Abstract
Imaging is a key step in evaluating the acute stroke patient in order to establish the correct diagnosis and to facilitate fast triage decisions regarding treatment with thrombolysis and endovascular therapy in potentially eligible patients. This chapter explores evidence-based guidelines for stroke imaging, discusses the role of CT and MRI in acute stroke assessment, offers strategies for streamlining imaging workflows, and provides insights into identifying stroke mimics.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
To assess current evidence in treatment of acute ischemic stroke.
-
To review the role of multimodal CT and MR imaging including vessel, collateral and perfusion imaging in “FAST positive” or “Code stroke” patients.
-
To describe optimal imaging workflows in acute stroke.
-
To recognize stroke mimics.
1 Introduction
Imaging is a key step in the evaluation of the acute stroke patient. While imaging strategies may vary between centers, the goal of acute stroke imaging remains the same, establishes the correct diagnosis, and facilitates fast triage decisions regarding treatment with thrombolysis and endovascular therapy in potentially eligible patients. Imaging allows evaluation for the following four critical points:
-
1.
Hemorrhage: Identification of intraparenchymal or subarachnoid hemorrhage is a key differentiator of hemorrhagic from the significantly more common ischemic stroke, both of which have widely different management strategies. In addition, exclusion of any intracranial hemorrhage is important in determining eligibility for thrombolysis.
-
2.
Infarction: Identification of any existing infarction, aging of same as acute, subacute, or chronic, and determining extent of acute infarction is crucial for decision-making regarding both intravenous thrombolysis and endovascular therapy (EVT).
-
3.
Arterial occlusion: Identification of any intracranial arterial occlusion, including large artery, medium and distal occlusions, as well as occlusion of carotid or vertebral arteries in the neck is critical in determining eligibility for EVT.
-
4.
Stroke mimics: A wide range of neurological conditions can present with similar symptoms to acute stroke at the outset and imaging can distinguish between stroke and stroke mimics.
2 Imaging in Acute Ischemic Stroke—Evidence-Based Guidelines
Over the years, multiple trials have changed the landscape of acute stroke treatment, it is vital that radiologists are familiar with these paradigm shifting studies. In 2015, five randomized controlled trials (RCTs) (MR CLEAN, ESCAPE, REVASCAT, SWIFT-PRIME, EXTEND-IA) [1,2,3,4,5] demonstrated benefit for EVT over best medical therapy (BMT) in patients with large vessel occlusion (LVO) of the anterior circulation within 6 h from symptom onset or last known well (LKW). These patients had moderate to severe stroke deficits (National Institutes of Health Score/NIHSS ≥6), absence of widespread established infarction on brain imaging (ASPECT score ≥6 on MRI or CT brain), and occlusion of the carotid terminus or proximal middle cerebral artery (MCA) on either CTA or MRA. As a result, endovascular thrombectomy became standard of care for patients with anterior circulation LVO meeting the above criteria [6, 7].
In 2018, two additional RCTs, DAWN and DEFUSE 3 [8, 9], demonstrated benefit of EVT over BMT in a later time window up to 24 h from onset/LKW. These late window trials used advanced stroke imaging techniques for patient selection, either CT perfusion (CTP) or MRI, and included evaluation of core infarct volume with automated post-processing software (RAPID, iSchemaView). The DAWN trial used clinical-core mismatch as a selection criterion between 6 and 24 h from symptom onset, with variable core volume allowed up to a limit of 51 mL depending on age and severity of stroke symptoms. The DEFUSE 3 trial allowed core volume up to 70 mL for patients from 6 to 16 h from symptom onset, with mismatch ratio >1.8, and mismatch volume >15 mL. These trials provided level 1a evidence for the benefit of EVT in the late time window and changed stroke guidelines, with new recommendations for imaging as well as treatment in delayed time windows [6, 7]. As per guidelines, in adult patients with anterior circulation LVO presenting up to 6 h from onset/LKW, advanced imaging is not necessary for patient selection. However, in patients presenting from 6 to 24 h, advanced imaging with either CT perfusion, diffusion weighted MRI (DW-MRI), or MR perfusion (MRP) is necessary.
Also using advanced imaging techniques, two major trials provided evidence for use of extended window IV thrombolysis beyond 4.5 h. Published in 2018, the WAKE-UP trial [10] randomized IV thrombolysis eligible patients who either awoke with stroke symptoms or had unclear time of onset >4.5 h from LKW. Imaging criteria required mismatch between MR-DWI and fluid-attenuated inversion recovery (FLAIR). The trial demonstrated benefit of IV tPA over placebo in patients with wake up or unknown time of onset stroke, and for whom EVT was not performed. Exclusion criteria included DW-MRI lesions larger than one-third of the MCA territory. Published in 2019, the EXTEND trial [11] evaluated use of IV thrombolysis in patients presenting 4.5–9 h from known stroke onset time, or after awakening with stroke if within 9 h from the midpoint of sleep. Advanced imaging with CTP or MRP was required for patient selection. Inclusion criteria included core infarct volume ≤70 mL and mismatch ratio of >1.2. The study showed better outcomes for IV tPA versus placebo. Guidelines for the use of IV thrombolysis in extended time window are now published [6, 12], with recommendations for advanced imaging techniques to be used in patient selection.
While EVT is performed for posterior circulation LVO, high level evidence has been lacking. Two recent RCTs have however shown positive results for EVT in basilar occlusion. The BAOCHE trial [13] randomized basilar occlusion patients presenting 6–24 h from onset, with NIHSS >6, and imaging-based inclusion criteria of posterior circulation ASPECTS score (PC-ASPECTS) of ≥6 and pons midbrain index score of ≤2. The ATTENTION trial [14] randomized patients with basilar occlusion presenting within 12 h of onset, with NIHSS >10 and PC-ASPECTS ≥6. It is likely that the benefit of EVT in basilar occlusion demonstrated in these trials will result in updates to the guidelines. Given that both trials included imaging-based selection criteria, the use of a scoring system for acute infarct burden such as PC-ASPECTS may well be incorporated into guideline recommendations.
Most recently, the “large core” trials have garnered significant attention to expand EVT in patients with a large burden of acute infarction. The recent publication of three RCTs has however given level 1 evidence that EVT can improve functional outcome over BMT, with all three trials showing positive effect, even in these severe strokes. RESCUE-Japan LIMIT [15], published in 2022, used predominantly MRI for triage, and included patients with ASPECT score 3–5, carotid terminus or first segment of MCA occlusion (M1), and presenting within 6 h from last known well or within 24 h if there was no early change on FLAIR. In the SELECT2 trial [16], published in 2023, patients had an ICA or M1 occlusion, presented within 24 h of onset, and had large ischemic core volume defined as ASPECT score 3–5 or a core volume of at least 50 mL on CT perfusion or DW-MRI. CT was used for triage in the majority. Published at the same time as SELECT2, the ANGEL-ASPECT trial [17] included patients within 24 h of stroke onset, with intracranial ICA or M1 occlusion. Imaging selection criteria included ASPECT score 3–5 on non-contrast CT with no limitation of infarct-core volume, but also ASPECT score 0–2 with infarct-core volume between 70 and 100 mL. The majority of included patients (85%) had an ASPECT score of 3–5. Together these 3 trials provide evidence that EVT in large core strokes with anterior circulation LVO is safe and more effective than BMT alone. Results from additional large core trials including LASTE (NCT03811769), TESLA (NCT03805308), and TENSION (NCT03094715) are pending. As of this writing, there are no current recommendations in the European and North American guidelines for mechanical thrombectomy in large cores. However, it is reasonable to anticipate future guidelines to incorporate this new evidence.
3 Imaging Paradigm
CT is often the workhorse for emergency stroke evaluation due to its speed, availability, fewer contraindications, lower cost, and ease of interpretation, despite MRI’s higher sensitivity for early ischemic changes and specific infarct types. MRI imaging times are longer and safety screening can be challenging in the acute stroke situation. Regardless of the modality chosen, a streamlined imaging protocol is essential for effective stroke triage, as discussed in later sections.
4 CT: Protocols, Findings, and Pitfalls
A CT-based protocol for acute stroke triage should include a non-contrast CT head (NCCT), CT angiogram (CTA) of both brain and neck vessels, and if available, the option of performing CT perfusion (CTP).
4.1 Non-contrast CT Head Imaging
A NCCT head should be performed as the first study to allow exclusion of hemorrhage and facilitate decision-making regarding intravenous thrombolysis in eligible patients. Findings of early infarction on CT include loss of gray white matter differentiation, low attenuation change, and sulcal effacement. In carotid terminus and M1 segment occlusions, the earliest change is frequently seen in the basal ganglia and insula due to lack of collateralization in lenticulostriate perforator territories. However, NCCT can be limited in identification of very early infarcts, and particularly in setting of extensive small vessel disease and older infarcts.
The Alberta stroke program early CT score (ASPECTS) is a widely used scoring system developed to allow objective analysis of the extent of acute infarction in the MCA territory. The MCA territory is segmented into 10 regions: caudate, lentiform, internal capsule, insula, and cortical zones M1–M6. Zones M1–M3 lie at the basal ganglia level, extending anteroposteriorly, while zones M4–M6 are supra-ganglionic and also extend from anterior to posterior. One point is deducted from the original score of 10 for every region involved by acute infarction. Many of the major RCTs providing evidence for the benefit of EVT included ASPECTS as a selection criterion. Higher ASPECT scores are associated with better outcomes following stroke treatment. While the scoring system is objective, there is poor inter-observer reliability and use is limited to MCA territory. The integration of AI in stroke imaging has expanded the capabilities of automated ASPECTS analysis. While these tools are designed to augment, not replace, radiologists, they enhance inter-rater agreement and boost non-expert performance.
A variation of the ASPECT scoring system has been developed for the posterior circulation, PC-ASPECTS. This is also a 10-point scoring system, with points lost for each region affected. Due to the high morbidity associated with brainstem infarction, the score is weighted to a degree for same with pons and midbrain both assigned 2 points each. Other zones include thalami (1 point each), occipital lobes (1 point each), and cerebellar hemispheres (1 point each). A limitation is the relative lack of sensitivity for CT identification of early brainstem infarction.
NCCT can also help identify site of occlusion, as intra-arterial thrombus may appear hyperdense depending on its composition. As well as identifying linear hyperdensity in a proximal artery such as the M1 (hyperdense artery sign), it is helpful to evaluate more distally in the sylvian fissure for a hyperdense “dot” sign which can indicate an M2 occlusion. Other locations of potential hyperdense thrombus include anterior interhemispheric fissure for an anterior cerebral occlusion, prepontine cistern for basilar thrombus, or ambient cistern for posterior cerebral artery occlusion.
4.2 CT Angiogram
CTA is quick and highly accurate in identification of LVO. It should be performed from the level of the aortic arch through to vertex, allowing evaluation of extracranial carotid and vertebral arteries for occlusion, stenosis, or dissection which may impact on interventional techniques used and patient outcome. If an extracranial arterial occlusion or stenosis is of an artery in the same territory as the intracranial occlusion, it is termed a tandem lesion.
The term LVO refers to occlusion of the carotid terminus (carotid T), first segment of MCA (M1), intracranial vertebral artery, and basilar artery, and all of these must be scrutinized for patency. There is debate in the literature as to whether occlusion of the second segment of the middle cerebral artery (M2) is a large or medium vessel occlusion (MeVO), particularly given that occlusion of a proximal dominant M2 segment, or more than one proximal M2 segment can behave as an M1 like occlusion in terms of volume of territory at risk of infarction and clinical severity of stroke syndrome. While evidence from RCTs for treatment of M2 occlusion is still lacking, there are many observational studies providing supportive evidence that EVT in M2 occlusions is beneficial and safe [18]. Treatment approaches for symptomatic MeVO (Medium Vessel Occlusions) vary across institutions, some following standard protocols despite limited evidence, while others opt for case-by-case decisions. Ongoing RCTs like ESCAPE-MeVO (NCT05151172) and DISTALS (NCT05152524) aim to establish the efficacy of thrombectomy in MeVO and more distal occlusions.
As a radiologist, it is important to have a strategy to identify medium and distal occlusions on CTA. The use of thin slice imaging (1 mm or less), multiplanar reconstruction, and maximal intensity projection (MIP) images with a slice thickness of 6–10 mm can all aid in the detection of these occlusions. For example, the sagittal plane is particularly useful for identifying medium and distal M2 occlusions, ACA occlusions, and PCA P2–P3 occlusions, and coronals are useful for proximal M2, proximal ACA and PCA occlusions.
CTA also provides vital insights into collateral pathways, either antegrade via the Circle of Willis (COW) or retrograde through leptomeningeal collaterals. For example, in ICA occlusion below the carotid terminus, collateral supply can come from the anterior communicating artery; if the occlusion is below the posterior communicating artery, it adds another route. However, in M1 or carotid T occlusions, antegrade COW collateralization is unavailable; any collateral supply must be retrograde, typically via leptomeningeal collaterals from ipsilateral ACA or PCA territory.
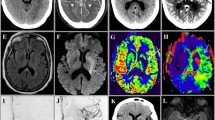
Numerous studies have shown that good leptomeningeal collateral supply confers benefit in stroke as it sustains brain tissue in the occluded vascular territory for a longer time with slower infarct growth (slow progressors), smaller final infarct volumes, and improved patient outcomes. Poor collateralization is associated with earlier infarction (fast progressors), larger infarcts, higher rates of hemorrhage, and poor outcomes [19, 20]. While there is no consensus on the type of grading scale, most are restricted to the MCA territory and vary from 2-point (good vs bad) to 5-point scales. The method is usually to compare the extent of arterial opacification in the occluded territory to the contralateral side, with degree of filling expressed as a fraction or percentage. For instance, <50% filling is considered poor, 50–75% moderate, and >75% good. Utilizing thick section MIP images in an axial plane facilitates rapid evaluation of collateral supply (Fig. 3.1).
A 77-year-old lady, wake-up stroke, over 12 h from LKW with NIHSS 15. Automated eASPECTS analysis from Brainomix software (a), and source images (b) ASPECTS 8 with infarction in caudate and lentiform nuclei. Hyperdense right distal M1 segment (c) which reflects thrombus and corresponds to site of occlusion on CTA (d). Moderate collateral filling on phase 1 (not shown) but good collaterals on phase 2 images with branches filling back to proximal M2 level at distal end of thrombus (d, e). Perfusion analysis (f) using RAPIDAI software shows small core infarct (purple shading) large territory at risk (green shading), therefore large penumbra with large mismatch ratio 17.3. Technically, adequate CTP study with contrast bolus demonstrating sharp rise and fall in attenuation with clear return to baseline on arterial and venous time density curves (g). Location for arterial ROI (h, red dot) is correct, usually the ICA terminus, proximal MCA, and ACA are appropriate. The blue dot (i) represents the location of venous ROI; superior sagittal sinus or torcula are commonly used sites
A pitfall of single-phase CTA is the lack of temporal resolution, which can underscore collateral supply, particularly in cases of poor cardiac output or proximal stenosis. Multiphase CTA with initial arterial phase from arch to vertex, delayed intracranial early venous, and later venous phases can resolve this issue by enabling temporal evaluation of collaterals. More comprehensive grading systems have been developed to facilitate interpretation with evaluation of degree of filling of the occluded territory as well as delay across phases [21]. The multiphase evaluation of collaterals can be a valuable tool in triage of LVO patients for thrombectomy, and together with NCCT ASPECTS was the selection criteria in the ESCAPE trial [2]. Multiphase CTA can also provide better depiction of the medium and distal vessel occlusions. Many of the stroke AI solutions include analysis and scoring of collateral supply in MCA territory, as well as automated LVO detection.
Another pitfall of single-phase CTA is pseudo-occlusion, most commonly seen in ICA or vertebral. This occurs with either a severe proximal stenosis or distal occlusion, where a slow flow situation arises with progressively poor contrast opacification of the artery distally giving a “false occlusion” sign, and can overestimate the length of thrombus. Most commonly seen with carotid T occlusion, where the more proximal intracranial and often much of the cervical segment of ICA are not opacified by contrast on a single-phase study, recognition of pseudo-occlusion is important as occlusion length is an important determinant of the efficacy and complication rates of mechanical thrombectomy [22]. Multiphase CTA can overcome this pitfall.
A less common CTA pitfall is the false patency sign, where a particularly hyperdense thrombus can appear isodense to the contrast filled artery, and can be mistaken for contrast opacification of the vessel (Fig. 3.2). Identification of hyperdense thrombus on NCCT is crucial to avoid this pitfall, while widening window settings and levels can also help. A similar problem can be encountered with calcified vessels and can be overcome in the same way.
CTA pitfall: false patency of vessel. A 55-year-old female, code stroke, transferred from outside hospital. NCCT in outside institution demonstrates hyperdense M2 (a), however this was not available during CTA interpretation. The CTA interpretation overlooked the vessel occlusion as it appears falsely patent (b, c). On careful review, there is slightly reduced attenuation on CTA compared to the other vessels (c)
Key Points: Review CTA Comprehensively to Assess For
-
Large vessel occlusion.
-
Medium or distal vessel occlusion.
-
Extracranial circulation including dissection, plaque, carotid web.
-
Access issues such as tortuosity, carotid disease, aneurysms.
-
Collateral circulation.
4.3 CT Perfusion
CTP is now increasingly incorporated into imaging protocols and allows evaluation of the volume of already infarcted tissue (ischemic core) and volume of tissue that is at risk due to hypoperfusion but not yet irreversibly infarcted (penumbra). It is performed by sequentially imaging a defined section of tissue after administration of a high-flow bolus of iodinated contrast material. Images are then postprocessed using either semi-automated or increasingly fully automated software that utilizes deconvolution algorithms to create perfusion maps, including cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), time to peak (TTP), and time to maximum residue (Tmax).
Many of the AI software solutions include automated post-processing of perfusion data. Generally, reduced CBF <30% of contralateral side is the parameter used to reflect ischemic core volume. The threshold of Tmax >6 s is the most commonly used to reflect critically hypoperfused territory at risk of infarction. Subtraction of core volume from volume of Tmax >6 s gives penumbra volume. Most software solutions will display a color-coded summary map which provides quantification of core, Tmax >6 s, mismatch and a mismatch ratio (Fig. 3.1). Additional variable thresholds of Tmax as well as CBF are also frequently provided. Further calculations such as the hypoperfusion index ratio (Tmax >10 s volume divided by Tmax >6 s volume) can be performed by automated software. Hypoperfusion index ratio (HIR) can be used to distinguish slow from fast progressors, with higher values in fast progressors, and is a good surrogate of tissue collaterals.
It is important to recognize the multiple pitfalls to automated CT perfusion [23]. There is inherent variation in outputs between different software solutions which can result in different calculations of core volume, territory at risk, penumbra, and mismatch ratio. The variation between different software is usually however not to the extent that it impacts decision-making. An exception can be in analysis of more distal occlusions, where the volumes of rCBF <30% and Tmax >6 s are inherently smaller and even minor variations have a greater impact on mismatch ratio calculation. Secondly, the CBF <30% threshold is not always an accurate reflection of ischemic core. This is particularly the case for very early presenters, in particular those presenting within 90 min from onset/LSW, where the ischemic core can be overestimated, i.e., ghost core (Fig. 3.3). The additional CBF thresholds can be helpful in this scenario, with recommendation to use CBF <20% threshold in ultra-early windows. Overestimation of core infarct can lead to incorrect exclusion of patients from EVT, potentially missing an opportunity for effective treatment. Poor cardiac output can result in significant delay in arrival of contrast bolus to intracranial vessels, with potential truncation of time density attenuation curves, and inaccuracy of generated perfusion maps. In addition, a chronic carotid occlusion/stenosis can result in a false positive penumbra (Fig. 3.4), while a proximal carotid stenosis can overestimate territory at risk. Use of additional Tmax threshold maps and close correlation with CTA findings aids interpretation in these circumstances.
Ghost core. A 76-year-old male, imaging including CTP at 55 min from witnessed stroke onset. No acute infarct on NCCT (a). Left M1 occlusion on CTA with poor collaterals on phase 1 (b), delayed collateral filling on phase 3 (c). CTP shows large volume of CBF <30%, suggesting large core infarct (d). Digital subtraction angiogram shows left M1 occlusion (e), complete recanalization on frontal (f) and lateral (g) view after single pass aspiration thrombectomy. No infarction on NCCT at 24 h (h), a delayed MRI 6 months later also shows no evidence of established infarction on FLAIR (i), confirming overestimation of core by CTP in this very early time window
It is important to check technical adequacy of a CTP before interpretation or decision-making (Fig. 3.1). The arterial input function (AIF) and venous output function (VOF) time density curves should always be inspected as part of the technical checklist of CTP interpretation to ensure they have returned to baseline. Further technical pitfalls include incorrect arterial input and venous output selections, motion artifact, poor signal to noise ratio if contrast bolus is poor, and limitation with many CT scanners to perform limited coverage perfusion rather than whole brain coverage perfusion.
Despite the above pitfalls, CTP remains key in acute ischemic stroke evaluation, but its role in LVO thrombectomy may change based on upcoming large core infarct trial outcomes. Its importance is growing for detecting at risk medium and distal occlusions, often missed on CTA.
Key Points
-
It is important to recognize technical and diagnostic pitfalls of an automated CTP.
-
Technical pitfalls include truncated time density curve, incorrect selection of arterial or venous input and patient motion.
-
Diagnostic pitfalls include under or overestimation of ischemic core or penumbra.
5 MRI: Protocols, Findings, Pitfalls, and Example Cases
An MRI-based paradigm for acute stroke imaging has advantages and disadvantages. A typical protocol for MRI in acute stroke is outlined in Table.
A distinct advantage of MRI is the increased sensitivity of DWI in detection of acute infarcts, particularly important in posterior circulation strokes. MRI also meets the criteria of advanced imaging selection in late window patients. Further advantages include the lack of ionizing radiation and ability to perform imaging without use of a contrast agent if needed. The use of MRI for triage may however result in less patients receiving thrombectomy due to detection of greater extent of acute ischemia on DWI [24]. Additional MRI pitfall is the potential reversibility of DWI lesions early in the course of stroke. Several studies have reported partial reversal of DWI after acute reperfusion treatment, with improved outcomes. Complete reperfusion and shorter imaging time to recanalization have been shown to be independently associated with DWI reversal among patients with LVO who received EVT [25].
Disadvantages include potential limited MR availability, and need for patient safety screening prior to scanning, both of which have been identified as a significant source of delay to imaging acquisition [26]. Another potential disadvantage can be longer imaging times, although MRI fast protocols, can be implemented in time frames as little as 6 min [27]. In MRI, as in CT, perfusion analysis automated software that allows fast post-processing is essential to prevent delay in decision-making. Decision-making with MRI for acute stroke includes evaluation for LVO, clinical diffusion mismatch (CDM), FLAIR diffusion mismatch (FDM) (Fig. 3.5), or perfusion diffusion mismatch (PDM).
DWI-FLAIR mismatch: a 63-year-old female presented with wake-up stroke. The initial CT workup was negative for early ischemia or vessel occlusion. MRI shows a DWI-FLAIR mismatch in the right post central gyrus. It is important not to heavily window the FLAIR sequences to look for subtle signal changes. A remote infarct is identified in the left post central gyrus
Key Points: MRI Protocol
• DWI | Acute infarcts demonstrate true diffusion restriction with high signal on B1000 images, and low signal on apparent diffusion coefficient (ADC), visible within 20 min of stroke onset |
• FLAIR | Acute infarcts appear hyperintense, may take up to 6 h, and indicator of irreversible changes |
• MR angiogram | Time of flight (TOF) technique, or contrast-enhanced MRA, requires imaging of COW as minimum, ideally include neck vessels |
• GRE/SWI | Evaluate for presence of acute or old hemorrhage, evaluate for blooming sign indicating intra-arterial thrombus similar to hyperdense sign on CT |
• MRP | Optional, if advanced imaging desirable or required |
• T2 weighted | Optional, preferential for evaluation of posterior fossa |
6 Approach to Interpreting “Code Stroke” Imaging
Regardless of imaging paradigm used, timely interpretation in the FAST positive/code stroke patient is crucial, whether done on the scanner console, PACS workstation or via AI for immediate triage and treatment decisions. While immediate questions like excluding hemorrhage and identifying LVO need quick answers, imaging must however also be thoroughly reviewed for additional and unexpected findings. Mirroring major trauma analogy, imaging interpretation in acute stroke is a two-tiered approach—a fast initial primary survey allowing rapid triage to treatment, followed by a less time pressured secondary definitive survey.
Key Points
-
Primary imaging survey
-
Hemorrhage: present or not
-
Acute infarction: present or not, location, extent, ASPECTS
-
Arterial occlusion: intracranial and extracranial sites
-
Proximal stenosis: significant or not
-
Collateral status: good or bad
-
Perfusion defect: core volume, mismatch present or not
-
Secondary imaging survey
-
Identify additional infarcts including subacute, chronic, and non-MCA territory
-
Additional brain findings—e.g., small vessel disease
-
Collateral scoring
-
Additional arterial occlusions, same vascular territory, different vascular territory
-
Atherosclerotic disease, associated stenosis, and degree of same if present
-
Consider mechanism of stroke and secondary prevention including artery to artery embolus, cardioembolic, soft plaque, carotid web, thrombus in aortic arch or in a vessel proximal to occlusion
-
Additional or unexpected perfusion findings, e.g., hyperemia
-
Consider additional differentials and stroke mimics
-
Incidental findings
7 Optimizing Imaging Workflow in Acute Stroke
The ability to do late window thrombectomy up to 24 h from onset/LKW does not negate the need to be fast. From the HERMES collaboration [28] for every 9-min delay in onset to reperfusion, one of every 100 patients will have greater functional disability at 90 days. There is an inverse relationship between in-hospital treatment speeds and functional independence, with reducing rate of good outcome as “door to reperfusion” time increases. Hence, it is critical to establish time efficient imaging workflow in order to reduce key time indicators such as “door to imaging,” “door to needle,” “door to puncture,” and ultimately “door to reperfusion” time. The radiology department is the cornerstone of stroke workflow, and it is important for radiologists to work on optimizing workflow together with imaging technologists, the stroke team, and neurointerventional team. Below are pointers to allow optimization of workflow.
-
A pre-alert from EMS to ER, stroke, and imaging teams via a single paging system enables immediate action. On arrival, the patient is met by the stroke team and directly taken to CT/MRI.
-
Parenchymal imaging, e.g., NCCT, performed first to allow triage to IV thrombolysis if eligible. Further angiographic and perfusion imaging should not delay administration of thrombolysis which can be given while the patient is in the CT or MRI scanner.
-
It is not necessary to delay iodinated contrast administration to check renal function.
-
A radiologist should be on standby to review images as patients are scanned, either at the scanner console or a PACS workstation. Automated sending to PACS should be set up, ensuring NCCT or DW-MRI is ready for review as angiograms or perfusion studies are underway.
-
AI software solutions with automated ASPECTS, LVO, and perfusion can be very helpful in streamlining workflow, with demonstrated positive impact on key time indicators [29]. Output from most AI software solutions can be viewed on hand-held devices with option for notifications to alert the team and text communication within the team. The current and new generation of radiologists will have to adapt the use of AI in imaging workflow.
8 Stroke Mimics
Almost one-third cases of new focal neurological deficits can be stroke mimics [30]. It is important to identify stroke mimics and differentiate from true strokes to avoid inappropriate treatment. MRI has inherent advantages over CT to identify mimics. The more common stroke mimics include seizures, migraines, posterior reversible encephalopathy syndrome, venous thrombosis, and brain tumors.
8.1 Seizures
The most frequent stroke mimic is seizures with Todd’s paralysis, paresis, or aphasia. The MRI changes are variable, ranging from focal, multifocal, hemispheric, or a diffuse cortical pattern of diffusion hyperintensity with variable ADC levels. Perfusion abnormalities can range from hyperperfusion in ictal phase to hypoperfusion or normal perfusion in the postictal phase. These shifts are due to neuronal activation or inhibition, respectively. Unlike strokes, seizure-related perfusion changes are not confined to a vascular territory, serving as a key clue. Additional distinguishing features of seizures include gyral or leptomeningeal enhancement, and absence of vessel occlusion.
8.2 Migraines
Migraine aura without headache can commonly present as a stroke mimic. MR imaging is usually normal, however in a few cases may show reversible restricted diffusion. The differentiating features include prior history of migraine, a nonvascular distribution, and lack of LVO. Perfusion abnormalities are variable with hypoperfusion in acute onset aura and normal or hyperperfusion in prolonged episodes. However, the perfusion abnormality does not usually correspond to a defined vascular territory and can span multiple vascular territories.
8.3 Posterior Reversible Encephalopathy Syndrome (PRES)
An acute onset neurological syndrome with vasogenic edema due to loss of auto-regulation and capillary leakage can mimic acute or subacute stroke. The PRES lesions are usually cortical/subcortical with hyperintensity on T2/FLAIR with predilection for parietooccipital lobes and a relatively symmetric pattern. Approximately 10–25% of cases can show restricted diffusion and around 15% have associated parenchymal hemorrhage.
8.4 Venous Thrombosis
Both a vasogenic and cytotoxic edema may be present with resultant variable diffusion restriction on DWI. Usually, edema due to venous thrombosis is easy to identify given the lack of arterial territorial distribution, with location of edema dependent on the location of the venous thrombus. T2-GRE with its susceptibility artifact can show abnormal hypointensity and blooming of the venous thrombus. Imaging may demonstrate a flame-shaped hemorrhage due to venous infarction (Fig. 3.6).
8.5 Tumors
Brain tumors can mimic code strokes by causing new neurological deficits, requiring prompt differentiation to avoid treatment delays. Small, cortical tumors in an arterial territory can be mistaken for infarcts on the initial imaging. Depending on the cellularity and grade, tumors have variable diffusion hyperintensity and contrast enhancement. Perfusion imaging may be helpful; cerebral blood volume (CBV) in tumors is generally high in tumors but low in acute infarcts.
Additional conditions mimicking stroke include toxic and metabolic etiologies, conversion disorder, infection, peripheral vertigo, syncope, transient global amnesia, and subdural hematoma.
9 Concluding Remarks
Efficient, rapid stroke imaging is essential for optimal triage of the acute ischemic stroke patient. The use of artificial intelligence with automated ASPECT score calculation, automated LVO detection, and automated post-processing of perfusion data is becoming increasingly more widespread. The boundaries of acute stroke treatment continue to expand with lengthening of treatment windows, and EVT in large core as well as distal occlusions. The evolving landscape of stroke imaging and treatment reinforces the radiologist’s vital role in translating these changes into practice.
Take-Home Messages
-
Rapidly identify acute infarct, vessel occlusions, and perfusion defects in acute ischemic stroke.
-
Optimize imaging workflows.
-
Differentiate true strokes from stroke mimics.
References
Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of Intraarterial treatment for acute ischemic stroke. N Engl J Med. 2014;372(1):11–20.
Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Saver JL, Goyal M, Bonafe A, et al. Stent-retriever Thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95.
Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418.
Turc G, Bhogal P, Fischer U, et al. European stroke organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical Thrombectomy in acute Ischaemic StrokeEndorsed by stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6–12.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017;378(1):11–21.
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–18.
Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611–22.
Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795–803.
Berge E, Whiteley W, Audebert H, et al. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I-LXII:I.
Jovin TG, Li C, Wu L, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387(15):1373–84.
Tao C, Nogueira RG, Zhu Y, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387(15):1361–72.
Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386(14):1303–13.
Sarraj A, Hassan AE, Abraham MG, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388(14):1259–71.
Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388(14):1272–83.
Wang J, Qian J, Fan L, et al. Efficacy and safety of mechanical thrombectomy for M2 segment of middle cerebral artery: a systematic review and meta-analysis. J Neurol. 2021;268(7):2346–54.
Sheth SA, Sanossian N, Hao Q, et al. Collateral flow as causative of good outcomes in endovascular stroke therapy. J Neurointerv Surg. 2016;8(1):2–7.
Maguida G, Shuaib A. Collateral circulation in ischemic stroke: an updated review. J Stroke. 2023;25(2):179–98.
Menon BK, d'Esterre CD, Qazi EM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275(2):510–20.
Gralla J, Burkhardt M, Schroth G, et al. Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29(2):247–52.
Vagal A, Wintermark M, Nael K, et al. Automated CT perfusion imaging for acute ischemic stroke: pearls and pitfalls for real-world use. Neurology. 2019;93(20):888–98.
Wisco D, Uchino K, Saqqur M, et al. Addition of hyperacute MRI AIDS in patient selection, decreasing the use of endovascular stroke therapy. Stroke. 2014;45(2):467–72.
Yoo J, Choi JW, Lee S-J, et al. Ischemic diffusion lesion reversal after endovascular treatment. Stroke. 2019;50(6):1504–9.
Atchaneeyasakul K, Shang T, Haussen D, et al. Impact of MRI selection on triage of endovascular therapy in acute ischemic stroke: the MRI in acute Management of Ischemic Stroke (MIAMIS) registry. Interv Neurol. 2020;8(2–6):135–43.
Nael K, Khan R, Choudhary G, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries. Stroke. 2014;45(7):1985–91.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Gunda B, Neuhaus A, Sipos I, et al. Improved stroke Care in a Primary Stroke Centre Using AI-decision support. Cerebrovasc Dis Extra. 2022;12(1):28–32.
Merino JG, Luby M, Benson RT, et al. Predictors of acute stroke mimics in 8187 patients referred to a stroke service. J Stroke Cerebrovasc Dis. 2013;22(8):e397–403.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Power, S., Vagal, A.S. (2024). Stroke and Its Mimics: Diagnosis and Treatment. In: Hodler, J., Kubik-Huch, R.A., Roos, J.E. (eds) Diseases of the Brain, Head and Neck, Spine 2024-2027. IDKD Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-031-50675-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-50675-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-50674-1
Online ISBN: 978-3-031-50675-8
eBook Packages: MedicineMedicine (R0)