation (20–1000 nm in diameter) allows efficient drug uptake into the intestinal lymphatic system due to the presence of lipids and their similar size to CM [114]. SLN can also be taken up by M-cells within PP, represented in Fig. 14.4b [115].
One example of the use of SLN to target the GALT is the work by Paliwal et al. [111]. The group loaded methotrexate into SLN made from glycerol monostearate, tristearin or Compritol 888 ATO, and the formulation was administered intraduodenally to rats. Methotrexate is used in the treatment of cancer and autoimmune diseases by antagonising folic acid metabolism [116, 117]. Drug concentration profiles in plasma and lymph were determined following intraduodenal administration of aqueous methotrexate solution and the four types of SLN loaded with methotrexate. The authors found that all SLN produced lead to increased drug bioavailability, a prolonged release compared to aqueous solution, and up to threefold higher plasma C max values. In addition, lymphatic uptake of methotrexate was up to tenfold higher with SLN compared to aqueous solution [111].
Polymeric Nanoparticles
When nanoparticles were first developed for use in drug delivery, synthetic polymers were the preferred choice for their outer coating. This was due to the varying purity of natural polymers (such as polysaccharides and proteins) at the time and their potential interaction and denaturation of contained drugs [118]. Among the most common and FDA-approved polymers used in polymeric nanoparticles are polyglycolic acid (PGA) and polylactic acid (PLA). PGA and PLA are considered biocompatible since they are degraded to glycolic and lactic acid, both of which are by-products of other metabolic pathways in the body [75, 119]. These polymers can also be combined into a copolymer, poly(lactide-co-glycoside) (PLGA). The ratio of PGA to PLA in PLGA can be fine-tuned to control degradation and drug release rates [118, 120, 121]. Polymeric nanoparticles could also be taken up by PP, in a manner similar to SLN [115].
Kim et al. [122] used PLGA nanoparticles entrapping type II collagen (CII) to study its ability to suppress collagen-induced arthritis in mice. Localisation of nanoparticles and CII, circulating immunoglobulin G (IgG) targeting CII, CII-specific T-cell proliferation and tumour necrosis factor α (TNFα) expression in PP and draining lymph nodes were assessed after a single oral administration of nanoparticles containing CII. The group found that CII-containing nanoparticles of 300 nm in diameter persisted in PP for 14 days after their administration and were able to reduce the incidence of arthritis by half (from 88.9 % to 43.8 %) and reduce IgG antibodies by more than half (28.6 ± 12.5 versus 78.5 ± 28.3 arbitrary units). The expression of TNFα was also up-regulated in PP cells when treated with nanoparticles, but down-regulated in the draining lymph nodes. The authors concluded that CII-containing PLGA nanoparticles were able to suppress the development of arthritis, as well as autoimmune responses.
Rebouças et al. [123] used polyanhydride nanoparticles (another biocompatible polymer) loaded with peanut extract to study oral immunotherapy for peanut allergies. Raw or roasted peanut proteins were orally administered to mice, and levels of different immunoglobulins (IgG, IgE, IgA), secretion of specific cytokines related to the immune response and the stimulation of T-helper cells were assessed. Polyanhydride nanoparticles containing peanut proteins were able to increase the production of IgG, IgE and IgA (compared to free protein and unloaded nanoparticles) and also up-regulate the expression of interleukin 10, an immunosuppressive cytokine that can reduce inflammation at sites of allergic reactions [124]. Finally, the nanoparticles were also shown to stimulate the appropriate prophylactic T-helper cell response twofold higher than free protein. While the effects of these nanoparticles in sensitised animals were not examined, the authors concluded that polyanhydride nanoparticles could be useful in food oral immunotherapy.
Prodrugs
A prodrug is a bio-reversible precursor of a drug that has an obstacle attenuating its therapeutic efficacy [125]. In regard to GALT targeting, prodrug can modify a drug’s physicochemical properties in such a way that would improve its delivery to the GALT. This can be achieved by a mechanism that involves the association of the prodrug with CM assembled in enterocytes. Therefore, a prodrug can be designed to have certain physicochemical properties, particularly log D7.4 ≥ 5 and high lipid solubility [16, 126], so that its intestinal lymphatic transport is enhanced when the prodrug is co-administered orally with LCT. Alternatively, a prodrug can be structured to be incorporated in one of the biochemical steps of lipid digestion processes. For example, prodrugs can be designed to be structurally similar to TG or phospholipids. In such circumstances, prodrugs can be hydrolysed, re-acylated and eventually incorporated with CM during lipoprotein assembly process in the enterocytes [127].
One of the ways to enhance the lipophilicity of a molecule is the synthesis of an ester, ether or amide-linked prodrug with large alkyl moiety [127]. Han et al. [128] described the synthesis of lipophilic prodrugs to promote the delivery of mycophenolic acid (MPA), a model immunomodulator, to the GALT after oral administration with oleic acid. The lipophilicity of MPA (log P 2.9) was increased by the synthesis of long-chain ester prodrug (MPA-C18E, log P 12.4) and long-chain amide prodrug (MPA-C18AM, log P 11.2). Oral administration of MPA-C18E and MPA-C18AM to mesenteric lymph duct-cannulated rats resulted in a 13- and 6-fold increase in lymphatic transport, respectively, compared to the parent compound. This approach enhanced the partitioning of alkyl chain prodrugs to CM and thereby promoted lymphatic transport. However, the least lipophilic medium-chain ester prodrug (MPA-C8E) did not increase the recovery of parent compound in lymph. In the same study, a TG-mimicking prodrug of MPA (2-MPA-TG, log P 17.8) was synthesised. The TG-mimicking prodrug leads to an 80-fold increase in lymphatic transport by a mechanism that involves the incorporation of the prodrug in TG hydrolysis-reacylation and CM assembly pathway. The authors suggested that metabolic instability and poor absorption were behind low lymphatic concentrations of alkyl prodrugs relative to TG-mimicking prodrug (Fig. 14.5).
Cumulative intestinal lymphatic transport of mycophenolic acid (MPA, open circles, n = 5), its medium-chain ester prodrug (MPA-C8E, triangles, n = 3), long-chain ester prodrug (MPA-C18E, inverted triangles, n = 6), long-chain amide prodrug (MPA-C18AM, squares, n = 4) and triglyceride mimic prodrug (2-MPA-TG, diamonds, n = 5) versus time following intraduodenal administration with oleic acid, Tween 80 and PBS in mesenteric lymph duct-cannulated anaesthetised rats. Data presented as mean ± SD (Reproduced with permission from Han et al. [128])
Future Directions
Currently, there is a high number of immunomodulators that show remarkable therapeutic benefits in the treatment of life-threatening immune disorders. If delivered to the GALT, these immunomodulators have potential to significantly improve future opportunities to treat these disorders. Examples of such molecules are statins which are widely used in clinical practice as cholesterol-lowering agents. Animal models of autoimmune diseases have shown that statins have therapeutic immunomodulatory effects in the treatment of multiple sclerosis [129–131], rheumatoid arthritis [132, 133], autoimmune myocarditis [134] and autoimmune uveitis [135]. However, doses of statins used in these experiments were higher than those usually used in humans. Thus, using an appropriate strategy to target GALT, statins might achieve sufficient concentrations to produce therapeutic immunomodulatory effects while inducing less systemic adverse effects in off-target tissues. In fact, researchers have used lipid-based formulations like SEDDS [136], SMEDDS [137], SNEDDS [138], SLN [139] and nanostructured lipid carriers (NLC, mentioned below) [140] to improve the oral bioavailability of statins, yet neither intestinal lymphatic transport nor immunomodulatory effects were assessed upon the administration of these formulations. Therefore, it is tempting to suggest that statins might have new therapeutic applications if properly targeted to GALT in patients with autoimmune diseases.
An important group of potential therapeutic immunomodulators are lipophilic cannabinoids, such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Pharmacodynamic studies have shown that both cannabinoids have broad spectrum of therapeutic activities [141–144]. Animal models studies of immune system disorders have reported that THC could be a promising drug in the treatment of multiple sclerosis [145], diabetes mellitus [146] and allergic asthma [147]. CBD also showed therapeutic efficacy in animal models of rheumatoid arthritis [148], diabetes mellitus [149] and allergic asthma [147]. Both THC and CBD are highly lipophilic molecules with log D7.4 of 7.25 and 6.99, respectively, which makes them good candidates for targeting to the GALT if orally co-administered with LCT.
Additional novel chemical or formulation-based strategies for targeting drugs to GALT could lead to increased targeting efficiency. One worth mentioning is nanostructured lipid carriers (NLC). After the development and success of SLN, a number of problems were identified. Using a single type of solid lipid in the core of SLN led to the formation of a crystalline lattice over time that potentially reduced drug-loading capacity [100]. Gelation of SLN also occurred after prolonged storage [101] and NLC were created as a way to reduce these problems. NLC use a mixture of solid and liquid lipids to create an imperfect core environment so there is more space to accommodate drugs, while still maintaining a solid state [104, 150]. It can therefore be said that NLC are a second-generation lipid-based nanoparticle formulation [100]. Due to the relatively new development of NLC, there has not been as extensive study into the targeting of NLC to the GALT, but research has begun in assessment for their targeting potential. One work studied the oral administration of NLC loaded with a lipophilic vasodilator [150]. The oral administration of these NLC resulted in a threefold increase in bioavailability of the loaded drug compared to an aqueous suspension. This suggests that NLC activated an alternative absorption pathway, possibly also avoiding first-pass metabolism, a frequent cause of low oral bioavailability for many lipophilic drugs [151]. It is therefore conceivable that immunomodulators can be loaded in NLC and targeted towards the GALT in a similar fashion.
Conclusions
Immunomodulatory drugs have advanced treatment protocols of a wide range of disorders where immune system is actively involved, such as rheumatoid arthritis and multiple sclerosis. However, despite considerable advances, some immunomodulators might cause serious adverse effects, which could be the cause of treatment failure for these therapies [152]. Systemic adverse effects are more prominent in non-targeted administration. Therefore, enhancing the delivery of immunomodulatory drugs to immune cells has potential to reduce systemic adverse effects as well as improve treatment efficacy [18].
Different approaches of targeting GALT by immunomodulatory drugs have successfully increased the concentration of drugs achieved in the GALT and, more importantly, significantly enhanced immunomodulatory effects. Immunomodulatory drugs of diverse physicochemical properties have been targeted to GALT, and this strategy presents a promising new way to treat diseases involving the immune system.
References
Loukas M, Bellary SS, Kuklinski M, Ferrauiola J, Yadav A, Shoja MM, et al. The lymphatic system: a historical perspective. Clin Anat. 2011;24(7):807–16. Epub 2011/05/06.
Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5(1):14–9. Epub 2001/01/09.
Eales NB. The history of the lymphatic system, with special reference to the Hunter-Monro controversy. J Hist Med Allied Sci. 1974;29(3):280–94. Epub 1974/07/01.
Chikly B. Who discovered the lymphatic system. Lymphology. 1997;30(4):186–93. Epub 1998/02/26.
Brown P. Lymphatic system: unlocking the drains. Nature. 2005;436(7050):456–8.
Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(1–2):3–20. Epub 2001/08/08.
Saroj P, Verma M, Jha K. An overview on immunomodulation. J Adv Sci Res. 2012;3(1):7–12.
Patil U, Jaydeokar A, Bandawane D. Immunomodulators: a pharmacological review. Int J Pharm Sci. 2012;4(1):30–6.
Masihi KN. Immunomodulatory agents for prophylaxis and therapy of infections. Int J Antimicrob Agents. 2000;14(3):181–91. Epub 2000/04/25.
Masihi KN. Immunomodulators in infectious diseases: panoply of possibilites. Int J Immunopharmacol. 2000;22(12):1083–91. Epub 2001/01/04.
Saladin KS. Anatomy & physiology: the unity of form and function. New York: McGraw-Hill; 2012.
Jurisic G, Detmar M. Lymphatic endothelium in health and disease. Cell Tissue Res. 2009;335(1):97–108. Epub 2008/07/24.
Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296(6):E1183–94. Epub 2009/01/23.
Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007;293(3):G519–24. Epub 2007/05/15.
Nordskog BK, Phan CT, Nutting DF, Tso P. An examination of the factors affecting intestinal lymphatic transport of dietary lipids. Adv Drug Deliv Rev. 2001;50(1–2):21–44. Epub 2001/08/08.
Charman WNA, Stella VJ. Estimating the maximal potential for intestinal lymphatic transport of lipophilic drug molecules. Int J Pharm. 1986;34(1–2):175–8.
von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–78. Epub 2003/12/12.
Trevaskis NL, Charman WN, Porter CJ. Targeted drug delivery to lymphocytes: a route to site-specific immunomodulation? Mol Pharm. 2010;7(6):2297–309. Epub 2010/10/21.
Clement C, Santambrogio L. The lymph proteome, peptidome, and degradome. In: Santambrogio L, editor. Immunology of the lymphatic system. New York: Springer; 2013. p. 65–79.
Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126(10):2167–77. Epub 2006/09/20.
McAllaster JD, Cohen MS. Role of the lymphatics in cancer metastasis and chemotherapy applications. Adv Drug Deliv Rev. 2011;63(10–11):867–75. Epub 2011/06/28.
Shields J. Lymph and lymphatic capillaries in cancer. In: Santambrogio L, editor. Immunology of the lymphatic system. New York: Springer; 2013. p. 121–42.
Ohl L, Bernhardt G, Pabst O, Förster R, editors. Chemokines as organizers of primary and secondary lymphoid organs. Semin Immunol. 2003;15(5):249–55. Elsevier.
Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. Epub 1999/06/08.
Pal I, Ramsey JD. The role of the lymphatic system in vaccine trafficking and immune response. Adv Drug Deliv Rev. 2011;63(10–11):909–22. Epub 2011/06/21.
Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34(5):599–608. Epub 2006/10/28.
Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3(4):331–41. Epub 2003/04/02.
Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93 Suppl 1:S41–8. Epub 2005/05/10.
Nakajima-Adachi H, Kikuchi A, Fujimura Y, Shibahara K, Makino T, Goseki-Sone M, et al. Peyer’s patches and mesenteric lymph nodes cooperatively promote enteropathy in a mouse model of food allergy. PLoS One. 2014;9(10), e107492. Epub 2014/10/08.
Wershil BK, Furuta GT. 4. Gastrointestinal mucosal immunity. J Allergy Clin Immunol. 2008;121(2 Suppl):S380–3; quiz S415. Epub 2008/02/05.
Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1(1):31–7. Epub 2008/12/17.
Varol C, Zigmond E, Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010;10(6):415–26. Epub 2010/05/26.
Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11(7):445–56. Epub 2011/06/18.
Miura S, Sekizuka E, Nagata H, Oshio C, Minamitani H, Suematsu M, et al. Increased lymphocyte transport by lipid absorption in rat mesenteric lymphatics. Am J Physiol. 1987;253(5 Pt 1):G596–600.
Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168(1):57–64. Epub 2001/12/26.
Watzl B, Girrbach S, Roller M. Inulin, oligofructose and immunomodulation. Br J Nutr. 2005;93 Suppl 1:S49–55. Epub 2005/05/10.
Miura S, Tsuzuki Y, Hokari R, Ishii H. Modulation of intestinal immune system by dietary fat intake: relevance to Crohn’s disease. J Gastroenterol Hepatol. 1998;13(12):1183–90. Epub 1999/01/26.
Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8(6):435–46. Epub 2008/05/27.
Spahn TW, Kucharzik T. Modulating the intestinal immune system: the role of lymphotoxin and GALT organs. Gut. 2004;53(3):456–65. Epub 2004/02/13.
Blanchard DK, Budde JM, Hatch 3rd GF, Wertheimer-Hatch L, Hatch KF, Davis GB, et al. Tumors of the small intestine. World J Surg. 2000;24(4):421–9. Epub 2000/03/09.
Porter CJ, Charman WN. Intestinal lymphatic drug transport: an update. Adv Drug Deliv Rev. 2001;50(1–2):61–80. Epub 2001/08/08.
Trevaskis NL, Charman WN, Porter CJ. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60(6):702–16. Epub 2007/12/25.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48. Epub 2007/03/03.
Wasan KM, Cassidy SM. Role of plasma lipoproteins in modifying the biological activity of hydrophobic drugs. J Pharm Sci. 1998;87(4):411–24. Epub 1998/04/21.
Miura S, Imaeda H, Shiozaki H, Ohkubo N, Tashiro H, Serizawa H, et al. Increased proliferative response of lymphocytes from intestinal lymph during long chain fatty acid absorption. Immunology. 1993;78(1):142–6. Epub 1993/01/01.
Calder PC, Yaqoob P, Newsholme EA. Triacylglycerol metabolism by lymphocytes and the effect of triacylglycerols on lymphocyte proliferation. Biochem J. 1994;298(Pt 3):605–11. Epub 1994/03/15.
Millar JH, Zilkha KJ, Langman MJ, Wright HP, Smith AD, Belin J, et al. Double-blind trial of linoleate supplementation of the diet in multiple sclerosis. Br Med J. 1973;1(5856):765–8. Epub 1973/03/31.
Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3(6):631–43. Epub 2006/12/05.
Hauss DJ. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59(7):667–76. Epub 2007/07/10.
Kuentz M. Lipid-based formulations for oral delivery of lipophilic drugs. Drug Discov Today Technol. 2012;9(2):e71–174. Epub 2012/07/01.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11 Suppl 2:S93–8. Epub 2000/10/18.
Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37(3):213–55. Epub 1999/10/08.
Armand M, Borel P, Ythier P, Dutot G, Melin C, Senft M, et al. Effects of droplet size, triacylglycerol composition, and calcium on the hydrolysis of complex emulsions by pancreatic lipase: an in vitro study. J Nutr Biochem. 1992;3(7):333–41.
Ruf H, Gould BJ. Size distributions of chylomicrons from human lymph from dynamic light scattering measurements. Eur Biophys J EBJ. 1999;28(1):1–11. Epub 1999/02/06.
Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenesis Res. 2010;2:14.
Noguchi T, Charman WNA, Stella VJ. Lymphatic appearance of DDT in thoracic or mesenteric lymph duct cannulated rats. Int J Pharm. 1985;24(2–3):185–92.
Gershkovich P, Fanous J, Qadri B, Yacovan A, Amselem S, Hoffman A. The role of molecular physicochemical properties and apolipoproteins in association of drugs with triglyceride-rich lipoproteins: in-silico prediction of uptake by chylomicrons. J Pharm Pharmacol. 2009;61(1):31–9.
Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. 2008;294(3):H1145–55. Epub 2008/01/08.
Arevalo-Martin A, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci Off J Soc Neurosci. 2003;23(7):2511–6. Epub 2003/04/10.
Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmun Pharmacol Off J Soc Neuroimmune Pharmacol. 2006;1(1):50–64. Epub 2007/11/28.
Achiron A, Miron S, Lavie V, Margalit R, Biegon A. Dexanabinol (HU-211) effect on experimental autoimmune encephalomyelitis: implications for the treatment of acute relapses of multiple sclerosis. J Neuroimmunol. 2000;102(1):26–31. Epub 2000/01/08.
Gershkovich P, Qadri B, Yacovan A, Amselem S, Hoffman A. Different impacts of intestinal lymphatic transport on the oral bioavailability of structurally similar synthetic lipophilic cannabinoids: dexanabinol and PRS-211,220. Eur J Pharm Sci Off J Eur Federation Pharm Sci. 2007;31(5):298–305. Epub 2007/06/15.
Takada K, Yoshimura H, Yoshikawa H, Muranishi S, Yasumura T, Oka T. Enhanced selective lymphatic delivery of cyclosporin A by solubilizers and intensified immunosuppressive activity against mice skin allograft. Pharm Res. 1986;3(1):48–51.
Fukui E, Kurohara H, Kageyu A, Kurosaki Y, Nakayama T, Kimura T. Enhancing effect of medium-chain triglycerides on intestinal absorption of d-alpha-tocopherol acetate from lecithin-dispersed preparations in the rat. J Pharmacobiodyn. 1989;12(2):80–6. Epub 1989/02/01.
Kovarik JM, Mueller EA, van Bree JB, Tetzloff W, Kutz K. Reduced inter- and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J Pharm Sci. 1994;83(3):444–6. Epub 1994/03/01.
Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, et al. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998;87(2):164–9.
Starzl TE, Klintmalm GBG, Porter KA, Iwatsuki S, Schröter GPJ. Liver transplantation with use of cyclosporin A and prednisone. N Engl J Med. 1981;305(5):266–9.
Takada K, Furuya Y, Yoshikawa H, Muranishi S. Biological and pharmaceutical factors affecting the absorption and lymphatic delivery of ciclosporin A from gastrointestinal tract. J Pharmacobiodyn. 1988;11(2):80–7. Epub 1988/02/01.
Wang CP, Hartman NR, Venkataramanan R, Jardine I, Lin FT, Knapp JE, et al. Isolation of 10 cyclosporine metabolites from human bile. Drug Metab Dispos Biol Fate Chemicals. 1989;17(3):292–6. Epub 1989/05/01.
Christians U, Strohmeyer S, Kownatzki R, Schiebel HM, Bleck J, Kohlhaw K, et al. Investigations on the metabolic pathways of cyclosporine: II. Elucidation of the metabolic pathways in vitro by human liver microsomes. Xenobiotica. 1991;21(9):1199–210. Epub 1991/09/01.
Tjia JF, Webber IR, Back DJ. Cyclosporin metabolism by the gastrointestinal mucosa. Br J Clin Pharmacol. 1991;31(3):344–6.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45(1):89–121.
Choc MG. Bioavailability and pharmacokinetics of cyclosporine formulations: Neoral® vs Sandimmune®. Int J Dermatol. 1997;36:1–6.
Zhang Z. Mechanism of enhanced oral absorption of morin by phospholipid complex based self-nanoemulsifying drug delivery system. Mol Pharm. 2015;12(2):504–13.
Ahmed K, Li Y, McClements DJ, Xiao H. Nanoemulsion- and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem. 2012;132(2):799–807.
Holm R, Porter CJH, Edwards GA, Müllertz A, Kristensen HG, Charman WN. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur J Pharm Sci. 2003;20(1):91–7.
Lian T, Ho RJY. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90(6):667–80.
Gabizon A, Dagan A, Goren D, Barenholz Y, Fuks Z. Liposomes as in vivo carriers of adriamycin: reduced cardiac uptake and preserved antitumor activity in mice. Cancer Res. 1982;42(11):4734–9.
Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1–2):143–56.
Perche F, Torchilin VP. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J Drug Deliv. 2013;2013:705265. Epub 2013/03/28.
Cho HY, Lee YB. Nano-sized drug delivery systems for lymphatic delivery. J Nanosci Nanotechnol. 2014;14(1):868–80. Epub 2014/04/16.
Júnior ÁDC, Mota LG, Nunan EA, Wainstein AJA, Wainstein APDL, Leal AS, et al. Tissue distribution evaluation of stealth pH-sensitive liposomal cisplatin versus free cisplatin in Ehrlich tumor-bearing mice. Life Sci. 2007;80(7):659–64.
Leite EA, Giuberti Cdos S, Wainstein AJ, Wainstein AP, Coelho LG, Lana AM, et al. Acute toxicity of long-circulating and pH-sensitive liposomes containing cisplatin in mice after intraperitoneal administration. Life Sci. 2009;84(19–20):641–9. Epub 2009/03/24.
Kneidl B, Peller M, Winter G, Lindner LH, Hossann M. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine. 2014;9:4387–98.
McCarley RL. Redox-responsive delivery systems. Ann Rev Anal Chem (Palo Alto Calif). 2012;5:391–411. Epub 2012/06/20.
Wang C, Liu P, Zhuang Y, Li P, Jiang B, Pan H, et al. Lymphatic-targeted cationic liposomes: a robust vaccine adjuvant for promoting long-term immunological memory. Vaccine. 2014;32(42):5475–83.
Eloy JO, Claro de Souza M, Petrilli R, Barcellos JP, Lee RJ, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf B Biointerfaces. 2014;123c:345–63. Epub 2014/10/05.
Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13(9):2722–7. Epub 2007/05/03.
Husseini GA, Pitt WG, Martins AM. Ultrasonically triggered drug delivery: breaking the barrier. Colloids Surf B Biointerfaces. 2014;123c:364–86. Epub 2014/12/03.
Wang JY, Wu QF, Li JP, Ren QS, Wang YL, Liu XM. Photo-sensitive liposomes: chemistry and application in drug delivery. Mini Rev Med Chem. 2010;10(2):172–81. Epub 2010/04/23.
Videira M, Almeida AJ, Fabra À. Preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effect. Nanomedicine Nanotechnol Biol Med. 2012;8(7):1208–15.
Barrowman JA. Physiology of the gastro-intestinal lymphatic system. Cambridge: Cambridge University Press; 1978.
Perry MA, Granger DN. Permeability of intestinal capillaries to small molecules. Am J Physiol. 1981;241(1):G24–30. Epub 1981/07/01.
Perrie Y, Obrenovic M, McCarthy D, Gregoriadis G. Liposome (Lipodine)-mediated DNA vaccination by the oral route. J Liposome Res. 2002;12(1–2):185–97. Epub 2003/02/27.
Masuda K, Horie K, Suzuki R, Yoshikawa T, Hirano K. Oral delivery of antigens in liposomes with some lipid compositions modulates oral tolerance to the antigens. Microbiol Immunol. 2002;46(1):55–8. Epub 2002/03/26.
Badiee A, Khamesipour A, Samiei A, Soroush D, Shargh VH, Kheiri MT, et al. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol. 2012;132(4):403–9.
Nishioka Y, Yoshino H. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev. 2001;47(1):55–64.
Eldridge JH, Meulbroek JA, Staas JK, Tice TR, Gilley RM. Vaccine-containing biodegradable microspheres specifically enter the gut-associated lymphoid tissue following oral administration and induce a disseminated mucosal immune response. Adv Exp Med Biol. 1989;251:191–202. Epub 1989/01/01.
Florence AT, Hussain N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv Drug Deliv Rev. 2001;50(Suppl 1(0)):S69–89.
Westesen K, Bunjes H, Koch MHJ. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J Control Release. 1997;48(2–3):223–36.
Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77.
Bargoni A, Cavalli R, Zara GP, Fundarò A, Caputo O, Gasco MR. Transmucosal transport of tobramycin incorporated in solid lipid nanoparticles (sln) after duodenal administration to rats. Part II—tissue distribution. Pharmacol Res. 2001;43(5):497–502.
Horne RW, Bangham AD, Whittaker VP. Negatively stained lipoprotein membranes. Nature. 1963;200(4913):1340.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76.
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60.
Yao M, Xiao H, McClements DJ. Delivery of lipophilic bioactives: assembly, disassembly, and reassembly of lipid nanoparticles. Ann Rev Food Sci Technol. 2014;5(1):53–81.
Garcia-Fuentes M, Prego C, Torres D, Alonso MJ. A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci. 2005;25(1):133–43. Epub 2005/04/28.
Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RSR. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release. 2005;105(3):185–98.
Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107(2):215–28.
Choi SH, Jin S-E, Lee M-K, Lim S-J, Park J-S, Kim B-G, et al. Novel cationic solid lipid nanoparticles enhanced p53 gene transfer to lung cancer cells. Eur J Pharm Biopharm. 2008;68(3):545–54.
Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine Nanotechnol Biol Med. 2009;5(2):184–91.
Chalikwar SS, Belgamwar VS, Talele VR, Surana SJ, Patil MU. Formulation and evaluation of Nimodipine-loaded solid lipid nanoparticles delivered via lymphatic transport system. Colloids Surf B Biointerfaces. 2012;97:109–16.
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–96.
Ali Khan A, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomedicine. 2013;8:2733–44. Epub 2013/08/09.
Qi J, Lu Y, Wu W. Absorption, disposition and pharmacokinetics of solid lipid nanoparticles. Curr Drug Metab. 2012;13(4):418–28. Epub 2012/03/27.
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343(22):1594–602. Epub 2000/11/30.
Shea B, Swinden MV, Tanjong Ghogomu E, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD000951. Epub 2013/06/04.
Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6(4):319–27.
Zakeri-Milani P, Loveymi BD, Jelvehgari M, Valizadeh H. The characteristics and improved intestinal permeability of vancomycin PLGA-nanoparticles as colloidal drug delivery system. Colloids Surf B Biointerfaces. 2013;103:174–81.
Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17(3):247–89. Epub 2006/05/13.
Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D, L-lactide-co-glycolide) and its derivatives. J Control Release Off J Control Release Soc. 2008;125(3):193–209. Epub 2007/12/18.
Kim W-U, Lee W-K, Ryoo J-W, Kim S-H, Kim J, Youn J, et al. Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002;46(4):1109–20.
De S, Rebouças J, Irache JM, Camacho AI, Gastaminza G, Sanz ML, Ferrer M, et al. Immunogenicity of peanut proteins containing poly(anhydride) nanoparticles. Clin Vaccine Immunol. 2014;21(8):1106–12.
Kosaka S, Tamauchi H, Terashima M, Maruyama H, Habu S, Kitasato H. IL-10 controls Th2-type cytokine production and eosinophil infiltration in a mouse model of allergic airway inflammation. Immunobiology. 2011;216(7):811–20.
Stella VJ. A case for prodrugs. Prodrugs: Springer New York; 2007. p. 3–33.
Gershkovich P, Hoffman A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci Off J Eur Federation Pharm Sci. 2005;26(5):394–404. Epub 2005/09/06.
Shackleford DM, Porter CJ, Charman WN. Lymphatic absorption of orally administered prodrugs. Prodrugs: Springer New York; 2007. p. 653–82.
Han S, Quach T, Hu L, Wahab A, Charman WN, Stella VJ, et al. Targeted delivery of a model immunomodulator to the lymphatic system: comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J Control Release Off J Control Release Soc. 2014;177:1–10. Epub 2014/01/09.
Nath N, Giri S, Prasad R, Singh AK, Singh I. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172(2):1273–86. Epub 2004/01/07.
Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. Epub 2002/11/08.
Stanislaus R, Singh AK, Singh I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental allergic encephalomyelitis. J Neurosci Res. 2001;66(2):155–62.
Leung BP, Sattar N, Crilly A, Prach M, McCarey DW, Payne H, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170(3):1524–30.
Barsante MM, Roffê E, Yokoro CM, Tafuri WL, Souza DG, Pinho V, et al. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. Eur J Pharmacol. 2005;516(3):282–9.
Liu W, Li W-M, Gao C, Sun N-L. Effects of atorvastatin on the Th1/Th2 polarization of ongoing experimental autoimmune myocarditis in Lewis rats. J Autoimmun. 2005;25(4):258–63.
Gegg ME, Harry R, Hankey D, Zambarakji H, Pryce G, Baker D, et al. Suppression of autoimmune retinal disease by lovastatin does not require Th2 cytokine induction. J Immunol. 2005;174(4):2327–35.
Patil P, Patil V, Paradkar A. Formulation of a self-emulsifying system for oral delivery of simvastatin: in vitro and in vivo evaluation. Acta Pharm. 2007;57(1):111–22. Epub 2007/03/01.
Kang BK, Lee JS, Chon SK, Jeong SY, Yuk SH, Khang G, et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004;274(1–2):65–73. Epub 2004/04/10.
Mahmoud H, Al-Suwayeh S, Elkadi S. Design and optimization of self-nanoemulsifying drug delivery systems of simvastatin aiming dissolution enhancement. Afr J Pharm Pharmacol. 2013;7(22):1482–500.
Padhye SG, Nagarsenker MS. Simvastatin solid lipid nanoparticles for oral delivery: formulation development and in vivo evaluation. Indian J Pharm Sci. 2013;75(5):591–8. Epub 2014/01/10.
Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415(1–2):232–43. Epub 2011/06/07.
Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharm (Basel). 2012;5(5):529–52. Epub 2012/01/01.
Pertwee RG. Pharmacological and therapeutic targets for Δ9 tetrahydrocannabinol and cannabidiol. Euphytica. 2004;140(1–2):73–82.
Ben AM. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105(1–2):1–25.
Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215(8):588–97.
Lyman W, Sonett J, Brosnan C, Elkin R, Bornstein M. Delta 9-tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol. 1989;23(1):73–81.
Li X, Kaminski NE, Fischer LJ. Examination of the immunosuppressive effect of delta9-tetrahydrocannabinol in streptozotocin-induced autoimmune diabetes. Int Immunopharmacol. 2001;1(4):699–712. Epub 2001/05/19.
Jan TR, Farraj AK, Harkema JR, Kaminski NE. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol Appl Pharmacol. 2003;188(1):24–35. Epub 2003/04/02.
Malfait A, Gallily R, Sumariwalla P, Malik A, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97(17):9561–6.
Weiss L, Zeira M, Reich S, Slavin S, Raz I, Mechoulam R, et al. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology. 2008;54(1):244–9. Epub 2007/08/24.
Zhuang C-Y, Li N, Wang M, Zhang X-N, Pan W-S, Peng J-J, et al. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm. 2010;394(1–2):179–85.
Szakacs T, Veres Z, Vereczkey L. In vitro-in vivo correlation of the pharmacokinetics of vinpocetine. Pol J Pharmacol. 2001;53(6):623–8. Epub 2002/05/03.
Sathish JG, Sethu S, Bielsky MC, de Haan L, French NS, Govindappa K, et al. Challenges and approaches for the development of safer immunomodulatory biologics. Nat Rev Drug Discov. 2013;12(4):306–24. Epub 2013/03/29.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zgair, A., Wong, J.C.M., Gershkovich, P. (2016). Targeting Immunomodulatory Agents to the Gut-Associated Lymphoid Tissue. In: Constantinescu, C., Arsenescu, R., Arsenescu, V. (eds) Neuro-Immuno-Gastroenterology. Springer, Cham. https://doi.org/10.1007/978-3-319-28609-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-28609-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28607-5
Online ISBN: 978-3-319-28609-9
eBook Packages: MedicineMedicine (R0)