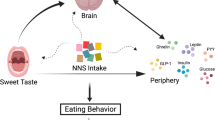
Abstract
Over the past decades, low-calorie sweeteners (LCSs) have emerged as the main source of sweetening food products and beverages. Evidences suggest mixed effects of LCSs intake. In addition, there is a paucity of data on long-term health outcomes of LCSs intake as well as their usefulness in certain groups of the population (e.g., pregnant women, children, and the elderly). Moreover, available unbiased studies conducted to date are fairly scanty, thereby restraining far-reaching conclusions. LCSs are biomolecules with cognate cellular receptors ubiquitously localized in many organs and tissues of the body. The T1R2-T1R3 receptor heterodimer is one of the widely known receptors of LCSs. Upon activation by LCSs, T1R2-T1R3 signals downstream that result in several cellular responses that characterize the biochemical and physiological effects of LCSs. In the gut, for instance, signaling of LCSs may be mediated via humoral and neural mechanisms (involving the gut-brain axis, gut-adipose tissue axis, gut-adipose tissue-brain triangle, gut-pancreas-adipose tissue or entero-adipo-insular triangle, gut-brain-adipose tissue triangle) influence obesity, overweight, appetite, satiety, cognition, and memory. Several interconnected signaling pathways are important in modulating the physiological outcomes of LCS intake. In this chapter, we discuss the signaling mechanisms of LCSs, and their relationship to metabolism, obesity, weight gain, satiety, appetite, cognition, and memory.
Similar content being viewed by others
Abbreviations
- AC:
-
Adenylate cyclase
- ACTH:
-
Adrenocorticotropic Hormone
- AgRP:
-
Agouti-related protein
- AMPK:
-
AMP-activated protein kinase
- AP:
-
Area postrema
- ATP:
-
Adenosine triphosphate
- CALHM1:
-
Calcium homeostasis modulator 1
- CAMK:
-
Calmodulin-dependent protein kinase
- cAMP:
-
Cyclic adenosine monophosphate
- CART:
-
Cocaine and amphetamine regulated transcript
- CREB:
-
cAMP-related element binding protein
- CRH:
-
Corticotropin releasing hormone
- DMN:
-
Dorsomotor nucleus
- GABA:
-
Gamma amino butyric acid
- GLP-1:
-
Glucagon-like peptide-1
- GLUT:
-
GLUcose Transporter
- IP3:
-
1,4,5-inositol trisphosphate
- Low-calorie sweeteners:
-
LCSs
- MC4R:
-
Melanocortin receptor type-4
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin 1
- NPY:
-
Neuropeptide Y
- NTS:
-
Nucleus tractus solitarius
- Ob-Rb:
-
Adiposity (obesity) receptor type b
- PDE:
-
Phosphodiesterase
- PI3K:
-
Phosphoinositide-3-kinase
- PKB:
-
Protein kinase B
- PLCβ:
-
Phospholipase Cβ
- POMC:
-
Proopiomelanocortin
- PVN:
-
Paraventricular nucleus
- PYY:
-
Peptide YY
- SGLT:
-
Sodium-dependent glucose cotransporter
- T1R2-T1R3:
-
Sweet-taste receptor heterodimer
- TRH:
-
Thyrotropin releasing hormone
- TRPM5:
-
Transient receptor potential cation channel, subfamily M, member 5
- α-MSH:
-
Alpha-melanin-stimulating hormone
References
Weihrauch MR, Diehl V (2004) Artificial sweeteners – do they bear a carcinogenic risk? Ann Oncol 15:1460–1465
Kant R (2005) Sweet proteins – potential replacement for artificial low calorie sweeteners. Nutr J 4:5
Roberts A (2016) The safety and regulatory process for low calorie sweeteners in the United States. Physiol Behav. doi:10.1016/j.physbeh.2016.02.039
Grembecka M (2015) Natural sweeteners in a human diet. Rocz Panstw Zakl Hig 66(3):195–202
Miller PE, Perez V (2014) Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 100:765–777
Gardner C (2014) Non-nutritive sweeteners: evidence for benefit vs. risk. Curr Opin Lipidol 25(1):80–84
Fitch C, Keim KS, Academy of Nutrition and Dietetics (2012) Position of the academy of nutrition and dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 112(5):739–758
Piernas C, Mendez MA, Ng SW, Gordon-Larsen P, Popkin BM (2014) Low-calorie- and calorie-sweetened beverages: diet quality, food intake, and purchase patterns of US household consumers. Am J Clin Nutr 99(3):567–577
Sylvetsky AC, Rother KI (2016) Trends in the consumption of low-calorie sweeteners. Physiol Behav. doi:10.1016/j.physbeh.2016.03.030
Foreyt J, Kleinman R, Brown RJ, Lindstrom R (2012) the use of low-calorie sweeteners by children: implications for weight management. J Nutr 142:1155S–1162S
Raben A, Richelsen B (2012) Artificial sweeteners: a place in the field of functional foods? Focus on obesity and related metabolic disorders. Curr Opin Clin Nutr Metab Care 15(6):597–604
Shankar P, Ahuja S, Sriram K (2013) Non-nutritive sweeteners: review and update. Nutrition 29(11–12):1293–1299
Anderson GH, Foreyt J, Sigman-Grant M, Allison DB (2012) The use of low-calorie sweeteners by adults: impact on weight management. J Nutr. 142(6):1163S–1169S
Gibson S, Drewnowski A, Hill J, Raben AB, Tuorila H, Widström E (2014) Consensus statement on benefits of low-calorie sweeteners. Nutr Bull 39:386–389
Mennella JA, Pepino MY, Reed DR (2005) Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 115(2):e216–e222
Tepper BJ, White EA, Koelliker Y, Lanzara C, d'Adamo P, Gasparini P (2009) Genetic variation in taste sensitivity to 6-n-propylthiouracil and its relationship to taste perception and food selection. Ann N Y Acad Sci 1170:126–139
Ventura AK, Mennella JA (2011) Innate and learned preferences for sweet taste during childhood. Curr Opin Clin Nutr Metab Care 14(4):379–384
Liem DG, de Graaf C (2004) Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol Behav 83(3):421–429
El-Sohemy A, Stewart L, Khataan N, Fontaine-Bisson B, Kwong P, Ozsungur S, Cornelis MC (2007) Nutrigenomics of taste – impact on food preferences and food production. Forum Nutr 60:176–182
Margolskee RF (2002) Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277:1–4
McLaughlin SK, McKinnon PJ, Robichon A, Spickofsky N, Margolskee RF (1993) Gustducin and transducin: a tale of two G proteins. Ciba Found Symp 179:186–200
Lindemann B (1996) Chemoreception: tasting the sweet and the bitter. Curr Biol 6(10):1234–1237
Margolskee RF (1993) The molecular biology of taste transduction. Bioessays 15(10):645–650
Depoortere I (2014) Taste receptors of the gut: emerging roles in health and disease. Gut 63(1):179–190
Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y (2009) Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol – Regul, Integr Comp Physiol 296(4):R960–R971
Wright EM, Turk E (2004) The sodium/glucose cotransport family SLC5. Pflugers Arch 447(5):510–518
Harada N, Inagaki N (2012) Role of sodium-glucose transporters in glucose uptake of the intestine and kidney. J Diab Invest 3(4):352–353
Takata K, Hirano H, Kasahara M (1997) Transport of glucose across the blood-tissue barriers. Int Rev Cytol 172:1–53
Janssen S, Depoortere I (2013) Nutrient sensing in the gut: new roads to therapeutics? Trends in Endocrin Metab 24(2):92–100
Folmes CD, Lopaschuk GD (2007) Role of malonyl-CoA in heart disease and the hypothalamic control of obesity. Cardiovasc Res 73(2):278–287
Riediger T, Zuend D, Becskei C, Lutz TA (2004) The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am J Physiol Regul Integr Comp Physiol 286(1):R114–R122
Riediger T (2012) The receptive function of hypothalamic and brainstem centres to hormonal and nutrient signals affecting energy balance. Proc Nutr Soc 71(4):463–477
Valassi E, Scacchi M, Cavagnini F (2008) Neuroendocrine control of food intake. Nutr Metab Cardiovas Dis 18:158–168
Cui H, Cai F, Belsham DD (2005) Anorexigenic hormones leptin, insulin, and alpha-melanocyte-stimulating hormone directly induce neurotensin (NT) gene expression in novel NT-expressing cell models. J Neurosci 25(41):9497–9506
Wellman PJ (2000) Norepinephrine and the control of food intake. Nutrition 16(10):837–842
Hanusch-Enserer U, Roden M (2005) News in gut-brain communication: a role of peptide YY (PYY) in human obesity and following bariatric surgery? Eur J Clin Invest 35(7):425–430
Watterson KR, Bestow D, Gallagher J, Hamilton DL, Ashford FB, Meakin PJ, Ashford ML (2013) Anorexigenic and orexigenic hormone modulation of mammalian target of rapamycin complex 1 activity and the regulation of hypothalamic agouti-related protein mRNA expression. Neurosignals 21(1–2):28–41
Yoshida R, Niki M, Jyotaki M, Sanematsu K, Shigemura N, Ninomiya Y (2013) Modulation of sweet responses of taste receptor cells. Sem in Cell Dev Biol 24(3):226–231
Gil-Campos M, Aguilera CM, Cañete R, Gil A (2006) Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr 96(2):201–226
Ghamari-Langroudi M, Colmers WF, Cone RD (2005) PYY3–36 inhibits the action potential firing activity of POMC neurons of arcuate nucleus through postsynaptic Y2 receptors. Cell Metab 2(3):191–199
Druce M, Bloom SR (2006) The regulation of appetite. Arch Dis Child. 91(2):183–187
Welcome MO, Pereverzev VA (2013) A mini-review of the mechanisms of glucose memory enhancement. Int J Med Pharm Sci 4(1):17–30
Welcome MO, Mastorakis NE, Pereverzev VA (2015) Sweet taste receptor signaling network: possible implication for cognitive functioning. Neurol Res Int (Hindawi Publishing Corporation), Article ID 606479, 13 pages. doi:10.1155/2015/606479. https://www.hindawi.com/journals/nri/2015/606479/
Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE (2009) Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3:12
de Araujo IE, Ren X, Ferreira JG (2010) Metabolic sensing in brain dopamine systems. Res Prob Cell Different 52:69–86
Hurtado-Carneiro V, Sanz C, Roncero I, Vazquez P, Blazquez E, Alvarez E (2012) Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behavior. Mol Neurobiol 45(2):348–361
Xu J, Ji J, Yan XH (2012) Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr 52(5):373–381
Ehninger D, de Vries PJ, Silva AJ (2009) From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res 53(10):838–851
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this entry
Cite this entry
Welcome, M.O., Mastorakis, N.E., Pereverzev, V.A. (2018). Sweet-Taste Receptor Signaling Network and Low-Calorie Sweeteners. In: Mérillon, JM., Ramawat, K. (eds) Sweeteners. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-27027-2_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-27027-2_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27026-5
Online ISBN: 978-3-319-27027-2
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics