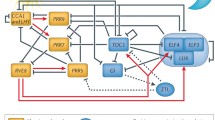
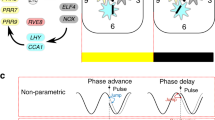
Abstract
Combining the unravelling of the molecular bases of functions in time and of organization in space in biology, on the one hand, with nonlinear dynamics as part of theoretical physics, on the other, is promising great progress in basic understanding of nonlinear spatial pattern formation from huge amounts of data becoming available in systems biology . In this chapter, this will be assessed in terms of the “tripod” (1) experimentation, (2) modelling and (3) theory.
-
1.
Empirical case studies of rhythmicity are derived from three areas of study, (i) Crassulacean acid metabolism, (ii) stomatal pore regulation by guard cells and (iii) plant memory. Biorhythmicity is underlying the former two, whose spatiotemporal dynamics can be documented by, among other techniques, chlorophyll fluorescence imaging. The third one, plant memory, is intimately related to rhythmicity and the biological clock with its set points and phase regulation. All three case studies reveal nonlinear performance with synchronization /desynchronization leading to modelling and theoretical concepts.
-
2.
In modelling, maximal models, providing perfectionist “photographic” imaging of nature, are distinguished from minimal models singling out essential domains in the parameter space of systems, with heuristic aims. The latter are explored in approaches based on experiment/theory feedback .
-
3.
Theoretical assessment dwells on the method of cellular automata , which are frameworks for simulating spatiotemporal patterns arising from local interactions. The theoretical concepts developed are based on the examination of stochasticity with the order-generating effects of noise in stochastic resonance and coherence resonance, where intermediate noise intensity generates quasi-rhythmic behaviour of systems from arrhythmicity.
This merges into a new path towards systems biology , where extensive data currently provided by analytical progress are integrated into the concept of universal dynamic principles. We illustrate this new path by using simple models of synchronization , this being one concept which systems biology can then exploit for the construction of more advanced models.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
Although ordinary differential equations are a frequent approach to modelling, a wide variety of other descriptions exists. One passes to partial differential equations when, in addition to changes in time, the spatial behaviour is taken into account. When a focus is on the influence of fluctuations on the dynamics, stochastic differential equations are analysed. Often, formulations which are discrete in space or time are selected due to their smaller computational demands and the capacity to incorporate local rules, which are not easily accommodated in the form of differential equations. Finite difference equations for the purely temporal case and cellular automata in the case of spatiotemporal patterns are examples of such formulations. We will briefly discuss cellular automata within the context of stomatal dynamics (Sect. 11.4).
References
Alon U (2007) Network motifs: theory and experimental approaches. Nat Rev Genet 8:450–461
Anishchenko V, Moss F, Neiman A, Schimansky-Geier L (1999) Stochastic resonance: noise induced order. Sov Phys Usp 42:7–36
Arenas A, Diaz-Guilera A, Kurths J, Moreno Y, Zhou C (2008) Synchronization in complex networks. Phys Rep 469:93–153
Bak P, Tang C, Wiesenfeld K (1988) Self-organized criticality. Phys Rev A 38:364–371
Barabási A-L, Oltvai ZN (2004) Network biology: understanding the cell’s functional organization. Nat Rev Genet 5:101–113
Barrs HD (1971) Cyclic variations in stomatal aperture, transpiration and leaf water potential under constant environmental conditions. Annu Rev Plant Physiol 22:223–236
Bassel GW, Gaudinier A, Brady SM, Hennig L, Rhee SY, De Smet I (2012) Systems analysis of plant functional, transcriptional, physical interaction, and metabolic networks. Plant Cell 24:3859–3875
Beck F, Blasius B, Lüttge U, Neff R, Rascher U (2001) Stochastic noise interferes coherently with a model biological clock and produces specific dynamic behaviour. Proc R Soc Lond B 268:1307–1313
Berridge MJ, Rapp PE (1979) A comparative survey of the function, mechanism and control of cellular oscillators. J Exp Biol 81:217–279
Berridge MJ, Bootman MD, Lipp P (1998) Calcium—a life and death signal. Nature 395:645–648
Betz A, Chance B (1965) Phase relationship of glycolytic intermediates in yeast cells with oscillatory metabolic control. Arch Biochem Biophys 109:585–594
Beyschlag W, Eckstein J (1997) Stomatal patchiness. Progr Bot 59:283–298
Bezrukov SM, Vodyanoy I (1995) Noise-induced enhancement of signal transduction across voltage-dependention channels. Nature 378:362–364
Bilichak A, Ilnystkyy Y, Hollunder J, Kovalchuk I (2012) The progeny of Arabidopsis thaliana plants exposed to salt exhibit changes in DNA methylation, histone modifications and gene expression. PLoS ONE 7:e30515
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16:6–21
Blasius B, Beck F, Lüttge U (1997) A model for photosynthetic oscillations in crassulacean acid metabolism (CAM). J Theor Biol 184:345–351
Blasius B, Beck F, Lüttge U (1998) Oscillatory model of crassulacean acid metabolism: structural analysis and stability boundaries with a discrete hysteresis switch. Plant, Cell Environ 21:775–784
Blasius B, Neff R, Beck F, Lüttge U (1999) Oscillatory model of crassulacean acid metabolism with a dynamic hysteresis switch. Proc R Soc Lond B 266:93–101
Bohn A, Hinderlich S, Hütt M-T, Kaiser F, Lüttge U (2003) Identification of rhythmic subsystems in the circadian cycle of crassulacean acid metabolism under thermoperiodic perturbations. Biol Chem 384:721–728
Bonasio R, Tu S, Reinberg D (2010) Molecular signals of epigenetic states. Science 330:612–616
Borland AM, Hartwell J, Jenkins GI, Wilkins MB, Nimmo HG (1999) Metabolic control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in crassulacean acid metabolism. Plant Physiol 121:889–896
Bornholdt S (2005) Less is more in large genetic networks. Science 310:449–451
Boxall SF, Foster JM, Bohnert HJ, Cushman JC, Nimmo HG, Hartwell J (2005) Conservation and divergence of circadian clock operation in a stress-inducible crassulacean acid metabolism species reveals clock compensation against stress. Plant Physiol 137:969–982
Busch H, Hütt M-Th, Kaiser F (2001) The effect of colored noise on networks of nonlinear oscillators. Phys Rev E 64:021105
Cardon ZG, Mott KA, Berry JA (1994) Dynamics of patchy stomatal movements, and their contribution to steady-state and oscillating stomatal conductance calculated using gas-exchange techniques. Plant, Cell Environ 17:995–1007
Chance B, Hess B, Betz A (1964) DPNH oscillations in a cell-free extract of S. carlsbergensis. Biochem Biophys Res Commun 16:182–187
Chew YH, Wenden B, Flis A, Mengin V, Taylor J, Davey CL, Tindal C, Thomas H, Ougham HJ, de Reffye P, Stitt M, Williams M, Muetzelfeldt R, Halliday KJ, Millar AJ (2014) Multiscale digital Arabidopsis predicts individual organ and whole-organism growth. Proc Nat Acad Sci 111:E4127–E4136
Chinnusamy V, Zhu J-K (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12:133–139
Collakova E, Yen JY, Senger RS (2012) Are we ready for genome-scale modeling in plants? Plant Sci 191–192:53–70
Cowan IR (1972a) Oscillations in stomatal conductance and plant functioning associated with stomatal conductance: observations and a model. Planta 106:185–219
Cowan IR (1972b) An electrical analogue of evaporation from, and flow of water in plants. Planta 106:221–226
Crutchfield JP, Mitchell M (1995) The evolution of emergent computation. Proc Natl Acad Sci U S A 92:10742–10746
Darrah C, Taylor BL, Edwards KD, Brown PE, Hall A, McWatters HG (2006) Analysis of phase of LUCIFERASE expression reveals novel circadian quantitative trait loci in Arabidopsis. Plant Physiol 140:1464–1474
Deco G, Jirsa VK, Mcintosh AR (2011) Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci 12:43–56
Demongeot J, Thomas R, Thellier M (2000) A mathematical model for storage and recall functions in plants. C R Acad Sci Paris (Sciences de la Vie/Life Sciences) 323:93–97
Demongeot J, Thellier M, Thomas R (2006) Storage and recall of environmental signals in a plant: modelling by use of a differential (continuous) formulation. CR Biologies 329:971–978
Desbiez MO, Kergosien Y, Champagnat P, Thellier M (1984) Memorization and delayed expression of regulatory messages in plants. Planta 160:392–399
Desbiez MO, Champagnat P, Thellier M (1986) Mécanismes de mise en mémoire et de rappel de mémoire chez Bidens pilosus. C R Acad Sci Paris 302:573–578
Desbiez MO, Gaspar T, Crouzillat D, Frachisse JM, Thellier M (1987) Effect of cotyledonary prickings on growth, ethylene metabolism and peroxidase activity in Bidens pilosus. Plant Physiol Biochem 25:137–143
Desbiez MO, Ripoll C, Pariot C, Thellier M (1991a) Elicitation of developmental processes in higher plants by hexoses or myo-inositol, in the presence of K+ or Ca2+. Plant Physiol Biochem 29:457–462
Desbiez MO, Tort M, Thellier M (1991b) Control of a symmetry-breaking process in the course of the morphogenesis of plantlets of Bidens pilosa L. Planta 184:397–402
Desbiez MO, Mikulecky D, Thellier M (1994) Growth messages in plants: principle of a possible modeling and further experimental characteristics. J Biol Syst 2:127–136
Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival and competitive advantage. Science 309:630–633
Dolmetsch RE, Lewis RS, Goodnow CC, Healy JJ (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855–858
Douglass JK, Wilkins L, Pantazelou E, Moss F (1993) Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature 365:337–340
Duarte HM, Jakovljevic I, Kaiser F, Lüttge U (2005) Lateral diffusion of CO2 in leaves of the crassulacean acid metabolism plant Kalanchoë daigremontiana Hamet et Perrier. Planta 220:809–816
Dunlap JC (1993) Genetic analysis of circadian clocks. Annu Rev Physiol 55:683–728
Frankhauser C, Staiger D (2002) Photoreceptors in Arabidopsis thaliana: light perception, signal transduction and entrainment of the endogenous clock. Planta 216:1–16
Fujiwara S, Oda A, Yoshida R, Niinuma K, Miyata K, Tomozoe Y, Tajima T, Nakagawa M, Hayashi K, Coupland G, Mizoguchi T (2008) Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell 20:2960–2971
Gallos L, Song C, Havlin S, Makse H (2007) Scaling theory of transport in complex biological networks. Proc Natl Acad Sci U S A 104:7746–7751
Gammaitoni L, Hänggi P, Jung P, Marchesoni F (1998) Stochastic resonance. Rev Mod Phys 70:223–287
Ghosh A, Chance B (1964) Oscillations of glycolytic intermediates in yeast cells. Biochem Biophys Res Commun 16:174–181
Gierer A (1998) Im Spiegel der Natur erkennen wir uns selbst. Wissenschaft und Menschenbild. Rowohlt, Reinbek bei Hamburg
Giersch C (1994) Photosynthetic oscillations: observations and models. Comments Theor Biol 3:339–364
Giersch C, Sivak MN, Walker DA (1991) A mathematical skeleton model of photosynthetic oscillations. Proc R Soc Lond B 245:77–83
Goldbeter A (1996) Biochemical oscillations and cellular rhythms. Cambridge University Press, Cambridge
Goldbeter A, Lefever R (1972) Dissipative structures from an allosteric model—application to glycolytic oscillations. Biophys J 12:1302–1315
Goldbeter A, Gérard C, Gonze D, Leloup JC, Dupont G (2012) Systems biology of cellular rhythms. FEBS Lett 586:2955–2965
Green RM, Tingay S, Wang Z-Y, Tobin EM (2002) Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol 129:576–584
Guerriero ML, Pokhilko A, Fernandez AP, Halliday KJ, Millar AJ, Hillston J (2012) Stochastic properties of the plant circadian clock. J Roy Soc Interface 9:744–756
Haefner JW, Buckley TN, Mott KA (1997) A spatially explicit model of patchy stomatal responses to humidity. Plant, Cell Environ 20:1087–1097
Harmer SL, Kay SA (2005) Positive and negative factors confer phase-specific circadian regulation of transcription in Arabidopsis. Plant Cell 17:1926–1940
Hartwell J (2005) The circadian clock in CAM plants. In: Hall AJW, McWatters HG (eds) Endogenous plant rhythms. Blackwell, Oxford, pp 211–236
Hauser MT, Aufsatz W, Jonak C, Luschnig C (2011) Transgenerational epigenetic inheritance in plants. Biochim Biophys Acta 1808:459–468
Helbing D, Keltsch J, Molnar P (1997) Modelling the evolution of human trail systems. Nature 388:47–50
Helbing D, Farkas I, Vicsek T (2000) Simulating dynamical features of escape panic. Nature 407:487–490
Henke K (2010) A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci 11:523–532
Henry-Vian C, Vian A, Dietrich A, Ledoigt G, Desbiez MO (1995) Changes in the polysomal mRNA population upon wound signal expression or storage in Bidens pilosa. Plant Physiol Biochem 33:337–344
Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AAR (2007) Modulation of environmental responses of plants by circadian clocks. Plant, Cell Environ 30:333–349
Hütt M-T (2014) Understanding genetic variation—the value of systems biology. Br J Clin Pharmacol 77:597–605
Hütt M-T, Dehnert M (2006) Methoden der Bioinformatik. Eine Einführung. Springer, Berlin
Hütt M-T, Lüttge U (2002) Nonlinear dynamics as a tool for modeling in plant physiology. Plant Biol 4:281–297
Hütt M-T, Lüttge U (2005a) The interplay of synchronization and fluctuations reveals connectivity levels in networks of nonlinear oscillators. Phys A 350:207–226
Hütt M-T, Lüttge U (2005b) Network dynamics in plant biology: current progress in historical perspective. Progr Bot 66:277–310
Hütt M-T, Neff R (2001) Quantification of spatio-temporal phenomena by means of cellular automata techniques. Phys A 289:498–516
Hütt M-T, Hilgetag CC, Kaiser M (2014) Network-guided pattern formation of neural dynamics. Philos Trans R Soc B Biol Sci 369(20130522):1–10
Ibáñez C, Kozarewa I, Johansson M, Ögren E, Rhode A, Eriksson ME (2010) Circadian clock components regulate entry and affect exit of seasonal dormancy as well as winter hardiness in Populus trees. Plant Physiol 153:1823–1833
Johnson CH, Golden SS (1999) Circadian programs in cyanobacteria: adaptiveness and mechanism. Ann Rev Microbiol 53:389–409
Kaiser H, Kappen L (2001) Stomatal oscillations at small apertures: indications for a fundamental insufficiency of stomatal feedback-control inherent in the stomatal turgor mechanism. J Exp Bot 52:1303–1313
Kergosien Y, Thellier M, Desbiez MO (1979) Préséances entre bourgeons axillaires chez Bidens pilosus L.: modélisation au niveau macroscopique en termes de catastrophes, ou au niveau microscopique en termes de “pompes et fuites” cellulaires. In: Delattre P, Thellier M (eds) Elaboration et justification des modèles: applications en biologie. Maloine, Paris, part I, pp 323–343
Kikis EA, Khanna R, Quail PH (2005) ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J 44:300–313
Kliemchen A, Schomburg M, Galla H-J, Lüttge U, Kluge M (1993) Phenotypic changes in the fluidity of the tonoplast membrane of crassulacean-acid metabolism plants in response to temperature and salinity stress. Planta 189:403–409
Kluge M, Kliemchen A, Galla H-J (1991) Temperature effects on crassulacean acid metabolism: EPR spectroscopic studies on the thermotropic phase behaviour of the tonoplast membrane of Kalanchoë daigremontiana. Bot Acta 104:355–360
Knight H, Brandt S, Knight MR (1998) A history of stress alters drought calcium signalling pathways in Arabidopsis. Plant J 16:681–687
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effect of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352:524–526
Kohl P, Noble D, Winslow RL, Hunter PJ (2000) Computational modelling of biological systems: tools and visions. Philos Trans R Soc A 358:579–610
Koukkari WL, Bingham C, Hobbs JD, Duke SH (1997) In search of a biological hour. J Plant Physiol 151:352–357
Kuramoto Y (1984) Chemical oscillations, waves, and turbulence. Springer, Berlin
Lee S-G, Neiman A, Kim S (1998) Coherence resonance in a Hodgkin–Huxley neuron. Phys Rev E 57:3292–3297
Liu Y, Tsinoremans NF, Johnson CH, Lebedeca NV, Golden SS, Ishiura M, Kondo TI (1995) Circadian orchestration of gene expression in cyanobacteria. Genes Dev 9:1469–1478
Liu J, Grieson CS, Webb AA, Hussey PJ (2010) Modelling dynamic plant cells. Curr Opin Plant Biol 13:744–749
Lloyd D (2008) Oscillations, synchrony and deterministic chaos. Progr Bot 70:69–91
Lloyd D, Kippert F (1993) Intracellular coordination by the ultradian clock. Cell Biol Int 17:1047–1052
Lloyd D, Murray DB (2006) The temporal architecture of eukaryotic growth. FEBS-Lett 580:2830–2835
Longtin A, Bulsara A, Moss F (1991) Time interval sequences in bistable systems and the noise induced transmission of information by sensory neurons. Phys Rev Lett 67:656–660
Love J, Dodd AN, Webb AAR (2004) Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 16:956–966
Lucas M, Laplaze L, Bennett MJ (2011) Plant systems biology: network matters. Plant, Cell Environ 34:535–553
Lüttge U (2000) The tonoplast functioning as a master switch for circadian regulation of crassulacean acid metabolism. Planta 211:761–769
Lüttge U (2003a) Circadian rhythmicity: is the biological clock hardware or software? Progr Bot 64:277–319
Lüttge U (2003b) Circadian rhythms. In: Thomas B, Murphy DJ, Murray BG (eds) Encyclopedia of applied plant sciences. Elsevier/Academic Press, Amsterdam, pp 1084–1096
Lüttge U (2012) The system vascular plant: whole plant physiology’s outlooks for systems biology with emergence by contrast to modularity. Progr Bot 74:165–190
Lüttge U, Beck F (1992) Endogenous rhythms and chaos in crassulacean acid metabolism. Planta 188:28–38
Lüttge U, Hertel Β (2009) Diurnal and annual rhythms in trees. Trees 23:683–700
Lüttge U, Hütt M-T (2004) High frequency or ultradian rhythms in plants. Progr Bot 65:235–263
Lüttge U, Hütt M-T (2006) Spatiotemporal patterns and distributed computation—a formal link between CO2 signalling, diffusion and stomatal regulation. Progr Bot 68:242–260
Lüttge U, Hütt M-T (2009) Talking patterns: communication of organisms at different levels of organization—an alternative view of systems biology. Nova Acta Leopoldina NF 96(357):161–174
McAinsh MR, Hetherington AM (1998) Encoding specificity in Ca2+ signalling systems. Trends Plant Sci 3:32–36
McAinsh MR, Brownlee C, Hetherington AM (1997) Calcium ions as second messengers in guard cell signal transduction. Physiol Plant 100:16–29
McAinsh MR, Webb AAR, Taylor JE, Hetherington AM (1995) Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7:1207–1219
McClung CR (2006) Plant circadian rhythms. Plant Cell 18:792–803
McClung CR, Gutierrez RA (2010) Network news: prime time for systems biology of the plant circadian clock. Current Opinion in Genetics Development 20:588–598
Michael TP, McClung CR (2002) Phase-specific circadian clock regulatory elements in Arabidopsis. Plant Physiol 130:627–638
Michael TP, McClung CR (2003) Enhancer trapping reveals widespread circadian clock transcriptional control in Arabidopsis. Plant Physiol 132:629–639
Mintz-Oron S, Meir S, Malitsky S, Ruppin E, Aharoni A, Shlomi T (2012) Reconstruction of Arabidopsis metabolic network models accounting for subcellular compartmentalization and tissue-specificity. Proc Natl Acad Sci 109:339–344
Mitchell M, Crutchfield JP, Hraber PT (1994) Evolving cellular automata to perform computations: mechanisms and impediments. Phys D 75:361–391
Moreira AA, Mathur A, Diermeier D, Amaral LAN (2004) Efficient system-wide coordination in noisy environments. Proc Nat Acad Sci U S A 101:12085–12090
Moss F (2000) Stochastic resonance: looking forward. In: Walleczek J (ed) Self-organized biological dynamics and nonlinear control. Cambridge University Press, Cambridge, pp 236–256
Moss F, Pierson D, O’Gorman D (1994) Stochastic resonance: tutorial and update. Int J Bifurc Chaos 4:1383–1397
Mott KA, Peak D (2006) Stomatal patchiness and task-performing networks. Ann Bot 99:219–226
Müller LM, von Korff M, Davis SJ (2014) Connections between circadian clocks and carbon metabolism reveal species-specific effects on growth control. J Exp Bot 65:2915–2923
Müller-Linow M, Hilgetag CC, Hütt M-T (2008) Organization of excitable dynamics in hierarchical biological networks. PLoS Computational Biol 4:e1000190
Nakamichi N (2011) Molecular mechanisms underlying the Arabidopsis circadian clock. Plant Cell Physiol 52:1709–1718
Neff R, Blasius B, Beck F, Lüttge U (1998) Thermodynamics and energetics of the tonoplast membrane operating as a hysteresis switch in an oscillatory model of crassulacean acid metabolism. J Membr Biol 165:37–43
Neger FW (1918) Die Wegsamkeit der Laubblätter für Gase. Flora 111:152–161
Nimmo HG (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5:75–80
Niwa Y, Yamashino T, Mizuno T (2009) The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol 50:838–854
Ogudi T, Sage-Ono K, Kamada H, Ono M (2004) Characterization of transcriptional oscillation of an Arabidopsis homolog of PnC401 related to photoperiodic induction of flowering in Pharbitis nil. Plant Cell Physiol 45:232–235
Olsen LF, Degn H (1985) Chaos in biological systems. Q Rev Biophys 18:165–225
Onai K, Okamoto K, Nishimoto H, Moroika C, Hirano M, Kami-Ike N, Ishiura M (2004) Large-scale screening of Arabidopsis circadian clock mutants by a high-throughput real-time bioluminescence monotoring system. Plant J 40:1–11
O’Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget F-Y, Reddy AB, Millar AJ (2011) Circadian rhythms persist without transcription in a eukaryote. Nature 469:554–558
OuyangY Andersson CR, Kondo T, Golden SS, Johnson CH (1998) Resonating circadian clocks enhance fitness in cyanobcteria. Proc Natl Acad Sci U S A 95:8660–8664
Peak D, West JD, Messinger SM, Mott KA (2004) Evidence for complex, collective dynamics and emergent, distributed computation in plants. Proc Natl Acad Sci U S A 101:918–922
Pikovsky AS, Kurths J (1997) Coherence resonance in a noise driven excitable system. Phys Rev Lett 78:775–778
Raikhel NV, Coruzzi GM (2003) Plant systems biology. Plant Physiol 132:403
Rapp PE (1986) Oscillations and chaos in cellular metabolism and physiological systems. In: Holden A (ed) Chaos. Manchester University Press, Manchester, pp 179–208
Rascher U, Lüttge U (2002) High-resolution chlorophyll fluorescence imaging serves as a non-invasive indicator to monitor the spatio-temporal variations of metabolism during the day-night cycle and during the endogenous rhythm in continuous light in the CAM plant Kalanchoe daigremontiana. Plant Biol 4:671–681
Rascher U, Blasius B, Beck F, Lüttge U (1998) Temperature profiles for the expression of endogenous rhythmicity and arrhythmicity of CO2 exchange in the CAM plant Kalanchoë daigremontiana can be shifted by slow temperature changes. Planta 207:76–82
Rascher U, Hütt M-T, Siebke K, Osmond B, Beck F, Lüttge U (2001) Spatio-temporal variation of metabolism in a plant circadian rhythm: the biological clock as an assembly of coupled individual oscillators. Proc Natl Acad Sci U S A 98:11801–11805
Raschke K (1965) Die Stomata als Glieder eines schwingungsfähigen CO2-Regelsystems. Experimenteller Nachweis an Zea mays L. Z Naturf 20b:1261–1270
Raschke K (1975) Stomatal action. Annu Rev Plant Physiol 26:309–340
Ripoll C, Le Sceller L, Verdus MC, Norris V, Tafforeau M, Thellier M (2009) Memorization of abiotic stimuli in plants: a complex role for calcium. In: Baluška F (ed) Plant-environment interactions. Springer, Berlin, pp 267–283
Roden LC, Song H-R, Jackson S, Morris K, Carré IA (2002) Floral responses to photoperiod are correlated with the timing of rhythmic expression relative to dawn and dusk in Arabidopsis. Proc Natl Acad Sci U S A 99:13313–13318
Roelfsema MRG, Hedrich R (2002) Studying guard cells in the intact plant: modulation of stomatal movement by apoplastic factors. New Phytol 153:425–431
Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G (2006) Electromagnetic fields (900 MHz) evoke consistent molecular responses in tomato plants. Physiol Plant 128:283–288
Salomé PA, Michael TP, Kearns EV, Fett-Neto AG, Sharrock RA, McClung CR (2002) The out of phase 1 mutant defines a role for PHYB in circadian phase control in Arabidopsis. Plant Physiol 129:1674–1685
Shabala S, Delbourgo R, Newman I (1997) Observations of bifurcation and chaos in plant physiological responses to light. Aust J Plant Physiol 24:91–96
Shabala L, Shabala S, Ross T, McMeekin T (2001) Membrane transport activity and ultradian ion flux oscillations associated with cell cycle of Thraustochytrium sp. Aust J Plant Physiol 28:87–99
Siebke K, Weis E (1995a) Assimilation images of leaves of Glechoma hederacea: analysis of non-synchronous stomata related oscillations. Planta 196:155–165
Siebke K, Weis E (1995b) Imaging of chlorophyll-a-fluorescence in leaves: topography of photosynthesis oscillations in leaves of Glechoma hederacea. Photosynth Res 45:225–237
Smith J, Hütt M-T (2010) Network dynamics as an interface between modeling and experiment in systems biology. In: Gebicke-Haerter PJ, Mendoza ER, Winterer G (eds) Tretter F. Systems biology in psychiatric research: from high-throughput data to mathematical modeling, Wiley-VCH, pp 234–276
Solé RV, Manrubia SC, Luque B, Delgado J, Bascompte J (1996) Phase transitions and complex systems. Complexity 2:13–29
Strogatz S (2004) Sync: the emerging science of spontaneous order. Hyperion, New York
Sweetlove LJ, Last RL, Fernia AR (2003) Predictive metabolic engineering: a goal for systems biology. Plant Physiol 132:420–425
Tafforeau M, Verdus MC, Norris V, White G, Demarty M, Thellier M, Ripoll C (2002) SIMS study of the calcium-deprivation step related to epidermal meristem production induced in flax by cold shock or radiation from a GSM telephone. J Trace Microprobe Tech 20:611–623
Tafforeau M, Verdus MC, Norris V, White GJ, Cole M, Demarty M, Thellier M, Ripoll C (2004) Plant sensitivity to low intensity 105 GHz electromagnetic radiation. Bioelectromagnetics 25:403–407
Tafforeau M, Verdus MC, Norris V, Ripoll C, Thellier M (2006) Memory processes in the response of plants to environmental signals. Plant Signal Behav 1:9–14
Tanner W, Grünes R, Kandler O (1970) Spezifität und Turnover des induzierbaren Hexose-Aufnahmesystems von Chlorella. Z Pflanzenphysiol 62:376–386
Teoh CT, Palmer JH (1971) Nonsynchronized oscillations in stomatal resistance among sclerophylls of Eucalyptus umbra. Plant Physiol 47:409–411
Terashima I (1992) Anatomy of non-uniform leaf photosynthesis. Photosynth Res 31:195–212
Thellier M (2011) A half-century adventure in the dynamics of living systems. Progr Bot 73:3–53
Thellier M, Lüttge U (2013) Plant memory: a tentative model. Plant Biol 15:1–12
Thellier M, Desbiez MO, Champagnat P, Kergosien Y (1982) Do memory processes also occur in plants? Physiol Plant 56:281–284
Thellier M, Demongeot J, Norris V, Guespin J, Ripoll C, Thomas R (2004) A logical (discrete) formulation for the storage and recall of environmental signals in plants. Plant Biol 6:590–597
Thellier M, Ripoll C, Norris V (2013) Memory processes in the control of plant growth and metabolism. Nova Acta Leopoldina NF 114(391):21–41
Trewavas A (2003) Aspects of plant intelligence. Ann Bot 92:1–20 (see especially the section “Plant memory and information retrieval”)
Twycross J, Band LR, Bennett MJ, King JR, Krasnogor N (2010) Stochastic and deterministic multiscale models for systems biology: an auxin-transport case study. BMC Syst Biol 4:1–11
Verdus MC, Thellier M, Ripoll C (1997) Storage of environmental signals in flax: their morphogenetic effect as enabled by a transient depletion of calcium. Plant J 12:1399–1410
Verdus MC, Le Sceller L, Norris V, Thellier M, Ripoll C (2007) Pharmacological evidence for calcium involvement in the long-term processing of abiotic stimuli in plants. Plant Signal Behav 2:212–220
Verdus MC, Ripoll C, Norris V, Thellier M (2012) The role of calcium in the recall of stored morphogenetic information by plants. Acta Biotheor 60:83–97
Vian A, Roux D, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G (2006) Microwave irradiation affects gene expression in plants. Plant Signal Behav 1:67–70
Walker DA (1992) Concerning oscillations. Photosynth Res 34:387–395
West JD, Peak D, Peterson JQ, Mott KA (2005) Dynamics of stomatal patchiness for a single surface of Xanthium strumarium L. leaves observed with fluorescence and thermal images. Plant, Cell Environ 28:633–641
Westermark PO, Herzel H (2013) Mechanism for 12 hr rhythm generation by the circadian clock. Cell Reports 3:1228–1238
Willmer CM (1988) Stomatal sensing of the environment. Biol J Linn Soc 34:205–217
Winfree AT (1967) Biological rhythms and the behavior of populations of coupled oscillators. J Theor Biol 16:15
Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH (2004) The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol 14:1481–1486
Wolfram S (2002) A new kind of science. Wolfram Media Publishing, Champaign, IL
Wyka TP, Lüttge UE (2003) Contribution of C3 carboxylation to the circadian rhythm of carbon dioxide uptake in a Crassulacean acid metabolism plant Kalanchoë daigremontiana. J Exp Bot 54:1471–1479
Wyka TP, Bohn A, Duarte HM, Kaiser F, Lüttge UE (2004) Perturbations of malate accumulation and the endogenous rhythms of gas exchange in the Crassulacean acid metabolism plant Kalanchoë dairemontiana: testing the tonoplast-as-oscillator model. Planta 219:705–713
Yaish MW, Colasnti J, Rothstein SJ (2011) The role of epigenetic processes in controlling flowering time in plants exposed to stress. J Exp Bot 62:3727–3735
Yerushalmi S, Green RM (2009) Evidence for the adaptive significance of circadian rhythms. Ecol Lett 12:970–981
Yerushalmi S, Yakir E, Green RM (2011) Circadian clocks and adaptation in Arabidopsis. Mol Ecol 20:1155–1165
Zhang Y, Reinberg D (2011) Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15:2343–2360
Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW-L, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Ecker JR (2006) Genome-wide high resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126:1189–1201
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hütt, MT., Lüttge, U., Thellier, M. (2015). Noise-Induced Phenomena and Complex Rhythms: A Test Scenario for Plant Systems Biology. In: Mancuso, S., Shabala, S. (eds) Rhythms in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-20517-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-20517-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20516-8
Online ISBN: 978-3-319-20517-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)