Abstract.
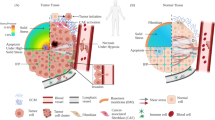
Mechanical forces, generated externally and internally, regulate cancer development. Cancer continues to be a disease that is difficult to be healed despite medical and scientific advancement. Advances in knowledge of the mechanical properties have led to new insights in cancer mechanobiology. This review is focused on four main directions: the physical forces found in the in vivo microenvironment, function of cellular and of tissue stiffness in cancer progression, and beneficial effects of physical exercise on cancer suppression. Physical forces in vivo include stretching, compression, shear stress, tension, contraction and relaxation. Due to the loss of contractile units such as tropomyosin 2.1 (Tpm 2.1), cancer cells are unable to transduce the physical force from the extracellular matrix (ECM), through ECM receptors such as β1 integrin, to cytoskeletal proteins like actomyosin. This results in reduced stiffness and loss of rigidity sensing ability in cancer cells, leading to uncontrolled cell proliferation. In order to reestablish homeostasis, cancer cells continue to grow, secrete growth factors, cytokines, and deposit ECM. Cancer-associated fibroblasts (CAFs) assist in ECM secretion and remodeling. On the one hand, the cancer tissue is being remodeled to a tumor, on the other hand, the remodeled tumor tissue also enhances the invasiveness of cancer cells. In summary, tumor development may be viewed as a process that reflects the mechanism employed in wound healing. This review emphasizes the role of physical forces that help shape tumor growth.
Article type: Review Article (Topic on Mechanobiology in Human Disease).
Similar content being viewed by others
References
Ono T, Higashihara A, Shinohara J, Hirose N, Fukubayashi T (2015) Estimation of tensile force in the hamstring muscles during overground sprinting. Int J Sports Med 36:163–168
Kohrt WM, Barry DW, Schwartz RS (2009) Muscle forces or gravity: what predominates mechanical loading on bone? Med Sci Sports Exerc 41:2050–2055
Poole K (2022) The diverse physiological functions of mechanically activated ion channels in mammals. Annu Rev Physiol 84:307–329
Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S et al (2006) Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol 85:165–173
Wolff MR, McDonald KS, Moss RL (1995) Rate of tension development in cardiac muscle varies with level of activator calcium. Circ Res 76:154–160
Liu S, Hur YH, Cai X, Cong Q, Yang Y, Xu C et al (2023) A tissue injury sensing and repair pathway distinct from host pathogen defense. Cell 186:2127–43.e22
Venables T, Griffith AV, DeAraujo A, Petrie HT (2019) Dynamic changes in epithelial cell morphology control thymic organ size during atrophy and regeneration. Nat Commun 10:4402
Schiaffino S (2017) Losing pieces without disintegrating: contractile protein loss during muscle atrophy. Proc Natl Acad Sci U S A 114:1753–1755
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z (2020) Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11:5120
Tijore A, Yao M, Wang YH, Hariharan A, Nematbakhsh Y, Lee Doss B et al (2021) Selective killing of transformed cells by mechanical stretch. Biomaterials 275:120866
Lin HH, Lin HK, Lin IH, Chiou YW, Chen HW, Liu CY et al (2015) Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget 6:20946–20958
Lekka M (2016) Discrimination between normal and cancerous cells using AFM. BioNanoScience 6:65–80
Cooper GM (2000) The cell: a molecular approach, 2nd edn. Sinauer Associates Inc. https://www.ncbi.nlm.nih.gov/books/NBK9894/
Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H et al (2022) Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell 40:818–34.e9
Wicks EE, Semenza GL (2022) Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest 132:e159839
Lunt SJ, Chaudary N, Hill RP (2009) The tumor microenvironment and metastatic disease. Clin Exp Metastasis 26:19–34
Kim TH, Gill NK, Nyberg KD, Nguyen AV, Hohlbauch SV, Geisse NA et al (2016) Cancer cells become less deformable and more invasive with activation of β-adrenergic signaling. J Cell Sci 129:4563–4575
Pon CK, Lane JR, Sloan EK, Halls ML (2016) The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J 30:1144–1154
Fjæstad KY, Rømer AMA, Goitea V, Johansen AZ, Thorseth M-L, Carretta M et al (2022) Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 41:1364–1375
Suresh S, O’Donnell KA (2021) Translational control of immune evasion in cancer. Trends Cancer 7:580–582
Kraning-Rush CM, Califano JP, Reinhart-King CA (2012) Cellular traction stresses increase with increasing metastatic potential. PLoS One 7:e32572
Jain RK, Martin JD, Stylianopoulos T (2014) The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 16:321–346
Natarajan S, Foreman KM, Soriano MI (2019) Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res 79:2271–2284
Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, Wirtz D et al (2013) Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res: MCR 11:456–466
Reid SE, Kay EJ, Neilson LJ, Henze AT, Serneels J, McGhee EJ et al (2017) Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J 36:2373–2389
Bauer J, Emon MAB, Staudacher JJ, Thomas AL, Zessner-Spitzenberg J, Mancinelli G et al (2020) Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci Rep 10:50
Seyfried TN, Huysentruyt LC (2013) On the origin of cancer metastasis. Crit Rev Oncog 18:43–73
Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM (1988) Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol 133:95–109
Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77:1745–1770
Henke E, Nandigama R, Ergün S (2019) Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 6:160
Wong P-P, Bodrug N, Hodivala-Dilke Kairbaan M (2016) Exploring novel methods for modulating tumor blood vessels in cancer treatment. Curr Biol 26:R1161–R11R6
Mittelstein DR, Ye J, Schibber EF, Roychoudhury A, Martinez LT, Fekrazad MH et al (2020) Selective ablation of cancer cells with low intensity pulsed ultrasound. Appl Phys Lett 116(1):013701. https://doi.org/10.1063/1.5128627
Tijore A, Margadant F, Yao M, Hariharan A, Chew CAZ, Powell S et al Ultrasound-mediated mechanical forces selectively kill tumor cells. bioRxiv 2020:2020.10.09.332726
Roberts WC (1997) Primary and secondary neoplasms of the heart. Am J Cardiol 80:671–682
Castillo JG, Silvay G (2010) Characterization and management of cardiac tumors. Semin Cardiothorac Vasc Anesth 14:6–20
Barnes JM, Przybyla L (2017) Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci 130:71–82
Abuhattum S, Kotzbeck P, Schlüßler R, Harger A, Ariza de Schellenberger A, Kim K et al (2022) Adipose cells and tissues soften with lipid accumulation while in diabetes adipose tissue stiffens. Sci Rep 12:10325
Gaetani R, Zizzi EA, Deriu MA, Morbiducci U, Pesce M, Messina E (2020) When stiffness matters: mechanosensing in heart development and disease. Front Cell Dev Biol 8:334
Kaushik G, Spenlehauer A, Sessions AO, Trujillo AS, Fuhrmann A, Fu Z et al (2015) Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci Transl Med 7:292ra99
Chapman MA, Pichika R, Lieber RL (2015) Collagen crosslinking does not dictate stiffness in a transgenic mouse model of skeletal muscle fibrosis. J Biomech 48:375–378
Rubio-Peirotén A, García-Pinillos F (2021) Relationship between connective tissue morphology and lower-limb stiffness in endurance runners. A prospective study. Int J Environ Res Public Health 18:8453
Ward M, Smart D, Roberts J, Monk P, Baugh JA, Jones MG et al (2019) TGFß1 induces collagen deposition and increased tissue stiffness in sarcoidosis fibroblasts grown in a novel, long term, 3D culture system. Eur Respir J 54:PA591
Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D et al (2015) Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun 6:8026
Barcus CE, O’Leary KA, Brockman JL, Rugowski DE, Liu Y, Garcia N et al (2017) Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res: BCR 19:9
Erdogan B, Webb DJ (2017) Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans 45:229–236
Nair N, Calle AS, Zahra MH, Prieto-Vila M, Oo AKK, Hurley L et al (2017) A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci Rep 7:6838
Miyazaki Y, Oda T, Mori N, Kida YS (2020) Adipose-derived mesenchymal stem cells differentiate into pancreatic cancer-associated fibroblasts in vitro. FEBS Open Bio 10:2268–2281
Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M et al (2017) Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214:579–596
Buchsbaum RJ, Oh SY (2016) Breast cancer-associated fibroblasts: where we are and where we need to go. Cancers 8:19
Yang B, Wolfenson H (2020) Stopping transformed cancer cell growth by rigidity sensing. Nat Mater 19:239–250
Downward J (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3:11–22
Lee CS, Siprashvili Z, Mah A, Bencomo T, Elcavage LE, Che Y et al (2021) Mutant collagen COL11A1 enhances cancerous invasion. Oncogene 40:6299–6307
Higgins G, Kim JE, Ferruzzi J, Abdalrahman T, Franz T, Zaman MH. Decreased cell stiffness facilitates detachment and migration of breast cancer cells in 3D collagen matrices: An exploratory study. bioRxiv 2021:2021.01.21.427639
Moreno-Vicente R, Pavón DM, Martín-Padura I, Català-Montoro M, Díez-Sánchez A, Quílez-Álvarez A et al (2018) Caveolin-1 modulates mechanotransduction responses to substrate stiffness through actin-dependent control of YAP. Cell Rep 25:1622–35.e6
Díaz MI, Díaz P, Bennett JC, Urra H, Ortiz R, Orellana PC et al (2020) Caveolin-1 suppresses tumor formation through the inhibition of the unfolded protein response. Cell Death Dis 11:648
Lu Z, Ghosh S, Wang Z, Hunter T (2003) Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4:499–515
Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E et al (2011) Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146:148–163
Hadjisavva R, Anastasiou O, Ioannou PS, Zheltkova M, Skourides PA (2022) Adherens junctions stimulate and spatially guide integrin activation and extracellular matrix deposition. Cell Rep 40:111091
Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, Pribyl J, Skládal P et al (2017) YAP regulates cell mechanics by controlling focal adhesion assembly. Nat Commun 8:15321
Hamidi H, Ivaska J (2018) Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 18:533–548
Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE (2008) The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res 68:7711–7717
Rens EG, Merks RMH (2020) Cell shape and durotaxis explained from cell-extracellular matrix forces and focal adhesion dynamics. iScience 23:101488
Weidenhamer NK, Tranquillo RT (2013) Influence of cyclic mechanical stretch and tissue constraints on cellular and collagen alignment in fibroblast-derived cell sheets. Tissue Eng Part C Methods 19:386–395
Chen L-K, Hsieh C-C, Huang Y-C, Huang Y-J, Lung C-F, Hsu W-E et al (2023) Mechanical stretch promotes invasion of lung cancer cells via activation of tumor necrosis factor-alpha. Biotechnol Bioprocess Eng 28:467–472
Tavian D, De Petro G, Colombi M, Portolani N, Giulini SM, Gardella R et al (1994) RT-PCR detection of fibronectin EDA+ and EDB+ mRNA isoforms: molecular markers for hepatocellular carcinoma. Int J Cancer 56:820–825
Kawamura S, Miyamoto S, Brown JH (2003) Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem 278:31111–31117
Kook SH, Lee HJ, Chung WT, Hwang IH, Lee SA, Kim BS et al (2008) Cyclic mechanical stretch stimulates the proliferation of C2C12 myoblasts and inhibits their differentiation via prolonged activation of p38 MAPK. Mol Cells 25:479–486
Liu N, Zhou M, Zhang Q, Yong L, Zhang T, Tian T et al (2018) Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif 51:e12478
Mousavi SJ, Hamdy DM (2015) Role of mechanical cues in cell differentiation and proliferation: a 3D numerical model. PLoS One 10:e0124529
Kippenberger S, Loitsch S, Guschel M, Müller J, Knies Y, Kaufmann R et al (2005) Mechanical stretch stimulates protein kinase B/Akt phosphorylation in epidermal cells via angiotensin II type 1 receptor and epidermal growth factor receptor*. J Biol Chem 280:3060–3067
Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A (2003) Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112:1486–1494
Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM et al (2014) Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A 111:10347–10352
Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT et al (2014) Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A 111:16148–16153
Bavi N, Richardson J, Heu C, Martinac B, Poole K (2019) PIEZO1-mediated currents are modulated by substrate mechanics. ACS Nano 13:13545–13559
Zhang S, Cao S, Gong M, Zhang W, Zhang W, Zhu Z et al (2022) Mechanically activated ion channel Piezo1 contributes to melanoma malignant progression through AKT/mTOR signaling. Cancer Biol Ther 23:336–347
Yu Y, Wu X, Liu S, Zhao H, Li B, Zhao H et al (2021) Piezo1 regulates migration and invasion of breast cancer cells via modulating cell mechanobiological properties. Acta Biochim Biophys Sin 53:10–18
Pickup MW, Mouw JK, Weaver VM (2014) The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15:1243–1253
Trinh MK, Pacyna CN, Kildisiute G, Thevanesan C, Piapi A, Ambridge K et al (2022) Precise identification of cancer cells from allelic imbalances in single cell transcriptomes. Commun Biol 5:884
Berger AJ, Renner CM, Hale I, Yang X, Ponik SM, Weisman PS et al (2020) Scaffold stiffness influences breast cancer cell invasion via EGFR-linked Mena upregulation and matrix remodeling. Matrix Biol 85–86:80–93
Wang YH, Chiu WT, Wang YK, Wu CC, Chen TL, Teng CF et al (2007) Deregulation of AP-1 proteins in collagen gel-induced epithelial cell apoptosis mediated by low substratum rigidity. J Biol Chem 282:752–763
Tang M-J, Hu J-J, Lin H-H, Chiu W-T, Jiang S-T (1998) Collagen gel overlay induces apoptosis of polarized cells in cultures: disoriented cell death. Am J Phys Cell Phys 275:C921–CC31
Harn HI, Ogawa R, Hsu CK, Hughes MW, Tang MJ, Chuong CM (2019) The tension biology of wound healing. Exp Dermatol 28:464–471
Harn HI, Chiu PY, Lin CH, Chen HY, Lai YC, Yang FS et al (2022) Topological distribution of wound stiffness modulates wound-induced hair follicle neogenesis. Pharmaceutics 14:1926
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
Li J, Burgess DJ (2020) Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm Sin B 10:2110–2124
Yardley DA (2013) Nab-paclitaxel mechanisms of action and delivery. J Control Release 170:365–372
Ventola CL (2017) Progress in nanomedicine: approved and investigational nanodrugs. P & T : a peer-reviewed journal for formulary management 42:742–755
Wigerup C, Påhlman S, Bexell D (2016) Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther 164:152–169
Romero-Garcia S, Lopez-Gonzalez JS, Báez-Viveros JL, Aguilar-Cazares D, Prado-Garcia H (2011) Tumor cell metabolism: an integral view. Cancer Biol Ther 12:939–948
Heller G, McCormack R, Kheoh T, Molina A, Smith MR, Dreicer R et al (2018) Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol 36:572–580
Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q et al (2021) Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther 6:404
Pavel M, Renna M, Park SJ, Menzies FM, Ricketts T, Füllgrabe J et al (2018) Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat Commun 9:2961
Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J et al (2015) Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol 35 Suppl:S25–s54
Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG et al (2017) Lactate metabolism in human lung tumors. Cell 171:358–71.e9
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41:211–218
Fadaka A, Ajiboye B, Ojo O, Adewale O, Olayide I, Emuowhochere R (2017) Biology of glucose metabolization in cancer cells. J Oncol Sci 3:45–51
Deng H, Chen Y, Li P, Hang Q, Zhang P, Jin Y et al (2023) PI3K/AKT/mTOR pathway, hypoxia, and glucose metabolism: potential targets to overcome radioresistance in small cell lung cancer. Cancer Pathog Ther 1:56–66
Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK et al (2012) Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci 109:911–916
Wullkopf L, West AV, Leijnse N, Cox TR, Madsen CD, Oddershede LB et al (2018) Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol Biol Cell 29:2378–2385
Liu C, Wu P, Zhang A, Mao X (2021) Advances in rodent models for breast cancer formation, progression, and therapeutic testing. Front Oncol 11:593337
Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ et al (2000) The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19:968–988
Zeng L, Li W, Chen CS (2020) Breast cancer animal models and applications. Zool Res 41:477–494
Berrueta L, Bergholz J, Munoz D, Muskaj I, Badger GJ, Shukla A et al (2018) Stretching reduces tumor growth in a mouse breast cancer model. Sci Rep 8:7864
Song Y, Chen J, Zhang C, Xin L, Li Q, Liu Y et al (2022) Mechanosensitive channel Piezo1 induces cell apoptosis in pancreatic cancer by ultrasound with microbubbles. iScience 25:103733
Zimmerman JW, Pennison MJ, Brezovich I, Yi N, Yang CT, Ramaker R et al (2012) Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer 106:307–313
Autenrieth CS, Baumert J, Baumeister SE, Fischer B, Peters A, Döring A et al (2011) Association between domains of physical activity and all-cause, cardiovascular and cancer mortality. Eur J Epidemiol 26:91–99
Park JH, Moon JH, Kim HJ, Kong MH, Oh YH (2020) Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med 41:365–373
Inoue M, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S (2008) Daily total physical activity level and total cancer risk in men and women: results from a large-scale population-based cohort study in Japan. Am J Epidemiol 168:391–403
Orsini N, Mantzoros CS, Wolk A (2008) Association of physical activity with cancer incidence, mortality, and survival: a population-based study of men. Br J Cancer 98:1864–1869
Wennerberg E, Lhuillier C, Rybstein MD, Dannenberg K, Rudqvist NP, Koelwyn GJ et al (2020) Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 11:452–461
McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D et al (2019) Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc 51:1252–1261
Emery A, Moore S, Turner JE, Campbell JP (2022) Reframing how physical activity reduces the incidence of clinically-diagnosed cancers: appraising exercise-induced immuno-modulation as an integral mechanism. Front Oncol 12:788113
Koevoets EW, Schagen SB, de Ruiter MB, Geerlings MI, Witlox L, van der Wall E et al (2022) Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomized controlled trial (PAM study). Breast Cancer Res 24:36
Ren X, Wang X, Sun J, Hui Z, Lei S, Wang C et al (2022) Effects of physical exercise on cognitive function of breast cancer survivors receiving chemotherapy: a systematic review of randomized controlled trials. Breast 63:113–122
Acknowledgements.
Special thanks to Professor Ming-Jer Tang for funding and resources assistance, submission of an abstract to the 4th ISMB would not be possible without his assistance. Sincere thanks to the International Center for Wound Repair and Regeneration at National Cheng Kung University for the laboratory resources.
Conflict of Interests.
The authors in this article have declared that there are no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Lee, CT., Hu, CS., Wong, T.Y. (2024). The Physical Factors Involved in Cancer Progression. In: Martinac, B., Cox, C.D., Poole, K., Baratchi, S., Kempe, D. (eds) Mechanobiology. ISMB 2022. Springer Series in Biophysics, vol 25. Springer, Cham. https://doi.org/10.1007/978-3-031-45379-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-45379-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-53904-6
Online ISBN: 978-3-031-45379-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)