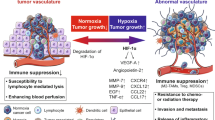
Abstract
Both innate and adaptive immune cells are involved in the mechanisms of endothelial cell proliferation, migration and activation, via production and release of a large spectrum of pro-angiogenic mediators, thus creating the specific microenvironment that favors increased rate of tissue vascularization. In this article, we focus on the immune cell component of the angiogenic process occurring during multiple myeloma progression. We also provide information on some anti-angiogenic properties of immune cells that may be applied for a potential pharmacological use as anti-angiogenic agents in the disease treatment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abdel-Majid RM, Marshall JS (2004) Prostaglandin E2 induces degranulation independent production of vascular endothelial growth factor by human mast cells. J Immunol 172:1227–1236
Aharinejad S, Abraham D, Paulus P et al (2002) Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res 62:5317–5324
Aharinejad S, Paulus P, Sioud M et al (2004) Colony-stimulating factor-1 blockade by antisense oligonucleotide and small interfering RNAs suppresses growth of human mammary tumor xenograft in mice. Cancer Res 64:5378–5384
Andreu P, Johansson M, Affara NI et al (2010) FcR gamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 17:121–134
Angiolillo AL, Sgadari C, Taub DD et al (1995) Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med 182:155–162
Aoki M, Pawankar R, Niimi Y et al (2003) Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int Arch Allergy Immunol 130:216–223
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217
Basile A, Moschetta M, Ditonno P et al (2013) Pentraxin 3 (PTX3) inhibits plasma cell/stromal cell cross-talk in the bone marrow of multiple myeloma patients. J Pathol 229:87–98
Benitez-Bribiesca L, Wong A, Utrera D et al (2001) The role of mast cell tryptase in neoangiogenesis of premalignant and malignant lesions of the uterine cervix. J Histochem Cytochem 49:1061–1062
Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196:254–265
Blair RJ, Meng H, Marchese MJ et al (1997) Human mast cells stimulate vascular tube formation: tryptase is a novel potent angiogenic factor. J Clin Invest 99:2691–2700
Boesiger J, Tsai M, Maurer M et al (1998) Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J Exp Med 188:1135–1145
Bowrey PF, King J, Magarey C et al (2000) Histamine, mast cells and tumour cell proliferation in breast cancer: does preoperative cimetidine administration have an effect? Br J Cancer 82:167–170
Cocco C, Morandi F, Airoldi J (2011) Interleukin-27 and interleukin-23 modulate human plasma cell functions. J Leukoc Biol 89:729–734
Colombo MP, Trinchieri G (2002) Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 13:155–168
Coussens LM, Raymond WW, Bergers G et al (1999) Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 13:1382–1397
Coussens LM, Tinkle CL, Hanahan D et al (2000) MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103:481–490
Denhardt DT, Noda M, O’Regan AW et al (2001) Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107:1055–1061
De Palma M, Venneri MA, Galli R et al (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8:211–226
Detmar M, Brown LF, Schön MP et al (1998) Increased microvascular density and enhanced leukocyte rolling and adhesionin the skin of VEGF transgenic mice. J Invest Dermatol 111:1–6
Detoraki A, Staiano RI, Granata F et al (2009) Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol 123:1142–1149
de Visser KE, Korets LV, Coussens LM (2005) De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7:411–423
Dias S, Boyd R, Balkwill F (1998) IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int J Cancer 78:361–365
Dineen SP, Lynn KD, Holloway SE et al (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68:4340–4346
Di Raimondo F, Azzaro MP, Palombo G et al (2000) Angiogenic factors in multiple myeloma: higher levels in bone marrow than in peripheral blood. Haematologica 85:800–805
Dvorak AM, Mihm MC Jr, Osage JE et al (1980) Melanoma. An ultrastructural study of the host inflammatory and vascular responses. J Invest Dermatol 75:388–393
Egami K, Murohara T, Shimada T et al (2003) Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest 112:67–75
Elpek GO, Gelen T, Aksoy NH et al (2001) The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the esophagus. J Clin Pathol 54:940–944
Fukushima N, Satoh T, Sano M et al (2001) Angiogenesis and mast cells in non-Hodgkin’s lymphoma: a strong correlation in angioimmunoblastic T-cell lymphoma. Leuk Lymphoma 42:709–720
Giraudo E, Inoue M, Hanahan D (2004) An aminobisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114:623–633
Glowacki J, Mulliken JB (1982) Mast cells in hemangiomas and vascular malformations. Pediatrics 70:48–51
Gounaris E, Erdman SE, Restaino C et al (2007) Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA 104:19977–19982
Graham R, Graham J (1996) Mast cells and cancer of the cervix. Surg Gynecol Obstet 123:3–9
Grimbaldesnton MA, Nakae S, Kalesnikoff K et al (2007) Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol 38:1095–1104
Gruber BL, Marchese MJ, Kew R (1995) Angiogenic factors stimulate mast cell migration. Blood 86:2488–2493
Grützkau A, Krüger-Krasagakes S, Baumeister H et al (1998) Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell 9:875–884
Guiducci L, Vicari AP, Sangaletti S et al (2005) Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 65:3437–3446
Hanada T, Nakagawa M, Emoto A et al (2000) Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 7:263–269
Hartveit F (1981) Mast cells and metachromasia in human breast cancer: their occurrence, significance and consequence: a preliminary report. J Pathol 134:7–11
Hideshima T, Mitsiades C, Tonon G et al (2007) Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer 7:585–598
Huang B, Lei Z, Zhang GM et al (2008) SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood 112:1269–1279
Imada A, Shijubo N, Koijma H et al (2000) Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Resp J 15:1087–1093
Imtiyaz HZ, Williams EP, Hickey MM et al (2010) Hypoxia-inducible factor 2 alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120:2699–2714
Jenkins DC, Charles IG, Thomsen LL et al (1995) Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA 92:4392–4396
Kanbe N, Kurosawa M, Nagata H et al (2000) Production of fibrogenic cytokines by cord blood-derived cultured human mast cells. J Allergy Clin Immunol 106:S85–S90
Klimp AH, Hollema H, Kempinga C et al (2001) Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res 61:7305–7309
Koide N, Nishio A, Sato T et al (2004) Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol 99:1667–1674
Kumar S, Witzig TE, Timm M et al (2004) Bone marrow angiogenic ability and expression of angiogenic cytokines in myeloma: evidence favouring loss of marrow angiogenesis inhibitory activity with disease progression. Blood 104:1159–1165
Lachter J, Stein M, Lichting C et al (1995) Mast cells in colorectal neoplasias and premalignant disorders. Dis Colon Rectum 38:290–293
Leali D, Dell’Era P, Stabile H et al (2003) Osteopontin (Eta-1) and fibroblast growth factor-2 cross-talk in angiogenesis. J Immunol 171:1085–1093
Leek RD, Lewis CE, Whitehouse R et al (1996) Association of macrophage infiltration with breast carcinoma. Cancer Res 56:4625–4629
Leek RD, Landers RJ, Harris AL et al (1999) Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 79:991–995
Lewis CE, Leek R, Harris A et al (1995) Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol 57:747–751
Lin EY, Nguyen AV, Russel RG et al (2001) Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193:727–740
Lin EY, Li JF, Gnatovskiy L et al (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66:11238–11246
Lin EY, Li JF, Bricard G et al (2007) Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol Oncol 1:288–302
Lissbrant IF, Stattin P, Wikstrom P et al (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17:445–451
Makitie T, Summanen P, Takkanen A et al (2001) Tumor-infiltrating macrophages (CD68+ cells) and prognosis in malignant uveal melanoma. Invest Ophtalmol Vis Sci 42:1414–1421
Mangieri D, Nico B, Benagiano V et al (2008) Angiogenic activity of multiple myeloma endothelial cells in vivo in the chick embryo chorioallantoic membrane assay is associated to a down-regulation in the expression of endogenous endostatin. J Cell Mol Med 12:1023–1028
Manthey CL, Johnson DL, Illig CR et al (2009) JNI-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther 8:3151–3161
Mantovani A, Sozzani S, Locati M (2002) Macrophage polarization: tumor associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Mazzieri R, Pucci F, Moi D et al (2008) Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 14:299–311
Metcalfe DD, Baram D, Mekori YA (1997) Mast cells. Physiol Rev 77:1033–1079
Molica S, Vacca A, Crivellato E et al (2003) Tryptase-positive mast cells predict clinical outcome of patients with B-cell chronic lymphocytic leukemia. Eur J Haematol 71:137–139
Moller A, Lippert U, Lessmann D et al (1993) Human mast cells produce IL-8. J Immunol 151:3261–3266
Movahedi K, Laoui D, Gysemans C et al (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70:5728–5739
Nakao S, Kuwano T, Tsutsumi-Miyahara C et al (2005) Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1beta-induced neovascularization and tumor growth. J Clin Inves 115:2979–2991
Nakayama T, Yao L, Tosato G (2004) Mast cell derived angiopoietin-1 plays a role in the growth of plasma cell tumors. J Clin Invest 114:1317–1325
Naldini A, Carraro F (2005) Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy 4:3–8
Naldini A, Leali D, Pucci A et al (2006) Cutting edge: IL-1beta mediates the proangiogenic activity of osteopontin-activated human monocytes. J Immunol 177:4267–4270
Nico B, Mangieri D, Crivellato E et al (2008) Mast cells contribute to vasculogenic mimicry in multiple myeloma. Stem Cells Dev 17:19–22
Nishie A, Ono M, Shono T et al (1999) Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 5:1107–1113
Nilsson G, Forsberg-Nilsson K, Xiang Z et al (1997) Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol 27:2295–2301
Norrby K, Jakobsson A, Sörbo J (1986) Mast cell-mediated angiogenesis: a novel experimental model using the rat mesentery. Virchows Arch B Cell Pathol Mol Pathol 52:195–206
Norrby K, Jakobsson A, Sörbo J (1989) Mast-cell secretion and angiogenesis, a quantitative study in rats and mice. Virchows Arch B Cell Pathol Mol Pathol 57:251–256
Ohno S, Ohno Y, Suzuki N et al (2004) Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res 24:3335–3342
Pittoni P, Tripodo C, Piconese S et al (2011) Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res 71:5987–5997
Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 7:803–815
Pucci F, Venneri MA, Biziato D et al (2009) A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood 114:901–914
Qian BZ, Pollard JM (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51
Qu Z, Liebler JM, Powers MR et al (1995) Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol 147:564–573
Qu Z, Huang X, Ahmadi P et al (1998a) Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor beta, tumor necrosis factor alpha, and stem cell factor. Int Arch Allergy Immunol 115:47–54
Qu Z, Kayton RJ, Ahmadi P et al (1998b) Ultrastructural immunolocalization of basic fibroblast growth factor in mast cells secretory granules. Morphological evidence for bfgf release through degranulation. J Histochem Cytochem 46:1119–1128
Reed JA, McNutt NS, Bogdany JK et al (1996) Expression of the mast cell growth factor interleukin-3 in the melanocytic lesions correlates with an increased number of mast cells in the perilesional stroma: implications for melanoma progression. J Cutan Pathol 23:495–505
Ribatti D, Roncali L, Nico B et al (1987) Effects of exogenous heparin on the vasculogenesis of the chorioallantoic membrane. Acta Anat 130:257–263
Ribatti D, Nico B, Vacca A et al (1998) Do mast cells help to induce angiogenesis in B-cell non-Hodgkin’s lymphomas? Br J Cancer 77:1900–1906
Ribatti D, Vacca A, Nico B et al (1999) Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer 79:451–455
Ribatti D, Vacca A, Marzullo A et al (2000) Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in B-cell non-Hodgkin’s lymphomas. Int J Cancer 85:171–175
Ribatti D, Crivellato E, Candussio L et al (2001) Mast cells and their secretory granules are angiogenic in the chick embryo chorioallantoic membrane. Clin Exp Allergy 31:602–608
Ribatti D, Polimero G, Vacca A et al (2002) Correlation of bone marrow angiogenesis and mast cells with tryptase activity in myelodysplastic syndromes. Leukemia 16:1680–1684
Ribatti D, Vacca A, Ria R et al (2003a) Neovascularization, expression of basic fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur J Cancer 39:666–675
Ribatti D, Molica S, Vacca A et al (2003b) Tryptase-positive mast cells correlate positively with bone marrow angiogenesis in B-cell chronic lymphocytic leukemia. Leukemia 17:1428–1430
Ribatti D, Vacca A, Ria R et al (2003c) Neovascularization, expression of fibroblast growth factor-2, and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur J Cancer 39:666–674
Ribatti D, Finato N, Crivellato E et al (2005) Neovascularization and mast cells with tryptase activity increase simultaneously with pathological progression in human endometrial cancer. Am J Obstet Gynecol 193:1961–1965
Ribatti D, Nico B, Vacca A (2006) Importance of the bone marrow microenvironment in inducing the angiogenic response in multiple myeloma. Oncogene 25:4257–4266
Ribatti D, Crivellato E (2009) The controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol 275:89–131
Ribatti D, Crivellato E (2012) Mast cells, angiogenesis, and tumor growth. Biochim Biophys Acta Mol Basis Dis 1822:2–8
Rojas IG, Spencer ML, Martinez A et al (2005) Characterization of mast cell subpopulations in lip cancer. J Oral Pathol Med 34:268–273
Romagnani P, Annunziato F, Lasagni L et al (2001) Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest 107:53–63
Sawatsubashi M, Yamada T, Fukushima N et al (2000) Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch B Cell Pathol Mol Pathol 436:243–248
Scavelli C, Nico B, Cirulli T et al (2008) Vasculogenic mimicry by bone marrow macrophages in patient with multiple myeloma. Oncogene 27:663–674
Sica A, Schioppa T, Mantovani A et al (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential target sof anti-cancer therapy. Eur J Cancer 42:717–727
Sörbo J, Jakobbson A, Norrby K (1994) Mast cell histamine is angiogenic through receptors for histamine 1 and histamine 2. Int J Exp Pathol 75:43–50
Soucek L, Lawlor ER, Soto D et al (2007) Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med 13:1211–1218
Starkey JR, Crowle PK, Taubenberger S (1988) Mast cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer 42:48–52
Stockmann C, Doedens A, Weidemann A et al (2008) Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456:814–818
Takanami I, Takeuchi K, Naruke M (2000) Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer 88:2686–2692
Talmadge JE, Donkor M, Scholar E (2007) Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Met Rev 26:373–400
Theoharides TC, Conti P (2004) Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol 25:235–241
Tomita M, Matsuzaki Y, Onitsuka T (2000) Effect of mast cells on tumor angiogenesis in lung cancer. Ann Thorac Surg 69:1686–1689
Tóth-Jakatics R, Jimi S, Takebayashi S et al (2000) Cutaneous malignant melanoma: correlation between neovascularization and peritumoral accumulation of mast cells overexpressing vascular endothelial growth factor. Human Pathol 31:955–960
Ullrich SE, Nghiem DX, Khaskina P (2007) Suppression of an established immune response by UVA-a critical role for mast cells. Photochem Photobiol 83:1095–1100
Vacca A, Ribatti D (2006) Bone marrow angiogenesis in multiple myeloma. Leukemia 20:193–199
Vacca A, Ribatti D, Presta M et al (1999) Bone marrow neovascularization, plasma cell angiogenic potential and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 93:3064–3073
Vacca A, Semeraro F, Merchionne F et al (2003) Endothelial cells in the bone marrow of patients with multiple myeloma. Blood 102:3340–3348
Walsh LJ, Trinchieri G, Waldorf HA et al (1991) Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA 88:4220–4224
Wanachantatak S (2003) Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med 23:195–199
Zeisberger SM, Odermatt B, Marty C et al (2006) Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new high and highly effective antiangiogenic therapy approach. Br J Cancer 95:272–281
Acknowledgments
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under Grant agreement n°278570 to DR and 278706 to AV.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Basel
About this chapter
Cite this chapter
Ribatti, D., Vacca, A. (2014). The Role of Inflammatory Cells in Angiogenesis in Multiple Myeloma. In: Aggarwal, B., Sung, B., Gupta, S. (eds) Inflammation and Cancer. Advances in Experimental Medicine and Biology, vol 816. Springer, Basel. https://doi.org/10.1007/978-3-0348-0837-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-0348-0837-8_14
Published:
Publisher Name: Springer, Basel
Print ISBN: 978-3-0348-0836-1
Online ISBN: 978-3-0348-0837-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)