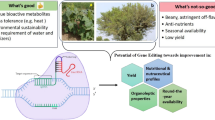
Abstract
Cichorium varieties are cultivated both as leafy vegetables as well as industrial root crop for extraction of the food fibre inulin. Cichorium is a typical European crop and grown on a relatively small scale. However, due to its distinctive taste and health benefits and its capacity to produce multiple bioactive compounds, Cichorium has great potential if varieties could be optimised for these properties by breeding. In recent years it has been demonstrated in several laboratories that chicory is very amenable to genome editing. Different protocols were developed and implemented to adapt bitterness as well as to accumulate medicinal terpenes, generating potential socio-economic benefits over the entire value chain from farmers to consumers, as well as for the environment. In addition, scientific knowledge on chicory biology, particularly on the biosynthesis of secondary metabolites was significantly increased. This demonstrates how genome editing can contribute to breeding of niche crops such as Cichorium, which have relatively little investment leverage for extensive breeding programs.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Cichorium Species and Their Sesquiterpene Lactones
The genus Cichorium belongs to the Asteraceae family of plants. About 10 Cichorium species have been described, with C. intybus being the best known and being cultivated for different applications (Fig. 21.1).
Overview of different Cichorium species and varieties: (a) C. intybus var. sylvestre (Catalogne); (b) C. endivia (endive); (c) C. intybus var. porphyreum (sugerloaf); (d, f) C. intybus var. latifolium (radicchio); (e) C. intybus var. latifolium (radicchio early red of Treviso); (g) C. intybus var. latifolium (radicchio late red of Treviso); (h) C. intybus var. foliosum (redloof); (i) C. intybus var. foliosum (witloof); (j) C. intybus ‘variegata di Castelfranco’; (k) C. intybus var. sativum (industrial chicory); (l) Flowers of C. intybus. (Pictures provided by Bram Van de Poel, KU Leuven, Belgium)
C. intybus var. foliosum (e.g., witloof), C. intybus var. latifolium (e.g., radicchio) and the closely related C. endivia (e.g., endive), are well-known leafy vegetables appreciated for their characteristic bitter taste and richness in nutritionally relevant compounds with beneficial effects on human health. However, the bitter taste can have a negative influence on consumer acceptability, especially among young adults and children. Industrial chicory (C. intybus var. sativum) is grown, particularly in the south of the Netherlands, Belgium and north of France, for its large taproots from which inulin is extracted. C. intybus is grown on a relatively small area with a total global acreage of around 14,500 ha [1, 2]. Inulin is a prebiotic food fibre which stimulates gut health [3] and is used as food ingredient in products such as yogurts and granola bars. Chicory is actually an ancient crop and its use as food and for its medicinal properties is documented already since Roman times [4]. Apart from inulin extraction, industrial chicory roots also have potential as raw material to make chicory flour for use in food industry as the flour will contain the nutritional benefits of inulin and does not contain gluten. But the use of this flour is still limited due to the high bitterness of the roots.
The bitter taste of the Cichorium crops is caused by the presence of sesquiterpene lactones (STLs) that accumulate both in leaves and roots. STLs represent a large and diverse group of terpenoids with more than 5000 different STLs being identified in Asteraceae [5]. STLs are specialized isoprenoid metabolites that contain 15 carbon atoms and which have a lactone function. The presence of a γ-lactone ring containing an α-methylene group, is a significant characteristic of STLs. They were shown to have a defence role in plants as protection against herbivorous insects and fungi [6,7,8].
Based on their skeleton, STLs are classified in six major groups namely germacranolides, eudesmanolides, eremophilanolides, guaianolides, pseudoguaianolides and hypocretenolides and many subtypes [8]. Leaves and especially roots of Cichorium species contain high concentrations of the bitter guaianolides lactucopicrin, lactucin, and 8-deoxylactucin. Eudesmanolides and germacranolides are present in smaller amounts [9]. 8-Deoxylactucin, lactucin, and lactucopicrin predominantly occur in the oxalated form [10], but also their derivatives such as 11,13-dihydro-analogues are present. Several health-related bioactivities of chicory STLs have been documented. Lactucin and lactucopicrin display analgesic and sedative effects [11] and have antimalarial activity [12] and natural extracts from chicory roots present antibacterial and antifungal properties [13]. Purified fractions from chicory roots containing a mixture of 8-deoxylactucin and dihydro-8-deoxylactucin possess promising anti-inflammatory properties [14]. Also dihydrolactucin, a lactucin derivative found in chicory, revealed anti-inflammatory potential [15]. It is not yet clear which STLs contribute the most to the bitterness perceived by consumers [5].
The biosynthesis of STLs in chicory has been partly elucidated (Fig. 21.2). The common sesquiterpene precursor farnesyl pyrophosphate (FPP) is first cyclized to germacrene A by the germacrene A synthase (CiGAS) [16]. The biosynthesis continues by oxygenation by presumably as many as six cytochrome P450 enzymes of which the genes encoding germacrene A oxidase (CiGAO), costunolide synthase (CiCOS), kauniolide synthase (CiKLS) and lactucin synthase (CiLCS) have been identified [17,18,19,20,21,22,23], all belonging to the CYP71 clan of cytochrome P450 enzymes. The STLs are further modified by conjugations with 4-hydroxyphenyl acetate and oxalate and by reduction of the 11,13-double bond. Enzymes involved in 4-hydroxyphenyl acetate and oxalate transfer and reduction of the double bond have not yet been identified. The basic STL structures in different chicory subspecies are identical but between varieties their relative abundance and the extent of modifications can differ. The downstream steps in the STL biosynthesis, from the tricyclic kauniolide onward, appear to take place in the latex itself, as the CiKLS and CiLCS genes show latex-specific expression [17, 18].
Biosynthetic pathway of chicory STLs. Enzymes to be inactivated for reduction of bitterness are indicated in red font and enzymes to be inactivated for accumulation of medicinal compounds are indicated by a blue arrow. Dashed arrows indicate uncharacterized enzymatic conversions. FPP – farnesyl pyrophosphate, CiGAS – germacrene A synthase, CiGAO – germacrene A oxidase, CiCOS – costunolide synthase, CiKLS – kauniolide synthase, CiLCS – lactucin synthase
In summary, chicory species are producing several compounds that are highly relevant for food, namely the dietary fibre inulin and the bitter tasting terpenes, with some of these terpenes displaying beneficial health properties. Generating varieties with an optimized composition of either of these compounds would therefore be highly interesting and open new markets for food industry with great economic impact. Today, for industrial chicory hot water extraction is used to extract inulin and the bitter tasting STLs are co-extracted in this process [24]. Since the presence of bitter compounds in inulin limits its use as a prebiotic dietary fibre and sweetener in food products, the STLs are removed in subsequent purification steps, increasing the costs and input for processing. Moreover, related to leafy Cichorium species as witloof and radicchio, product differentiation by creating a more diverse range of flavours would maximize witloof acceptance and benefit of its health promoting compounds. However, chicory species are relatively small crops with little investment leverage to enter lengthy breeding programs of about 10 years to produce a new cultivar. Although Cichorium species are self-incompatible, selfing at a low rate can still occur [25, 26]. Thanks to the high number of flower heads produced during a generative period, a quite high amount of selfed seeds can thus still be formed. However, creating true hybrids remains cumbersome. New breeding techniques, e.g. CRISPR/Cas genome editing, could make a difference to create chicory varieties with consumer traits in a faster and more efficient manner.
2 Genome Editing in Cichorium Species
Genome editing methods for Cichorium have first been described in 2019 [27]. Since then several studies describe the use of CRISPR/Cas-based genome editing in Cichorium species (Table 21.1). Protocols for four different delivery methods of CRISPR/Cas reagents have been established. High efficiency of genome editing using Agrobacterium rhizogenes-mediated stable transformation in hairy roots was first demonstrated for the chicory phytoene desaturase gene (CiPDS). This optimized protocol was later used to elucidate the function of biosynthetic enzymes involved in the synthesis of tetracoumaroyl spermine in chicory [28]. Introduction of CRISPR/Cas system via Agrobacterium tumefaciens-mediated stable transformation was first described targeting the CiGAS gene [29]. While stable transformation methods lead to high editing efficiency, thanks to the continuous editing after stable genome integration of CRISPR/Cas, they have a disadvantage for chicory breeding due to chicory self-incompatibility (although incomplete) and long time needed for outcrossing the T-DNA. Genome editing of the same gene in two compatible varieties is needed followed by segregation of transgenes after crossing. In addition, detailed genotyping of the resulting plants revealed that plants obtained by stable transformation often showed chimerism and a mixture of CiGAS genotypes in the same plant was observed.
In parallel, transient delivery methods for chicory protoplasts combined with whole plant regeneration were established. Transient delivery of plasmids encoding CRISPR/Cas reagents was used for editing of the CiPDS gene and genes involved in the biosynthesis of chicory STLs [17, 18, 21, 23, 27, 30]. Although efficient generation of biallelic mutants was described even for multi-gene families of the STL pathway, unintended integration of plasmid DNA into the chicory genome in up to 30% of plants was observed in several studies [17, 18, 27, 30]. Alternatively, delivery of preassembled ribonucleoprotein complexes (RNPs) was established for chicory protoplasts [17, 29, 30]. This DNA-free delivery method is efficient while at the same time eliminating the risk of unintended DNA integration. Therefore, it can be concluded that delivery by RNPs is the most suitable delivery method for genome editing in chicory.
When comparing the three methods, stable Agrobacterium transformation, transient plasmid delivery and RNP transfection, for off-target editing at six potential off-target sites in the chicory genome showing similarity to the target sequence in the CiGAS gene, no off-target editing was observed for either of the three delivery methods [29]. In conclusion, both stable transformation and transient transfection have successfully been used in chicory with high efficiency, also for editing multi-gene families (Table 21.1). This advance will facilitate functional analysis and genetic improvement of chicory.
3 Genome Editing for Reduction of Bitterness in Cichorium Crops
Although there is some natural variation in the STL content among Cichorium species [31, 32], variants with a considerable low STL content have not yet been identified. Using CRISPR/Cas targeting genes involved in the STL biosynthetic pathway would facilitate the generation of less or zero bitterness in Cichorium.
The first dedicated step of the pathway (Fig. 21.2) is catalysed by CiGAS enzyme which is encoded in the chicory genome in four gene copies of the terpene synthase gene family, i.e., CiGAS-S1, CiGAS-S2, CiGAS-S3 and CiGAS-L [33]. Genome and transcriptome analysis identified two additional CiGAS paralogs belonging to the same gene clade [23]. Efficient editing of the CiGAS genes was achieved by several approaches (Table 21.1). The interruption of all CiGAS-S1, CiGAS-S2, CiGAS-S3 and CiGAS-L alleles has been achieved and led to elimination of the STLs in chicory leaves and taproots [30]. The edited plants showed no other directly observable morphological phenotypic changes under greenhouse conditions.
To investigate if inactivation of downstream steps in the STL biosynthetic pathway could modulate bitterness, genes from the CYP71 clan of the cytochrome P450 gene family CiGAO, CiCOS and CiKLS were functionally characterized in Cichorium [17,18,19,20, 22, 23]. In conclusion, taken all these studies together, two paralogous CiGAO genes, three paralogous CiCOS genes, and three paralogous CiKLS genes displayed catalytic activity in the upstream STL pathway to produce costunolide and kauniolide. De Bruyn et al. [23] used CRISPR/Cas genome editing to simultaneously target multiple CYP71 genes in protoplasts of witloof, followed by regeneration of plants. A broad spectrum of plant genotypes containing different (loss of function) mutants in multiple genes were obtained. Several genotypes containing mutations in one or more paralogous CYP71 genes (CiGAO mutants, CiCOS mutants and CiKLS mutants) were profiled for their STL composition. The production of 14 out of 16 detectable STL metabolites [34] was eliminated in the mutant types containing a homozygous loss of function mutation in the three functional paralogous CiKLS genes [22, 23] as was also observed when the three CiKLS paralogs were edited in root chicory [17]. Other mutants only showed shifts in STL compounds concentrations, but no clear effects or decreases were detected. It is likely that the mutation effect on STL compound quantities is masked if at least one functional allele and/or paralog is still present.
Studies indicate that the perception of bitterness is not only related to the guaianolide STL metabolite quantities and composition. François et al. [35] showed a strong correlation between glucose and sucrose with crunchiness and bitterness and a correlation between fructose and sweetness. Also the balance between STL compounds and phenolic compounds is described to affect bitterness [36]. So STLs are probably not the only metabolites to consider when aiming to modify bitterness in Cichorium. Cellular assays and human sensory tests should be used to link bitterness to the STL concentrations in CRISPR/Cas mutants [37].
4 Genome Editing to Produce Medicinal Terpenes in Chicory
The use of industrial chicory for extraction of inulin fibre is well established, with the chicory STLs being discarded during the purification of inulin due to their bitterness. These terpenes could however be obtained from the chicory root as a secondary product via a procedure compatible with inulin processing, yielding both inulin and terpenes which can be used for e.g., health applications. Depending on the homogeneity and quality of the terpene fraction and on the application, this fraction might need further processing. Recently, a sustainable method of terpene extraction from chicory taproots using supercritical CO2 was established [14]. The socio-economic impacts of a multi-product process compared to a reference inulin process indicate that a multi-product process leads to higher added value and increased socio-economic impact compared to the reference inulin process [24]. Therefore, it is interesting to increase not only the inulin content of the roots by breeding, but also the terpene amounts. Genome editing can be used to facilitate the generation of chicory varieties that accumulate higher amounts of medicinal terpenes.
A first example is a chicory variety with an increased accumulation of costunolide [17], which is a natural STL from the Asteraceae family and exhibits anti-cancer activity through its functional moiety α-methylene-γ-butyrolactone that reacts with the cysteine sulfhydryl group of various proteins [38]. In chicory, costunolide is an intermediate in the biosynthesis of STLs and does not accumulate since it is efficiently converted to downstream STLs. To accumulate costunolide the kauniolide synthase (CiKLS) that converts costunolide to kauniolide, needs to be inactivated. Genome editing of the three CiKLS paralogs in chicory was performed by transient transfection of both plasmids and RNPs to chicory protoplasts, both leading to small indel mutations but only the former sometimes to unintended DNA integration. As a result the biosynthesis of the chicory sesquiterpenes was interrupted and costunolide accumulation was observed [17]. Interestingly, next to free costunolide predominantly costunolide conjugates were found to be accumulating, presumably mitigating the toxicity of costunolide to chicory cells.
A second example of chicory varieties with increased medicinal compounds are chicory lines with increased amounts of anti-inflammatory 8-deoxylactucin and its derivatives [18]. 8-deoxylactucin is a natural STL of chicory that accumulates in chicory taproots next to lactucin and lactucopicrin, predominantly in oxalated form [10]. To increase 8-deoxylactucin accumulation in the taproots the gene encoding the lactucin synthase (CiLCS) that hydroxylates 8-deoxylactucin on position 8 to generate lactucin needs to be inactivated. Genome editing was used to inactivate three candidate cytochrome P450 genes with putative LCS activity resulting in the identification of one of the genes as CiLCS and generation of chicory lines that predominantly accumulate 8-deoxylactucin and its derivatives [18].
These two examples show that genome editing can be successfully applied in chicory to inactivate single genes or gene families, elucidating enzyme function and resulting in novel terpene chemotypes. In both examples the target medicinal STLs in genome edited roots accumulated to the similar high amounts of total STLs as found in wild type roots, namely about 1.5 mg/g FW. The availability of the chicory lines with modified terpene composition can also be used to generate more knowledge on the natural role of terpenes in chicory development and defence.
5 Potential of Genome Editing for Chicory Breeding
Chicory is very amenable to genome editing. Highly efficient protocols have recently been developed in different laboratories. Using these protocols scientific knowledge on chicory biology, particularly of the biosynthesis of secondary metabolites was increased. Implementation of these protocols resulted in generation of multiple chicory lines with altered terpene profiles. These lines could have potential benefits for consumers (healthy terpenes, varieties with altered taste), food producers (lack of bitter compounds simplifies inulin extraction or alternatively, bitter terpenes currently discarded as waste can be used as secondary product), farmers (agricultural diversification), breeders (creation of new cultivars with added value), the environment (reduction in energy demand and greenhouse gas emissions due to avoiding bitter compound extraction), and the economy (higher added value and more jobs).
Using the now available genome editing protocols as breeding tool, also other important biological processes for Cichorium breeding, e.g., male sterility, haploid induction, inulin quality and yield, etc., can be investigated and used to facilitate the long breeding process and generate novel cultivars in relatively shorter time frame with commercial interesting traits related to e.g., root shape, root weight, inulin chain length, disease resistance, bolting resistance, etc. In a broader perspective, genome editing can be a powerful tool to help to stimulate agricultural biodiversity in Europe by improving niche crops, like chicory, which have relatively little investment leverage. In synergism with other breeding and farming methods, this is highly relevant for securing crop diversity and improving sustainable production while at the same time dealing with the challenges like that of climate change.
References
Wilson, R.G., Smith, J.A., Yonts, C.D.: Chicory root yield and carbohydrate composition is influenced by cultivar selection, planting, and harvest date. Crop Sci. 44(3), 748–752 (2004)
FAOstat Chicory root production area and yield in 2017 in Food and Agriculture Organization of the United Nations database Downloaded on 1st April 2022. Available at: https://www.fao.org/faostat/en/#data/QCL. (2017)
Roberfroid, M.B.: Inulin-type fructans: functional food ingredients. J. Nutr. 137(11 Suppl), 2493S–2502S (2007)
Puhlmann, M.L., de Vos, W.M.: Back to the roots: revisiting the use of the fiber-rich Cichorium intybus L. taproots. Adv. Nutr. 12(4), 1598–1598 (2021)
Chadwick, M., Trewin, H., Gawthrop, F., Wagstaff, C.: Sesquiterpenoids lactones: benefits to plants and people. Int. J. Mol. Sci. 14(6), 12780–12805 (2013)
Prasifka, J.R., Spring, O., Conrad, J., Cook, L.W., Palmquist, D.E., Foley, M.E.: Sesquiterpene lactone composition of wild and cultivated sunflowers and biological activity against an insect pest. J. Agric. Food Chem. 63(16), 4042–4049 (2015)
Huber, M., Epping, J., Schulze Gronover, C., Fricke, J., Aziz, Z., Brillatz, T., Swyers, M., Kollner, T.G., Vogel, H., Hammerbacher, A., Triebwasser-Freese, D., Robert, C.A., Verhoeven, K., Preite, V., Gershenzon, J., Erb, M.: A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol. 14(1), e1002332 (2016)
Padilla-Gonzalez, G.F., dos Santos, F.A., Da Costa, F.B.: Sesquiterpene lactones: more than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 35(1), 18–37 (2016)
de Kraker, J.W., Franssen, M.C., de Groot, A., Konig, W.A., Bouwmeester, H.J.: (+)-Germacrene A biosynthesis. The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol. 117(4), 1381–1392 (1998)
Sessa, R.A., Bennett, M.H., Lewis, M.J., Mansfield, J.W., Beale, M.H.: Metabolite profiling of sesquiterpene lactones from Lactuca species. Major latex components are novel oxalate and sulfate conjugates of lactucin and its derivatives. J. Biol. Chem. 275(35), 26877–26884 (2000)
Wesolowska, A., Nikiforuk, A., Michalska, K., Kisiel, W., Chojnacka-Wojcik, E.: Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 107(2), 254–258 (2006)
Bischoff, T.A., Kelley, C.J., Karchesy, Y., Laurantos, M., Nguyen-Dinh, P., Arefi, A.G.: Antimalarial activity of lactucin and lactucopicrin: sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 95(2–3), 455–457 (2004)
Hakkinen, S.T., Sokovic, M., Nohynek, L., Ciric, A., Ivanov, M., Stojkovic, D., Tsitko, I., Matos, M., Baixinho, J.P., Ivasiv, V., Fernandez, N., Nunes Dos Santos, C., Oksman-Caldentey, K.M.: Chicory extracts and sesquiterpene lactones show potent activity against bacterial and fungal pathogens. Pharmaceuticals (Basel). 14(9), 941 (2021)
Baixinho, J.P., Anastacio, J.D., Ivasiv, V., Cankar, K., Bosch, D., Menezes, R., de Roode, M., Santos, C.N.D., Matias, A.A., Fernandez, N.: Supercritical CO2 extraction as a tool to isolate anti-inflammatory sesquiterpene lactones from Cichorium intybus L. roots. Molecules. 26(9), 2583 (2021)
Matos, M.S., Anastacio, J.D., Allwood, J.W., Carregosa, D., Marques, D., Sungurtas, J., McDougall, G.J., Menezes, R., Matias, A.A., Stewart, D., Santos, C.N.D.: Assessing the intestinal permeability and anti-inflammatory potential of sesquiterpene lactones from chicory. Nutrients. 12(11), 3547 (2020)
Bouwmeester, H.J., Kodde, J., Verstappen, F.W., Altug, I.G., de Kraker, J.W., Wallaart, T.E.: Isolation and characterization of two germacrene A synthase cDNA clones from chicory. Plant Physiol. 129(1), 134–144 (2002)
Cankar, K., Hakkert, J.C., Sevenier, R., Campo, E., Schipper, B., Papastolopoulou, C., Vahabi, K., Tissier, A., Bundock, P., Bosch, D.: CRISPR/Cas9 targeted inactivation of the kauniolide synthase in chicory results in accumulation of costunolide and its conjugates in taproots. Front. Plant Sci. 13, 940003 (2022)
Cankar, K., Hakkert, J.C., Sevenier, R., Papastolopoulou, C., Schipper, B., Baixinho, J.P., Fernandez, N., Matos, M.S., Serra, A.T., Santos, C.N., Vahabi, K., Tissier, A., Bundock, P., Bosch, D.: Lactucin synthase inactivation boosts the accumulation of anti-inflammatory 8-deoxylactucin and its derivatives in chicory (Cichorium intybus L.). J. Agric. Food Chem. 71(15), 6061–6072 (2023)
Liu, Q., Majdi, M., Cankar, K., Goedbloed, M., Charnikhova, T., Verstappen, F.W., de Vos, R.C., Beekwilder, J., van der Krol, S., Bouwmeester, H.J.: Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS One. 6(8), e23255 (2011)
Nguyen, D.T., Gopfert, J.C., Ikezawa, N., Macnevin, G., Kathiresan, M., Conrad, J., Spring, O., Ro, D.K.: Biochemical conservation and evolution of germacrene A oxidase in Asteraceae. J. Biol. Chem. 285(22), 16588–16598 (2010)
De Bruyn, C., Ruttink, T., Eeckhaut, T., Jacobs, T., De Keyser, E., Goosens, A., Van Laere, K.: Establishment of CRISPR/Cas9 genome editing in witloof (Cichorium intybus var. foliosum). Front. Genome Ed. 2, 604876 (2020)
De Bruyn, C.: Unravelling of the sesquiterpene lactone biosynthetic pathway in Cichorium Intybus using CRISPR/Cas9 genome editing. Ghent University, Faculty of Sciences, Belgium (2022)
De Bruyn, C., Ruttink, T., Lacchini, E., Rombouts, S., Haegeman, A., De Keyser, E., Van Poucke, C., Desmet, S., Jacobs, T.B., Eeckhaut, T., Goosens, A., Van Laere, K.: Identification and characterization of CYP71 subclade cytochrome P450 enzymes involved in the biosynthesis of bitterness compounds in Cichorium intybus. Front. Plant Sci. 14, 1200253 (2023)
Hingsamer, M., Kulmer, V., de Roode, M., Kernitzkyi, M.: Environmental and socio-economic impacts of new plant breeding technologies: a case study of root chicory for inulin production. Front. Genome Ed. 4, 919392 (2022)
de Nettancourt, D.: The genetics of self-incompatibility. In: Incompatibility and Incongruity in Wild and Cultivated Plants. Springer, Berlin, Heidelberg (2001)
Lucchin, M., Varotto, S., Barcaccia, G.: Vegetables I: Asteraceae, brassicaceae, chenopodicaceae and cucurbitaceae. In: Handbook of Plant Breeding. Springer, New York (2008)
Bernard, G., Gagneul, D., Alves Dos Santos, H., Etienne, A., Hilbert, J.L., Rambaud, C.: Efficient genome editing using CRISPR/Cas9 technology in chicory. Int. J. Mol. Sci. 20(5), 1155 (2019)
Bernard, G., Buges, J., Delporte, M., Molinie, R., Besseau, S., Bouchereau, A., Watrin, A., Fontaine, J.X., Mathiron, D., Berardocco, S., Bassard, S., Quero, A., Hilbert, J.L., Rambaud, C., Gagneul, D.: Consecutive action of two BAHD acyltransferases promotes tetracoumaroyl spermine accumulation in chicory. Plant Physiol. 189(4), 2029–2043 (2022)
Salvagnin, U., Unkel, K., Sprink, T., Bundock, P., Sevenier, R., Bogdanovic, M., Todorovic, S., Cankar, K., Hakkert, J.C., Schijlen, E., Nieuwenhuis, R., Hingsamer, M., Kulmer, V., Kernitzkyi, M., Bosch, D., Martens, S., Malnoy, M.: A comparison of three different delivery methods for achieving CRISPR/Cas9 mediated genome editing in Cichorium intybus L. Front. Plant Sci. 14, 1111110 (2023)
Cankar, K., Bundock, P., Sevenier, R., Hakkinen, S.T., Hakkert, J.C., Beekwilder, J., van der Meer, I.M., de Both, M., Bosch, D.: Inactivation of the germacrene A synthase genes by CRISPR/Cas9 eliminates the biosynthesis of sesquiterpene lactones in Cichorium intybus L. Plant Biotechnol. J. 19(12), 2442–2453 (2021)
Foster, J.G., Cassida, K.A., Sanderson, M.A.: Seasonal variation in sesquiterpene lactone concentration and composition of forage chicory (Cichorium intybus L.) cultivars. Grass Forage Sci. 66(3), 424–433 (2011)
Testone, G., Mele, G., Di Giacomo, E., Gonnella, M., Renna, M., Tenore, G.C., Nicolodi, C., Frugis, G., Iannelli, M.A., Amesi, G., Schiappa, A., Giannino, D.: Insights into the sesquiterpenoid pathway by metabolic profiling and de novo transcriptome assembly of stem-chicory (Cichorium intybus cultigroup “Catalogna”). Front. Plant Sci. 7, 1676 (2016)
Bogdanović, M., Cankar, K., Todorović, S., Dragicević, M., Simonović, A., van Houwelingen, A., Schijlen, E., Schipper, B., Gagneul, D., Hendriks, T., Quillet, M., Bouwmeester, H., Bosch, D., Beekwilder, J.: Tissue specific expression and genomic organization of bitter sesquiterpene lactone biosynthesis in Cichorium intybus L. (Asteraceae). Ind. Crop. Prod. 129, 253–260 (2019)
Kips, L.: Characterization and processing of horticultural byproducts: a case study of tomato and Belgian endive roots. Ghent University, Faculty of Bioscience Engineering, Belgium (2017)
Francois, I.M., Wins, H., Buysens, S., Godts, C., Van Pee, E., Nicolai, B., De Proft, M.: Predicting sensory attributes of different chicory hybrids using physico-chemical measurements and visible/near infrared spectroscopy. Postharvest Biol. Technol. 49(3), 366–373 (2008)
D’Antuono, L.F., Ferioli, F., Manco, M.A.: The impact of sesquiterpene lactones and phenolics on sensory attributes: an investigation of a curly endive and escarole germplasm collection. Food Chem. 199, 238–245 (2016)
Yanagisawa, T., Misaka, T.: Characterization of the human bitter taste receptor response to sesquiterpene lactones from edible Asteraceae species and suppression of bitterness through pH control. Acs Omega. 6(6), 4401–4407 (2021)
Li, Q., Wang, Z., Xie, Y., Hu, H.: Antitumor activity and mechanism of costunolide and dehydrocostus lactone: two natural sesquiterpene lactones from the Asteraceae family. Biomed. Pharmacother. 125, 109955 (2020)
Acknowledgement
This work has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No. 760891 [H2020-NMBP-BIOTEC-07-2017: New Plant Breeding Techniques (NPBT) in molecular farming: Multipurpose crops for industrial bioproducts]. The authors want to thank all researchers and technicians that contributed to this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Cankar, K., Van Laere, K., Bosch, D. (2024). Genome Editing for Reduction of Bitterness and for Production of Medicinal Terpenes in Cichorium Species. In: Ricroch, A., Eriksson, D., Miladinović, D., Sweet, J., Van Laere, K., Woźniak-Gientka, E. (eds) A Roadmap for Plant Genome Editing . Springer, Cham. https://doi.org/10.1007/978-3-031-46150-7_21
Download citation
DOI: https://doi.org/10.1007/978-3-031-46150-7_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-46149-1
Online ISBN: 978-3-031-46150-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)