Abstract
Both relative (due to vasodilatation and leaky capillaries) and absolute hypovolemia are common in patients with septic shock, and fluid infusion remains the first-line resuscitation measure. However, fluid overload is a potential consequence of fluid infusion, especially left unmonitored. Early vasopressor infusion may be useful in specific situation. Every effort should be made to limit cumulative fluid balance in every stage of resuscitation.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- EROS concept for septic shock management
- Fluid boluses
- Fluid responsiveness
- Early vasopressors
- Limit cumulative balance
Sepsis is a life-threatening organ dysfunction caused by a maladaptive and dysregulated host response to an infection in the bloodstream. That infection can be a virus (e.g., COVID), a bacteria, or a fungus. Without prompt recognition and adequate intervention, this can lead to septic shock, in which several organs usually fail simultaneously, resulting in death. It is therefore crucial to be alert from the first subtle clinical signs and symptoms. According to the World Health Organization, about 50 million people suffer from sepsis each year, resulting in 11 million deaths. Sepsis is responsible for as much as 20% of all deaths worldwide. This means that every 2.8 s someone dies from sepsis. However, as we speak, sepsis is poorly dealt with in many countries and education and training of healthcare workers can be substantially improved.

Proportion (in %) of departments (emergency room, general ward, or intensive care unit) with a standardized protocol or screening tool in place specifically for the (early) recognition or detection of sepsis. Adapted from the International Fluid Academy presentation by Scheer C. “Preliminary results of a global survey on sepsis acute care awareness with Christian Scheer” under the Open Access CC BY License 4.0 (video recording minute 11:10 https://whova.com/portal/ifad_202211/videos/3cTO2czN4YTM/).
Often, basic information and data are lacking regarding how many patients have had sepsis, where they were admitted, what the cause was, what treatment or organ support they received (mechanical ventilation, renal replacement therapy, etc.), and whether they died or not. Sepsis nevertheless has a huge impact on our society and the cost of healthcare. In addition, the physical and psychological complaints of sepsis survivors can drag on for a long time with an incremental cost and impact on work reintegration. Just to name a few, think of the post-intensive care syndrome and the recent emergence of long COVID.
Different quality improvement initiatives have been started via the Global and European Sepsis Alliance and the Surviving Sepsis Campaign. The introduction of sepsis stewardship is advocated in analogy with fluid and antimicrobial stewardship. Stewardship is a combination of coordinated actions whereby the right diagnosis is made and the correct treatment, medication, antibiotics, or fluids are administered to the right patient at the right time, in order to prevent complications and adverse effects, improve outcomes, and reduce costs. There are several early clinical signs that can point to sepsis and that can be used in early warning scores. Hospital staff, general practitioners, and even citizens should be trained to recognize them early. Depending on those scores, we can establish general and specific guidelines (for hospitals and primary care) on how best to intervene. Early recognition and prompt treatment of sepsis are essential to prevent permanent damage and save lives. The World Health Organization therefore passed a resolution in 2017 to urge governments to develop a national sepsis plan with guidelines for early diagnosis, treatment, and aftercare. Several European countries, including Germany, the Netherlands, and the United Kingdom, have already undertaken appropriate action by including measurable quality indicators on sepsis in healthcare. The guidelines of the European Society of Intensive Care Medicine include the guiding principle with five main points for immediate resuscitation and treatment in patients with sepsis and septic shock:
-
1.
Protocol-driven early recognition of sepsis and septic shock, with specific attention in case of immune depression or neutropenia. Measuring the amount of lactate in the blood (as a parameter for diagnosing sepsis and septic shock).
-
2.
Taking blood cultures (preferably at least two sets) before administering antibiotics to determine the source of bloodstream infections and sepsis.
-
3.
Immediately starting an IV for adequate fluid resuscitation up to 30 ml/kg.
-
4.
Early administration of vasoactive medication in patients with persistent septic shock despite fluid resuscitation.
-
5.
Administration of antibiotics based on local antibiotic guidelines.
Specifically for patients admitted to the emergency department with sepsis or septic shock, rapid administration of antibiotics (within 1 h in case of shock or within 3 h in other cases) is recommended as one of the important quality indicators, with the aim of treatment as short as possible.
Septic shock is a subset of sepsis in which underlying circulatory, cellular, and metabolic abnormalities are profound enough to substantially increase mortality [1]. In order to address the circulatory dysfunction, early aggressive fluid therapy has been one of the cornerstones in the treatment of septic shock using an early goal-directed therapy [2]. The revised Surviving Sepsis Campaign guidelines advocate the start of 30 ml/kg of IV fluid within the first hour [3]. However, adequate fluid management in sepsis requires a thoughtful approach. While early aggressive fluid therapy is generally required in the very early phases of patients with profound shock, one should also be aware of the risks of overzealous administration of large volumes of intravenous fluids as the body of evidence has grown that positive daily and cumulative fluid balances during ICU stay increase morbidity and mortality [4]. Furthermore, studies have shown that the type of IV fluid given during resuscitation also has an impact on the patients’ outcome [5–7].
This chapter will discuss the pathophysiologic mechanisms of sepsis and the different diagnostic tools to assess volemic status, perfusion, and fluid responsiveness. It also emphasizes the importance of looking at fluids as drugs. A new framework is suggested, with the acronym EROS looking at early recognition, resuscitation, optimization, and source control. However, sepsis should be also seen within the overarching framework that can be divided in four distinct phases, each requiring a different fluid strategy: resuscitation, optimization, stabilization, and evacuation. Phase-by-phase guidance using this ROSE conceptual model is proposed. This led to two important concepts. The first concept being the fact that fluids should be considered as drugs. They come with indications, contraindications, and potential adverse effects. Similar to antibiotic stewardship, a more thoughtful administration of fluids is necessary, hence giving birth to the concept fluid stewardship. This addresses the importance of choosing the right fluid, applying the right dose, using it for the correct duration, and thinking timely about de-escalation. This concept is named the four D’s of fluid therapy referring to drug, dose, duration, and de-escalation [8].
Even more important, the second concept states that adequate fluid therapy during sepsis requires a different strategy depending on the phase of illness. The first phase is one of a more aggressive resuscitation to rescue the patient’s life; second, we need to optimize organ perfusion by more diligently titrating fluids. In a third phase, we aim at stabilizing the fluid balance to a neutral daily fluid balance, and in the final phase we try to evacuate the potentially accumulated fluids. Hence, the ROSE acronym has been proposed as a mnemonic for this conceptual model [8, 9].
-
Fluids in sepsis are drugs and should be treated accordingly with indications, contraindications, and adverse effects.
-
One should consider the four D’s of fluid therapy: drug, dose, duration, de-escalation.
-
We need to consider the four dynamic phases of fluid therapy in sepsis applying the ROSE conceptual model: resuscitation, optimization, stabilization, evacuation.
Suggested Reading
-
1.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315(8):801–10.
-
2.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001;345(19):1368–77.
-
3.
Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 2018;44(6):925–8.
-
4.
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006;34(2):344–53.
-
5.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 2012;367(2):124–34.
-
6.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358(2):125–39.
-
7.
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367(20):1901–11.
-
8.
Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care 2018;8(1):66.
-
9.
Mekeirele M, Vanhonacker D, Malbrain MLNG. Fluid Management in Sepsis. In: Prabhakar H, S Tandon, M., Kapoor, I., Mahajan, C. (eds) Transfusion practice in clinical neurosciences. Springer, Singapore. 2022. https://doi.org/10.1007/978-981-19-0954-2_20.
The learning objectives of this chapter are:
-
1.
To learn the basic pathophysiology and hemodynamic changes observed in septic shock.
-
2.
To get an insight about the steps towards diagnosis of septic shock.
-
3.
To learn about timing, choice, and dosing of resuscitation fluids in septic shock.
-
4.
To learn about the interaction between fluids and vasopressors and timing of initiation of vasopressors in septic shock.
-
5.
To understand goals of resuscitation in septic shock.
Mr. X, a 64-year-old male, was brought to ED with complaints of burning micturition and pain in the groin for the past two days. He is a known hypertensive and has history of coronary artery disease with poor LV function (LVEF ~ 45%). On examination, he is drowsy but easily arousable with HR of 110/min, blood pressure of 70/40 mmHg, RR of 20/min, and SpO2 of 96% on room air. His extremities are cold and clammy. The ED physician decides to infuse him with 500 ml of Ringer’s lactate solution. Even after fluid bolus, he continues to be dull, with HR of 104/min, BP of 88/48 mmHg, and SpO2 of 95%. Blood gas analysis shows metabolic acidosis with lactate value of 4 mmol/ liter. The ED physician calls you to assess the patient and discuss further resuscitation plan.
Questions
-
Q1: How do you plan to resuscitate this gentleman now?
Introduction
Sepsis and septic shock remain a common and potentially lethal entity among the critically ill adult patients requiring prompt recognition and management [1]. The new Sepsis-3 definition has defined sepsis as a life-threatening organ dysfunction due to dysregulated host response to infection [2]. Septic shock is defined as persisting hypotension requiring vasopressors to maintain a mean arterial pressure more than 65 mmHg or a lactate of more than 2 mmol/l despite adequate fluid resuscitation [2]. Key principles in the management of septic shock are early recognition, administration of adequate antimicrobial(s), source control, organ support, and early aggressive resuscitation. As recommended by international guidelines, resuscitation starts with rapid intravenous fluid bolus, followed by further fluid administration guided by physiological parameters [3]. However, an inevitable consequence of aggressive fluid resuscitation is fluid overload and its complications, especially when the resuscitation is not monitored carefully. In this chapter, we shall be discussing on key aspects of fluid resuscitation in septic shock, potential ways to limit fluid overload including timely initiation of vasopressors, and goals of septic shock resuscitation. This chapter will focus on adult patients, and more information on fluid therapy in children can be found in Chap. 20. The next chapters will discuss fluids in specific populations: heart failure (Chap. 15), trauma (Chap. 16), neurocritical care (Chap. 17), perioperative setting (Chap. 18), burns (Chap. 19), liver failure (Chap. 21), abdominal hypertension (Chap. 22), and COVID-19 (Chap. 26).
Septic Shock: Pathophysiology
The fundamental features of septic shock include vasodilation, relative and true hypovolemia, increased permeability, and myocardial dysfunction. Vasodilatation in septic shock is due to underlying inflammatory state compounded by decreased responsiveness to natural catecholamines and relative deficiency of vasopressin [4, 5]. Profound vasodilation leads to decreased stress volume and lower mean systemic filling pressure, in turn leading to a decrease in venous return, cardiac output, and arterial pressure. In some patients, true hypovolemia may worsen the scenario, e.g., patients with abdominal sepsis and GI losses.
Increased capillary permeability is a hallmark feature in nearly all patients of sepsis. In response to infection, there is activation of neutrophils, release of a large number of inflammatory mediators, reactive oxygen species, and activation of coagulation pathways [6]. All these lead to endothelial dysfunction and an increase in vascular permeability [7]. Aggressive fluid resuscitation may compound this vascular permeability further by producing an increase in capillary transmural hydrostatic pressure worsening interstitial edema and organ dysfunction [8]. This explains why patients with sepsis and high fluid balances have worse outcomes [9].
An increasingly recognized entity that contributes to the pathophysiology of septic shock is septic cardiomyopathy, a condition characterized by transient decrease in left ventricular contractility with normal filling pressure. Incidence of septic cardiomyopathy widely varies in the literature from 10% to as high as 70% and reflects lack of standardized definition as well as variations in the patient population studies. [10] Right ventricular dysfunction can complicate septic shock further. It is characterized by poor right ventricular contractility with reduction in tricuspid annular plane systolic excursion (TAPSE) and dilatation of right ventricle observed in echocardiography. Using a definition of RV fractional area change (FAC) <35% or TAPSE <1.6 cm, a recent study has reported incidence of right ventricular dysfunction in septic shock as 48%.[11] Reduction in cardiac output resulting from left and right ventricular dysfunction worsens tissue perfusion further. Tachycardia observed in septic shock patients may sometimes be inappropriate and results in poor diastolic filling and loss of ventriculo-aortic coupling. [12].
Apart from endothelial dysfunction and capillary leak, septic shock is also characterized by lack of homogeneity in the distribution of blood flow at the level of microcirculation with both poorly perfused and adequately perfused areas in close vicinity to each other. [13] These alterations at the microvascular level have profound impact in the pathophysiology of septic shock, worsening organ dysfunction further and unfavorable outcomes. In the early stages of septic shock, improvement in systemic hemodynamics (macrocirculation) leads to improvement in microcirculation (“hemodynamic coherence”). However, in the late stages, this coherence between macro- and microcirculation is lost, leading to refractory shock. [14].
Septic Shock: Diagnosis
Early recognition of shock state is of utmost importance to improve hemodynamics and restore tissue perfusion [3]. Detailed history and clinical examination are essential first step towards diagnosing septic shock and identifying the source of infection. Look for history of altered sensorium, decreased urine output, and signs of poor peripheral perfusion such as cold extremities, increased capillary refill time, or mottling. Low blood pressure and a need for vasopressor to maintain MAP of at least 65 mmHg is a must to define septic shock as per current definition [2]. However, hypotension may not be there at the time of presentation in some patients especially in previously hypertensive patients on irregular or no medication in some patients because of compensatory response. In fact, high lactate values without hypotension in patients with septic shock (the so-called “cryptic shock”) have similar bad prognosis as overt shock with hypotension. [15] Invasive blood pressure monitoring not only helps in accurate BP monitoring but also gives important information by giving variables for fluid responsiveness such as pulse pressure variation and systolic pressure variation or can be used for cardiac output monitoring.
Current definition of septic shock mandates measurement of lactate. High lactate may be an indicator for global tissue hypoperfusion, especially when correlated with other clinical hypoperfusion parameters. Serial measurement of lactate is also of value in following resuscitation process and is recommended by guideline. [3, 16] However, lactate may not be elevated in every patient of septic shock; but this lack of lactate elevation is associated with better prognosis. [15] One should also remember that lactate may be elevated in several other conditions apart from tissue hypoperfusion (type B lactic acidosis), e.g., hepatic dysfunction, mitochondrial dysfunction, thiamine deficiency, and medications (metformin, antiretroviral drugs). Elevated lactate may also be related to hyperglycemia and increased production of pyruvate, stress-related adrenergic hyperactivity, and increased glycolysis. Other markers of global hypoperfusion states used for monitoring resuscitation are mixed venous (or central venous) oxygen saturation (SvO2 or ScvO2) or venoarterial carbon dioxide pressure difference (Pv-aCO2). SvO2>65% or ScvO2>70% has been suggested by sepsis guideline as end point of resuscitation [3].
Point-of-care ultrasound can now be considered as an extension of clinical examination in critically ill patients. A focused ultrasonographic examination in appropriate clinical context is extremely useful in identifying mechanism of circulatory shock at the bedside and to determine appropriate mean to rectify it. [17] Apart from identifying type of shock, detailed ultrasonographic examination is helpful in recognizing possible source of infection in patients with septic shock, for example, consolidation pattern in lung ultrasound for pneumonia or fluid collection in body cavities, localized abscess in liver, or hydronephrosis of kidney. In appropriate patient, respirophasic variation in inferior caval diameter can help in taking a decision about further fluid resuscitation.
In some patients, more advanced hemodynamic monitoring may become necessary. Despite its limitations, pulmonary artery (PA) catheter is still considered as the gold standard for advanced hemodynamic monitoring. But with the availability of advanced noninvasive or minimally invasive diagnostic tests and potential concern about the safety of PA catheter, it is now gradually falling out of favor [18, 19].
Septic Shock: Management
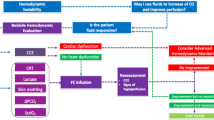
The four pillars of septic shock management are (1) Early recognition of shock state and identification of source of infection, (2) Resuscitation and rapid establishment of tissue reperfusion, (3) Providing support to failing Organs, and (4) Source control including early adequate antibiotics and drainage/debridement of infectious focus (if feasible). The pneumonic “EROS” may be useful as a checklist at the bedside (Fig. 14.1).
EROS principle: summarizing principles of managing septic shock (Adapted from Ghosh S. with permission [20])
Resuscitation
The goal of resuscitation is to rapidly establish tissue reperfusion. This can be achieved by increasing delivery of oxygen and/or by maintaining oxygen content of blood and/or by increasing mean arterial pressure (upstream pressure for organ perfusion). Different tools are available to achieve these goals: fluid infusion, vasopressors, inotropic support, and blood transfusion, if required. In this chapter, we shall be focusing on fluid resuscitation in septic shock, its potential interaction with vasopressors, and appropriate indications to initiate inotropic support.
Despite several caveats associated with it, intravenous fluid infusion remains the first-line resuscitation option in septic shock resuscitation. Physiological principles behind fluid resuscitation are described elsewhere in this book. Briefly, intravenous fluid infusion can potentially increase cardiac output by increasing circulatory stressed volume and may also increase mean arterial pressure provided ventriculo-aortic coupling is maintained.
Which Fluid?
Colloids Versus Crystalloids
Current evidence does not support colloids as the resuscitation fluid of choice in septic shock.
-
Theoretical advantage of colloids remaining in the intravascular compartment for a longer period of time and thus potentially limiting overall resuscitation fluid volume has not been shown to be clinically significant. In the SAFE study, overall ratio of resuscitation volume in 4% albumin and 0.9% saline groups in the first four days of resuscitation was approximately 1:1.4. [21].
-
Resuscitation with colloids has not shown to improve patient-centered outcome in clinical studies. [22].
-
Compared to crystalloids, resuscitation with hydroxyethyl starch (HES) has shown increasing number of adverse events including renal failure, need for renal replacement therapy, coagulopathy, need for blood transfusion, and allergic reactions. [23,24,25] In the 6S study, compared to Ringer’s acetate, infusion with hetastarch in Ringer’s acetate was associated with higher 90-day mortality. [25].
-
There are limited high-quality data available for resuscitation with gelatin or dextran. In a recent Cochrane review, meta-analysis of all available evidence does not find any advantage of both these colloids over the control group. [26] Moreover, both gelatin and dextrans are associated with number of adverse effects including renal failure, anaphylaxis (or anaphylactoid) reaction, and coagulopathy.
-
Hypo- or iso-oncotic (4% or 5%) albumin is safe for resuscitation of septic shock patients and may possibly have some beneficial effect. [21, 27] However, cost–benefit ratio must be considered while prescribing 4% or 5% albumin for volume resuscitation in septic shock. Albumin should not be used for resuscitation of a patient with underlying traumatic brain injury or any evidence of raised intracranial pressure. [28].
-
In a small study, hyperoncotic (20 or 25%) albumin infusion was found to be beneficial in limiting resuscitation volume compared to hypo- or iso-oncotic albumin. [29] However, this finding needs to be validated in a larger multicenter study, before recommending it for wider use.
-
However, effects of albumin infusion in septic shock may not only be limited to improvement of hypoperfusion state. In the multicenter Italian ALBIOS trial, supplemental 20% albumin infusion in addition to crystalloid infusion was associated with mortality benefit in subgroup of patients with septic shock. [30].
Crystalloids: Saline or Balanced
Over the years, 0.9% saline has been the most commonly administered crystalloid. But in recent years, its safety has been questioned because of its high chloride load and potential for renal hypoperfusion through tubuloglomerular feedback mechanism. Moreover, with a strong ion difference of 0, large volume 0.9% saline infusion may lead to hyperchloremic metabolic acidosis. Based on the current evidence, the Surviving Sepsis Campaign guidelines recommend balanced crystalloids as the first-line resuscitation fluid in septic shock [3].
-
In a study on human volunteers, 0.9% saline has shown to reduce renal perfusion. [31].
-
Restricting use of chloride-liberal fluids have shown to improve renal outcome in before and after studies. [32, 33].
-
In a large observational study, increasing utilization of balanced salt solutions during septic shock resuscitation was shown to be associated with better survival outcome. [34].
-
In the single-center SMART randomized control study, resuscitation with balanced crystalloids (mostly Ringer’s lactate) had shown to lower composite outcome of death, renal dysfunction, and new requirement of RRT at 30 days (major advanced kidney event at 30 days or MAKE30) compared to 0.9% saline. [35].
-
In the SALT-ED study, compared to 0.9% saline, resuscitation with balanced salt solutions was shown to reduce hospital length of stay in adult patients who needed fluid infusion in the ED and were admitted in the wards. [34] Balanced fluid group also had lower incidence of MAKE30. [36].
-
In four more randomized control studies, balanced salt solutions were not associated with worse clinical outcome compared to 0.9% saline. [37,38,39,40].
-
However, 0.9% saline is still useful in certain clinical scenarios like resuscitating patients with raised intracranial pressure, hypovolemic hyponatremia, hypovolemia with metabolic alkalosis, or in resource-limited settings.
Which Balanced Fluid?
Currently, there is no high-quality clinical evidence comparing different balanced salt solutions. Choosing the right balanced fluid is largely based on physiological data and cost.
-
Balanced salt solutions vary significantly in their composition including electrolyte content, buffer base, and strong ion difference. Lactate present in Ringer’s lactate is largely metabolized in the liver and the metabolism may get overwhelmed, at least theoretically, in patients with severe hepatic dysfunction. In contrast, acetate present in Plasma-Lyte, Ringer’s acetate, and Sterofundin is metabolized in almost all tissues.
-
With higher SID, Plasma-Lyte can potentially correct metabolic acidosis faster. [41] However, clinical benefit of this earlier resolution of metabolic acidosis needs to be further evaluated in larger human studies.
-
However, cost and availability of a particular balance fluid solution also should be considered before deciding the choice of fluid.
To Summarize
Current evidence supports use of balanced salt solution as the preferred resuscitation fluid in most clinical circumstances.
-
Synthetic colloids (HES, gelatins, and dextrans) should be avoided in septic shock resuscitation because of potential safety concern.
-
Albumin can be given safely in patients requiring large volumes of fluid, thus decreasing cumulative fluid balance. However, high cost of albumin needs to be balanced with this small benefit.
Dose of Fluid
As discussed earlier, the rationale for fluid resuscitation is to possibly increase mean systemic filling pressure in massively vasodilated patients and also to cater for volume already lost or to replace ongoing losses. But volume infusion must be balanced against the possible increase in interstitial edema resulting from the capillary leak. Studies have shown a strong association between over-resuscitation and positive fluid balance with worse patient-centric outcomes. [42, 43] To maintain a balance between benefit and harm associated with fluid infusion, patients should be assessed frequently and every effort should be made to limit overall cumulative fluid volume without compromising tissue perfusion.
-
SSC Guidelines recommend liberal fluid resuscitation with at least 30 ml/kg of crystalloids in the first 3 h of resuscitation in a septic shock patient [3]. However, the recommendation is not based on sound clinical evidence and has been widely criticized. [44].
-
Instead of going ahead with empirical weight-based fluid resuscitation, fluid dosing should be individualized, based on clinical and hemodynamic parameters and evidence of no apparent harm associated with it. We suggest small frequent fluid boluses, with frequent monitoring of clinical response to the bolus, as proposed in the original description of intravenous fluid therapy. [45] Minimal fluid bolus to be given for substantive improvement in hemodynamic parameter is at least 4 ml/kg body weight. [46].
-
After initial bolus, all subsequent fluid boluses should be administered only after assessment of fluid responsiveness parameters. [47].
-
When fluid responsiveness parameters are not available or not applicable, it is advisable to perform a standard fluid challenge or mini-fluid challenge to guide further fluid therapy. [48, 49] Further fluid bolus should be administered only if the patient is fluid responsive or fluid challenge (or mini-fluid challenge) is positive. Fluid challenge should not be repeated if the initial response is negative or equivocal. [50].
-
Underlying clinical status and possible harm associated with fluid infusion also should be considered before administering further fluid boluses. One should be extra cautious in patients at a higher risk of harm from fluid, for example, patients with profound baseline hypoxia or raised intra-abdominal pressure.
Interaction with Vasopressors
Prolonged hypotension is associated with increased mortality in septic shock and early initiation of vasopressors has shown to improve outcome, but not before infusion of some fluid. [51,52,53] Infusion of norepinephrine can potentially increase cardiac output by its positive effect on stressed volume through venoconstrictor effect. [54] Early initiation of norepinephrine infusion has also shown to reverse shock state earlier. [55] But these potential beneficial effects of early vasopressor initiation must be balanced against potentially adverse effects.
-
Norepinephrine infusion may actually decrease cardiac output by increasing resistance to venous return, particularly in the presence of hypovolemia (both relative and absolute). [56].
-
Vasopressors can potentially increase organ ischemia by vasospasm. In the multicenter SEPSISPAM study, incidences of acute myocardial infarction, mesenteric ischemia, and digital ischemia were 1.8%, 2.3%, and 2.6%, respectively, in the “high MAP” target group. [57].
-
All vasopressors are associated with risk of potentially life-threatening cardiac arrhythmias. In the SOAP II study, overall incidences of arrhythmias were 24.1% in “dopamine arm” and 12.4% in “norepinephrine arm.” [58].
-
Other metabolic disturbances like hyperlactatemia are also known with certain vasopressor infusion. In the multicenter CAT study, 12.9% patients included in the “epinephrine group” withdrew because of transient but significant metabolic side effects especially hyperlactatemia. [59].
When to Initiate Vasopressor Infusion?
The Surviving Sepsis Guidelines suggest initiation of vasopressor infusion after adequate volume resuscitation [3]. However, at the bedside, timing of vasopressor initiation should be individualized. We suggest the following guidelines based on available evidence:
-
In patients with diastolic blood pressure of <50 mmHg, vasopressor infusion should be started along with fluid boluses. DBP is the upstream pressure for coronary perfusion. Rise in cardiac enzymes was observed in pregnant ladies with persistent DBP <50 mmHg following postpartum hemorrhage. [60].
-
Vasopressors also should be initiated simultaneously with fluid infusion, in patients with profound vasodilatation as suggested by diastolic shock index >2.3. [61] Diastolic shock index is defined as the ratio between heart rate and diastolic blood pressure.
-
Otherwise, it is reasonable to start vasopressor infusion after initial 1–2 liters of crystalloid infusion. In the REFRESH study, a strategy of initiating norepinephrine infusion after one liter of fluid had shown to reduce cumulative fluid balance. [62] In an observational study, dose of vasopressor in the first six hours after septic shock onset was shown to be associated with increased mortality, and this effect was mitigated if vasopressors were initiated only after two liters of fluids. [53] However, further fluid administration should not be limited after starting norepinephrine infusion.
Septic Shock: Monitoring
Throughout the process of resuscitation, patients should be monitored for improvement of physiological parameters including heart rate, mean arterial pressure, increase in urine output, improvement of peripheral perfusion parameters (especially capillary refill time), gradual normalization of central venous (or mixed venous) oxygen saturation, and gradual improvement in lactate. Monitoring should also be done for harmful consequences of resuscitation including evidence of fluid overload (fall in oxygen saturation, B-profile in lung ultrasound, extravascular lung water, rising intra-abdominal pressure), or adverse effects of vasopressors (myocardial, mesenteric, digital ischemia, or arrhythmias).
-
In the landmark early goal-directed therapy (EGDT) study, Rivers and colleagues applied protocolized resuscitation aiming to achieve certain hemodynamic and other parameters like central venous pressure, mean arterial pressure, oxygen saturation, and hemoglobin with an ultimate goal of ScvO2 > 65%, very early in septic shock resuscitation at ED. [63] This early protocolized resuscitation achieved remarkable mortality benefit compared to usual care. However, three recent studies conducted across several continents could not confirm the benefits of EGDT strategy targeting ScvO2 > 70%. [64,65,66].
-
Serial measurement of lactate has shown to improve clinical outcome. [16] Guideline has suggested lactate measurement for initial diagnosis of sepsis, for following progress of resuscitation, and as an end point of resuscitation [3]. However, in a randomized study of septic shock resuscitation in emergency department, a strategy aiming to normalize lactate clearance did not show any outcome benefit compared to a strategy of normalizing ScvO2. [67].
-
Capillary refill time is an easily available bedside clinical parameter with no additional cost involved to monitor. It is shown to have the fastest recovery kinetics among all available physiological parameters, and its normalization is shown to be associated with better regional perfusion and better prognosis. [14] In the ANDROMEDA-SHOCK trial, patients with septic shock were randomized to either be resuscitated based on capillary refill time (CRT) monitoring every 30 minutes or to 2-hourly lactate measurement. Compared to lactate group, CRT group received lower volume of fluid and also had less organ dysfunction. [68] In a subsequent Bayesian analysis of the ANDROMEDA-SHOCK data, mortality rate was better in the CRT group. [69].
-
To summarize, a holistic approach should be followed while monitoring a patient with septic shock and multiple parameters should be considered to decide specific treatment measure during resuscitation process and to take a final call for the end point of resuscitation.
Limiting Cumulative Fluid Balance
The relationship between fluid volume and mortality follows a “U”-shaped curve with both too little and too much fluid increasing death. Every effort should be made to limit fluid infusion without compromising perfusion of organs.
-
Protocolized “restrictive fluid” strategy has yielded mixed result so far with CLASSIC pilot study showing beneficial effect and larger study showing no beneficial effect. [70, 71].
-
A more prudent approach perhaps is to apply restrictive strategies in every stage of resuscitation [8]. We suggest the following approach in limiting cumulative fluid balance in different stages of shock (Fig. 14.2).
Suggested approach to limit cumulative fluid balance in different stages of resuscitation. (Adapted from Ghosh S. with permsission [20])
Case Vignette
Mr. X is likely a case of urosepsis with septic shock. He has already received 1 L of fluids. Despite that, he continues to be hypotensive with high lactates. Vasopressors should be initiated early along with appropriate antibiotics after obtaining cultures. He has a low ejection fraction. Hence, further fluid should be in the form of small boluses with frequent assessment for fluid responsiveness.
Conclusion
Balanced isotonic crystalloids are generally the fluid of choice in sepsis resuscitation, while there might be a place for human albumin in patients with septic shock and an albumin level below 30 g/l. Hypotonic crystalloids are the maintenance fluids of choice when enteral or parenteral feeding is insufficient. There is strong evidence against using HES or NaCl 0.9% in sepsis. There is no convincing evidence to support the use of Gelatins or Dextran. Early use of vasopressors can increase the stressed volume hence limiting the need for ongoing fluid resuscitation.
Take-Home Messages
-
Fluid resuscitation remains the vital part of management of septic shock patients.
-
Resuscitation should be initiated early with balanced crystalloids with frequent assessment of clinical parameters followed by variables to measure fluid responsiveness.
-
Care must be taken to frequently assess for end point of hemodynamic resuscitation, thus avoiding increase in cumulative fluid balance and overload.
-
Early introduction of vasopressors may improve clinical outcome.
-
The goal is to achieve early and adequate resuscitation to prevent end-organ damage followed by measures to evacuate fluid and minimize cumulative balance once the shock has settled.
References
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. 2020;395:200–11.
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:762–74.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247.
Benedict CR, Rose JA. Arterial norepinephrine changes in patients with septic shock. Circ Shock. 1992;38:165–72.
Cumming AD, Dredger AA, McDonald JW, Lindsay RM, Solez K, Linton AL. Vasoactive hormones in the renal response to systemic sepsis. Am J Kidney Dis. 1988;11:23–32.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:2063.
Khakpour S, Wilhelmsen K, Hellman J. Vascular endothelial cell toll-like receptor pathways in sepsis. Innate Immun. 2015;21(8):827–46.
Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal P-J, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care [Internet]. 2018;8(1)
Silversides JA, Fitzgerald E, Manickavasagam US, Lapinsky SE, Nisenbaum R, Hemmings N, et al. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med. 2018;46:1600–7.
Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, Brown SM. Septic cardiomyopathy. Crit Care Med. 2018;46:625–34.
Lanspa MJ, Cirulis MM, Wiley BM, Olsen TD, Wilson EL, Beesley SJ, Brown SM, Hirshberg EL, Grissom CK. Right ventricular dysfunction in early sepsis and septic shock. Chest. 2021;159:1055–63.
Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–91.
De Backer D, Ricottilli F, Ospina-Tascón GA. Septic shock: a microcirculation disease. Curr Opin Anaesthesiol. 2021;34:85–91.
Hernández G, Teboul J-L. Is the macrocirculation really dissociated from the microcirculation in septic shock? Intensive Care Med. 2016;42:1621–4.
Hernandez G, Bruhn A, Castro R, Regueira T. The holistic view on perfusion monitoring in septic shock. Curr Opin Crit Care. 2012;18:280–6.
Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J, LACTATE study group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61.
Lichtenstein D. Fluid administration limited by lung sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Med. 2012;6:155–62.
Connors AF Jr, Speroff T, Dawson NV, Thomas C, Harrell FE Jr, Wagner D, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA. 1996;276:889–97.
Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnaud D, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled Trial. JAMA. 2003;290:2713–20.
Ghosh S. Fluid resuscitation in septic shock. In: Handbook of intravenous fluids. Singapore: Springer; 2022. https://doi.org/10.1007/978-981-19-0500-1_11.
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56.
Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declère AD, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310:1809–17.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39.
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–11.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34.
Lewis SR, Pritchard MW, Evans DJW, Butler AR, Alderson P, Smith AF, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Systematic Rev. 2018;8:CD000567.
Finfer S, McEvoy S, Bellomo R, McArthur C, Myburgh J, Norton R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37:86–96.
Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–84.
Mårtensson J, Bihari S, Bannard-Smith J, Glassford NJ, Lloyd-Donald P, Cioccari L, et al. Small volume resuscitation with 20% albumin in intensive care: physiological effects: the SWIPE randomised clinical trial. Intensive Care Med. 2018;44:1797–806.
Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–21.
Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24.
Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–72.
Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–64.
Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan MS, Setoguchi S, Beadles C, Lindenauer PK. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–91.
Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–39.
Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–28.
Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–10.
Semler MW, Wanderer JP, Ehrenfeld JM, Stollings JL, Self WH, Siew ED, et al. Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med. 2017;195:1362–72.
Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% Saline Solution on mortality in critically ill patients: the Basics randomized clinical trial. JAMA. 2021;326(29):818.
Finfer S, Micallef S, Hammond N, Navarra L, Bellomo R, Billot L, et al. Balanced multielectrolyte solution versus saline in critically ill adults. N Engl J Med. 2022;386:815–26.
Rauserova-Lexmaulova L, Prokesova B, Blozonova A, Vanova-Uhrikova I, Rehakova K, Fusek M. Effects of the administration of different buffered balanced crystalloid solutions on acid–base and electrolyte status in dogs with gastric dilation–volvulus syndrome: a randomized clinical trial. Top Companion Anim Med. 2022;46:100613. https://doi.org/10.1016/j.ajem.2016.10.007.
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75.
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65.
Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10(1):37–47.
Latta T. Saline venous injection in cases of malignant cholera, performed while in the vapour-bath. Lancet. 1832;19(480):208–9.
Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit Care Med. 2017;45:e161–8.
Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2016;6(1):111.
Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med. 2006;34:1333–7.
Muller L, Toumi M, Bousquet PJ, Riu-Poulenc B, Louart G, Candela D, et al. AzuRéa group. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology. 2011;115:541–7.
Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41:1529–37.
Bai X, Yu W, Ji W, Lin Z, Tan S, Duan K, Dong Y, Xu L, Li N. Early versus delayed administration of norepinephrine in patients with septic shock. Crit Care. 2014;18:532.
Waechter J, Kumar A, Lapinsky SE, Marshall J, Dodek P, Arabi Y, et al. Interaction between fluids and vasoactive agents on mortality in septic shock: a multicenter, observational study. Crit Care Med. 2014;42:2158–68.
Roberts RJ, Miano TA, Hammond DA, Patel GP, Chen JT, Phillips KM, et al. Evaluation of vasopressor exposure and mortality in patients with septic shock. Crit Care Med. 2020;48:1445–53.
Persichini R, Silva S, Teboul JL, Jozwiak M, Chemla D, Richard C, Monnet X. Effects of norepinephrine on mean systemic pressure and venous return in human septic shock. Crit Care Med. 2012;40:3146–53.
Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T. Udompanturak S. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A randomized trial. Am J Respir Crit Care Med. 2019;199:1097–105.
Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol. 1972;34:13–46.
Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–93.
De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–89.
Myburgh JA, Higgins A, Jovanovska A, Lipman J, Ramakrishnan N, Santamaria J, CAT Study investigators. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34:2226–34.
Karpati PCJ, Rossignol M, Pirot M, Cholley B, Vicaut E, Henry P, et al. High incidence of myocardial ischemia during postpartum hemorrhage. Anesthesiology. 2004;100:30–6.
Ospina-Tascón GA, Teboul JL, Hernandez G, Alvarez I, Sánchez-Ortiz AI, Calderón-Tapia LE, et al. Diastolic shock index and clinical outcomes in patients with septic shock. Ann Intensive Care. 2020;10:41.
Macdonald SPJ, Keijzers G, Taylor DM, Kinnear F, Arendts G, Fatovich DM, et al. Restricted fluid resuscitation in suspected sepsis associated hypotension (REFRESH): a pilot randomised controlled trial. Intensive Care Med. 2018;44:2070–8.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–93.
Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506.
Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–11.
Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–46.
Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321:654–64.
Zampieri FG, Damiani LP, Bakker J, Ospina-Tascón GA, Castro R, Cavalcanti AB, Hernandez G. Effects of a resuscitation strategy targeting peripheral perfusion status versus serum lactate levels among patients with septic shock. A Bayesian reanalysis of the ANDROMEDA-SHOCK trial. Am J Respir Crit Care Med. 2020;201:423–9.
Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettilä V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695–705.
Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 2022;386:2459–70.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Ghosh, S., Arora, G. (2024). Fluid Management in Septic Shock. In: Malbrain, M.L., Wong, A., Nasa, P., Ghosh, S. (eds) Rational Use of Intravenous Fluids in Critically Ill Patients. Springer, Cham. https://doi.org/10.1007/978-3-031-42205-8_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-42205-8_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42204-1
Online ISBN: 978-3-031-42205-8
eBook Packages: MedicineMedicine (R0)