Abstract
The book “Rational Fluid Therapy” aims to provide guidance on the appropriate use of intravenous (IV) fluids, which are often overlooked as medications despite being a cornerstone of patient care. In this introductory chapter, the importance of seeing IV fluids as drugs with indications, contra-indications, and potential adverse effects is emphasized. Inappropriate fluid therapy can have deleterious effects and cause patient morbidity and mortality. Therefore, the need for careful oversight and guidance is stressed in fluid prescription, as well as the implementation of fluid stewardship and organ function monitoring. Different terms related to fluid therapy, such as fluid overload and hypervolemia, are often used interchangeably despite indicating different clinical situations. Clear definitions are provided for these terms to avoid misunderstandings and misinterpretations that may lead to inappropriate therapeutic decisions. These definitions will be repeated throughout the book to ensure clarity and consistency.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara IFA CommentaryThe chapter on terms and definitions of fluid therapy and monitoring in the book “Rational Fluid Use in the Critically Ill” provides an essential foundation for understanding the importance of intravenous (IV) fluid therapy in critically ill patients. The authors effectively highlight the need to view IV fluids as drugs and not simply as a routine treatment option. The chapter’s approach is practical, providing clear definitions of essential and common terms used in fluid therapy and organ function monitoring, emphasizing the significance of appropriate fluid therapy such as volume status, resuscitation, maintenance fluids, colloids and crystalloids. This practical approach is critical for clinicians as it provides them with a clear understanding of what they are administering and how to administer it appropriately.
Introduction
This introductory chapter will list the common terms and definitions used throughout this book. Intravenous fluid (IV) therapy is a cornerstone to treating shock status and providing water, electrolytes and glucose needs. Worldwide thousands of litres of IV fluids are administered every day. However, IV fluids are not yet treated in the same way as other medications given to our patients. We need to see them as drugs, and they come with a dose, duration and de-escalation, and they need to be given in a timely manner, but only when needed and when the patient cannot have oral fluid intake. They have indications, contra-indications and potential adverse effects. Inappropriate fluid therapy is one of the main concerns.
Thus, the appropriate use of IV fluids is an essential part of patient safety and deserves careful oversight and guidance, given the association between fluid (mis)use and the deleterious effects causing patient morbidity and mortality [1]. Correct definitions, implementation of a fluid stewardship and organ function monitoring may limit the deleterious effects of inappropriate fluid prescription and fluid overload [2]. The literature on fluid therapy in the critically ill is continuously expanding, however, sometimes, different definitions are used. For example, fluid overload, fluid accumulation, hypervolemia and hyperhydration are often used interchangeably, while, they may indicate different clinical situations [3]. Using wrong definitions can lead to misunderstandings, misinterpretations and inappropriate therapeutic decisions regarding fluid administration or fluid removal.
In this chapter, we provide definitions of the different terms important in the context of fluid therapy in hospitalized patients, and intensive care units [4,5,6,7,8,9,10]. These definitions will be repeated throughout the different chapters when these conditions are discussed in more detail.
Terms and Definitions
4 compartments: This can be dealt with in different ways, classically you have fat–water–protein–and minerals while water on its own is also distributed into four compartments: intracellular water (ICW), interstitial water, intravascular water and transcellular, with extracellular water (ECW) calculated as the sum of interstitial + intravascular + transcellular water content.
4 D’s: Fluids are medications in which one should take into account the 4D’s in analogy to antimicrobial stewardship: Drug–Dose–Duration–De-escalation.
4 hits: The four hits are:
-
first hit = initial insult,
-
second hit = ischemia reperfusion,
-
third hit = global increased permeability syndrome (GIPS).
-
fourth hit = potential risk of hypoperfusion during de-resuscitation.
4 indications: Fluids can be given for four reasons: resuscitation, maintenance, replacement and nutrition.
4 fluid losses: Traditionally four ways can be taken into account with regard to fluid losses: insensible loss, urine output, gastrointestinal losses and third space. Additional losses can occur in trauma with overt bleeding or in severely burned patients.
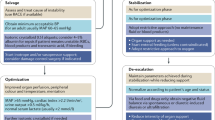
4 phases: The four dynamic fluid phases are: resuscitation, optimization, stabilization and evacuation.
4 questions: The four main questions surrounding fluid therapy that need to be solved are:
-
when to start IV fluids?
-
when to stop IV fluids?
-
when to start fluid removal?
-
when to stop fluid removal?
4 spaces: There are traditionally four fluid spaces:
-
first space = intravascular.
-
second space = interstitial.
-
third space = pleural or peritoneal space.
-
fourth space = transcellular fluid, and not to forget the lymphatic system.
Abdominal compartment syndrome (ACS): Abdominal compartment syndrome (ACS) is defined as a sustained intra–abdominal pressure (IAP) >20 mmHg (with or without an abdominal perfusion perfusion (APP) <60 mmHg) that is associated with new organ dysfunction/failure. Primary ACS is a condition associated with injury or disease in the abdomino-pelvic region that frequently requires early surgical or interventional radiological intervention. Secondary ACS refers to conditions that do not originate from the abdomino-pelvic region. Recurrent ACS refers to the condition in which ACS redevelops following previous surgical or medical treatment of primary or secondary ACS [11].
Abdominal perfusion pressure (APP): Abdominal perfusion pressure (APP) = mean arterial pressure (MAP) − IAP. The Filtration Gradient (FG) = glomerular filtration pressure (GFP) − proximal tubular pressure (PTP) = MAP – 2 × IAP [11].
Abdominal West zones: West zones describe areas of the abdomen based upon variations in IAP, central venous pressure (CVP) and inferior vena cava pressure (IVCP). These differences result from vascular flow, gravity and pressure transmission from the abdominal to the thoracic compartment. The concept of abdominal vascular zones may be present in the patient with IAH, analogous to the pulmonary vascular zone conditions described by West. In this concept, an increased IAP increases venous return when the transmural IVCP (defined as IVCP minus IAP) at the thoracic inlet significantly exceeds the critical closing transmural pressure (= zone 3 abdomen). This is most often the case in hypervolemic patients with a high IVCP. In zone 3 conditions, the abdominal venous compartment functions as a capacitor. In contrast, when the transmural IVCP at the thoracic inlet is below the critical closure transmural pressure (= zone 2 abdomen), venous return is significantly decreased. This is most often the case in hypovolemic patients and by extension in most non-cardiogenic shock patients. In zone 2 conditions, the abdominal venous compartment functions as a collapsible starling transistor [12] (Fig. 1.1). This model clearly illustrates why hypovolemia (and especially in combination with positive pressure ventilation and high levels of PEEP) predisposes patients to lower cardiac output (CO) in response to elevated IAP than normovolemia. In real life, the model may be more complex and should also take into account, volemia status, compliance and positive pressure ventilation with PEEP settings.
Abdominal West zones. The abdomen can be divided into discrete regions according to the interplay between IAP, CVP and IVCP. These regions are zone 1, where IAP is higher than IVCP or CVP; zone 2, where the IAP is lower than the CVP but higher than the IVCP and zone 3, where both CVP and IVCP are higher than IAP. Other contributing factors (but more difficult to assess) are the compliance of the lungs (CL), chest wall (CCW) and abdominal wall (CAB) as well as the use of positive pressure ventilation with PEEP and intrathoracic pressure. CVP central venous pressure, IAP intra-abdominal pressure, ITP intrathoracic pressure, IVCP inferior vena cava pressure, PEEP positive end-expiratory pressure
Acid: A molecule or substance that is able to increase the concentration of hydrogen ions (H+) when dissolved in water or an aqueous solution.
Acidemia: A blood pH that is lower than the normal physiologic range.
Acidosis: A process in the body in which there is a net accumulation of acid.
Acute kidney injury (AKI): According to the KDIGO guidelines [13], acute kidney injury is defined by either an increase in serum creatinine ≥0.3 mg/dL within 48 h or an increase in serum creatinine ≥1.5 times baseline or a urine output ≤0.5 mL/kg/h for 6 h (Table 1.1).
Albumin leak index: Laboratory index correlated with ongoing infection or poor source control, can be calculated by dividing the urine albumin by the urine creatinine level.
Alkalemia: A blood pH that is greater than the normal physiologic range.
Alkalosis: A process in the body in which there is a net accumulation of base.
Anion: A negatively charged atom or molecule, such as chloride (Cl−) and bicarbonate (HCO3−).
Anion Gap (AG): The calculated difference between the major cations and anions in plasma. The anion gap is calculated by the following formula: AG = (Na+ + K+) − (Cl− + HCO3−). Anion gap is useful to help narrow down the potential causes of metabolic acidosis.
Autotransfusion: A blood conservation strategy used during haemorrhage or surgery where blood is collected, filtered and reinfused into the patient. This process is also referred to as blood salvage or cell salvage. The passive leg raising test can be seen as a sort of autotransfusion with 300 mL blood coming from the legs and mesenteric venous pool increasing venous return into the central circulation. Another example of autotransfusion is the use of vasopressors in vasoplegia with relative hypovolemia, increased unstressed volume and a decreased stressed volume. See also under vasoplegia.
Balanced solution: An intravenous crystalloid fluid that contains an electrolyte composition similar to normal plasma. An iso-osmotic and isotonic balanced solution maintains the acid–base status and does not induce inappropriate fluid shifts. Recently, fluids with low chloride content are also labelled as “balanced” solutions. Hence, a balanced solution can be categorized into (1) IV fluids with strong ion difference (SID) close to that of plasma, i.e. 24–29 mEq/L, causing a minimal effect on the acid–base equilibrium and (2) IV fluids containing normal or sub-normal chloride content (i.e. serum chloride ≤110 mEq/L).
Base: A molecule or substance that is able to increase the concentration of hydroxyl ions (OH−) when dissolved in water or an aqueous solution. The hydroxyl ions (OH−) will interact with hydrogen ions (H+) to form water molecules (OH− + H+ = H2O), so said in other words a base is a substance or molecule that combines with hydrogen ions (H+) already present in the solution.
Base deficit: The amount of a strong base that must be added in vitro to 1 L of oxygenated blood to return the pH to 7.40, at a partial pressure of carbon dioxide of 40 mmHg, and temperature of 37 °C, in the presence of metabolic acidosis.
Base excess: The amount of a strong acid in mmol/L that must be added in vitro to 1 L of oxygenated blood to return the pH to 7.40, at a partial pressure of carbon dioxide of 40 mmHg, and temperature of 37 °C, in the presence of metabolic alkalosis.
Breathing ongoing loss: As we breathe, we exhale moisture in the form of water vapour. This is particularly noticeable in cold weather, when our breath may condense and form visible clouds. This is a form of ongoing fluid loss. See also replacement fluids.
Buffered solution: An intravenous crystalloid fluid that contains an acid–base buffer in order to help maintain the SID. The most common buffers are bicarbonate or organic anions (e.g. lactate, acetate, gluconate).
Capillary leak index: This index is correlated with ongoing infection or poor source control, and can be calculated by dividing the serum C-reactive protein (CRP) by the serum albumin level, the higher the CRP, the more the inflammation and the lower the albumin, the more the leak to the interstitium.
Cardiac output (CO): The amount of volume that is present at the end of the diastole and that is ejected from the left ventricle (stroke volume) multiplied by the number of heartbeats per minute, usually around 5–6 L/min. The cardiac index is CO normalized per body surface area (in m2). The main drivers of cardiac output are preload, afterload and contractility (Fig. 1.2).
The main drivers of cardiac output are preload, afterload and contractility and they affect oxygen delivery and mean arterial pressure. CO cardiac output, CVP central venous pressure, DO2 oxygen delivery, MAP mean arterial pressure, MSFP mean systemic filling pressure, RVR resistance to venous return, VR venous return
Cardio-abdominal-renal syndrome (CARS): An organ–organ interaction between heart, kidney and abdomen through elevated IAP has been proposed. Therefore, the traditional perception of worsening renal failure secondary to hypoperfusion of the kidneys through low-flow states in critically ill patients, especially those with advanced decompensated heart failure, has been challenged. The low cardiac output with venous congestion of heart failure is proposed to cause elevated CVP, IAP, sodium and water retention, and decreased renal perfusion pressure, leading to the concept of “Congestive Kidney Failure” or “Cardio-Abdomino-Renal Syndrome (CARS)” (Fig. 1.3) [14].
Pathophysiological effect of heart failure (in RED forward failure) related venous congestion (in BLUE backward failure) on organ function and net effects on salt and water homeostasis (in ORANGE). CVP central venous pressure, EDV end-diastolic volume, IAH intra-abdominal hypertension, IAP intra-abdominal pressure, ITP intra-thoracic pressure, IVC inferior vena cava, MAP mean arterial pressure, Na sodium, RAAS renin–angiotensin–aldosterone system, RH right heart, RPP renal perfusion pressure, RVP renal venous pressure. (Adapted with permission from Dabrowski et al. according to the Open Access CC BY License 4.0 [15])
Cation: A positively charged atom or molecule, such as chloride (Cl+), sodium (Na+), potassium (K+), calcium (Ca2+) and bicarbonate (NH4+).
Classification of fluid dynamics: With respect to the different phases of fluid resuscitation (early vs. late) one can classify the dynamics of fluid management by combining early adequate (EA) or early conservative (EC) and late conservative (LC) or late liberal (LL) fluid management. Based on this theoretical concept, four distinct strategies can be defined: EALC, EALL, ECLC and ECLL. The EALC and ECLC groups carry the best prognosis (Fig. 1.4).
Circulating blood volume (CBV): The total (intravascular) volume of blood contained within the circulatory system.
Coherence: Coherence between microcirculation and macrocirculation in order to obtain adequate regional and tissue oxygen delivery [16]. The hemodynamic coherence depends on the type of the shock state and can be either: hypovolemic, cardiogenic, distributive or obstructive. Four different types of microcirculatory alterations have been described: heterogeneity, hemodilution, hyperpermeability and vasoconstriction [17].
Colloid solution: Solutions constituted of macromolecules (with a molecular weight >30 kDa) that are preferentially retained in the intravascular space following intravenous administration. There are natural colloids (e.g. plasma and albumin) and synthetic colloids (e.g. hydroxyethyl starches, dextrans and gelatins).
Colloid osmotic pressure (COP): The osmotic force exerted by large molecules (colloids) in a solution when separated by a semipermeable membrane from a region with a different colloid concentration. The colloid osmotic pressure provided by plasma proteins is also referred to as oncotic pressure. The normal value for COP is around 20 mmHg and should be at least maintained above 16 mmHg.
Crystalloid: A solution that contains electrolytes and other small water-soluble molecules, and/or dextrose or glucose. Crystalloids are categorized by their tonicity relative to plasma: isotonic, hypotonic and hypertonic.
Cumulative fluid balance: The cumulative fluid balance is the amount of fluid accumulated by calculating the sum of daily fluid balances over a set period of time. Usually, the first week of ICU stay is taken into account for prognostication. A positive cumulative fluid balance is a state where cumulative fluid intakes exceed cumulative fluid outputs.
Daily fluid balance: Daily fluid balance is the difference between all fluids given to a patient during a 24-h period and combined output. As a consequence, daily fluid balance can be negative, neutral or positive. The daily fluid balance does not usually include insensible losses unless the patient is being cared for on an ICU bed that can weigh the patient. Caution should be exercised when using daily weight as a surrogate of fluid balance because muscle and tissue loss cannot be easily measured.
De-escalation: Refers to not initiating extra fluids (withhold) or lowering of the dose or speed of administration (withdraw/reduction) of previously started fluid therapy due to improvement in the clinical condition of the patient.
Dehydration (see also fluid underload): Defined as excessive loss of body water, with or without salt, at a rate greater than the body can replace it. Dehydration has a wide range of aetiologies, including gastrointestinal loss of fluid (vomiting or diarrhoea), heat exposure, prolonged vigorous exercise, kidney disease and medication (e.g. diuretics). A drop in weight might be an indication of dehydration, though regular weight monitoring is often difficult in ICU. The percentage of fluid loss is defined by dividing the cumulative fluid balance in litres by the patient’s baseline body weight and multiplying it by 100%. Dehydration is defined by a minimum value of 5% fluid loss. Dehydration is considered mild (5–7.5%), moderate (7.5–10%), or severe (>10%).
De-resuscitation: Correction of fluid accumulation or fluid overload by the active removal of the excess fluids using non-pharmacological (e.g. dialysis with net ultrafiltration) or pharmacological (e.g. diuretics) methods.
Diffusion: See osmosis.
Digestive processes ongoing loss: The digestive system processes food and drink, absorbing nutrients and water from the food and excreting waste products. The amount of fluid lost through digestion can vary depending on the type and amount of food consumed, as well as individual digestive function. See also replacement fluids.
Drug: A medical substance or therapeutic that comes with indications, contra-indications and potential adverse effects. It should be given judiciously and its effect should be monitored. The dose must be appropriate as well as the duration. The drug should be stopped when no longer needed.
Early adequate goal-directed fluid management (EAFM): EAFM is the initial hemodynamic resuscitation of patients with septic shock by administering fluids within the first 6 h of the initiation of therapy. Most studies looking at the treatment of septic shock define the early goal as giving 25–50 mL/kg (on average around 30 mL/kg) of fluids within the first 6 h. The recent update of the surviving sepsis campaign guidelines defines EAFM as the administration of 30 mL/kg of IV fluids within the first 1–3 h. However, it has been suggested that fluid resuscitation using such large volumes of fluid in all patients may cause “iatrogenic salt water drowning” and a more conservative strategy for fluid resuscitation might be warranted.
Early goal directed (fluid) therapy (EGDT): A protocol-driven treatment algorithm introduced by Rivers et al. aiming to guide fluids, vasopressors, inotropes, blood products and other resuscitation therapy towards specific hemodynamic end-points, with the goal of maintaining and improving hemodynamic stability, adequate tissue perfusion and optimizing oxygen delivery [18]. See also EAFM.
Ebb phase: This refers to the initial phase of septic shock when the patient shows hyperdynamic circulatory shock with decreased systemic vascular resistance due to vasodilation, increased capillary permeability, and severe absolute or relative intravascular hypovolemia. Fluids are mandatory and lifesaving in this phase. The patient in this stage needs EAFM.
Edema: Peripheral and generalized oedema (anasarca) is not only of cosmetic concern, as believed by some, but is harmful to the patient, as it can cause organ oedema and dysfunction. Oedema mirrors fluid overload that has potential harmful consequences on different end-organ systems, with consequential effects on patient morbidity and mortality. As such, fluid therapy can be considered a double-edged sword.
Effective osmole: An electrolyte (ion) that exerts an osmotic force across a semi-permeable membrane and determines a solution’s tonicity. Sodium ion is the predominant effective osmole in the body. See also under tonicity.
E:I ratio: The ratio of extracellular water to intracellular water (ECW/ICW) is normally below 1 (0.7–0.8). An increase in ICW will result in a decrease in the E:I ratio and is seen in patients with heart failure, liver cirrhosis or renal failure, especially in early stage. A decrease in ICW will result in a increase in the E:I ratio and is generally due to osmotic leakage. Finally, an increase in ECW will also increase the E:I ratio and occurs due to shift from intra to extracellular space or capillary leak and resulting second (interstitial) and third space fluid accumulation and/or oedema.
Electrolyte: Dissolved anions and cations in solution carrying a positive or negative electric charge, such as sodium, potassium, chloride and calcium amongst others.
End-expiratory occlusion test: This is a test of fluid responsiveness that consists of pausing the flow of mechanical ventilation at end-expiration for 15 s and measuring the resultant changes in cardiac output. The test increases cardiac preload by stopping the cyclic impediment of venous return that occurs at each insufflation of the ventilator. An increase in cardiac output above the threshold of 5% indicates preload/fluid responsiveness. A continuous monitoring of cardiac output is recommended for an accurate measurement of the change. When the test is performed with echocardiography, it is better to add the effects of an end-inspiratory occlusion and if the combined change in velocity-time integral is greater than or equal to 13% in total, fluid responsiveness is accurately predicted. This threshold is more compatible with the precision of echocardiography than that obtained by end-expiratory occlusion alone, because the diagnostic threshold of changes in stroke volume may vary with precision of echocardiography.
Endothelial glycocalyx (EG): The EG is a thin negatively charged proteinaceous mesh-like layer, a gel-like matrix that surrounds all vascular endothelium on the luminal surface. The endothelial glycocalyx (EG) is easy to imagine. It is like sea grass, a virtual structure that lies flat on the ground where the river or sea is shallow (during low tide). Seagrass stands tall when the river or sea is flooded (Fig. 1.5). It is composed of membrane-bound glycoproteins and proteoglycans. The EG was previously thought to be inert but plays a key role in vascular integrity and function; regulation of vascular permeability, endothelial anticoagulation and modulation of interactions between the endothelium and the vascular environment. Thus, the EG prevents free movement of water and electrolytes. Disruption or degradation of the glycocalyx may be an important mediator of inflammation, oedema, sepsis syndromes and capillary leak syndromes. Hence, in various surgical and disease states the glycocalyx has the potential to be a novel therapeutic target.
Euvolemia: Normal circulating blood volume.
Evacuation phase: A phase during fluid therapy in critically ill patients with a focus on organ recovery and resolving fluid overload (in case of no flow state), characterized by active late goal-directed fluid removal (LGFR) by means of either diuretics or renal replacement therapy with net ultrafiltration. The fluid removal may be performed in combination with hypertonic solutions (hypertonic saline 3% or 7.2% or albumin 20%) in order to obtain a negative fluid balance. See also under, late conservative fluid management, late goal-directed fluid removal and de-resuscitation.
Extracellular water (ECW): Extracellular water is the body water that exists outside of the cell membrane (like blood, interstitial fluid, etc.). The extracellular water can be further subdivided into interstitial, lymphatic fluid, trans-cellular water and blood. This accounts for up to 40% of total body water.
Extravascular fluid (EVF): Fluids that exist or accumulate outside the intravascular space. This accounts for up to 75% of ECW.
Flow phase: This refers to the phase of septic shock after initial stabilization where the patient will mobilize the excess fluid spontaneously, a classic example is when a patient enters a polyuric phase recovering from acute kidney injury (AKI). In contrast to the “ebb” phase, the “flow” phase refers to the time period after the acute circulatory shock has been resolved. In this post-shock phase, the metabolic turnover is increased, the innate immune system is activated, and a hepatic acute-phase response is induced. This hypercatabolic metabolic state is characterized by an increase in oxygen consumption and energy expenditure.
Fluid accumulation: An increase in net fluid balance resulting in the accumulation of excess fluids in body tissues and weight gain and in some cases, peripheral oedema. This results from pathophysiological processes of renal fluid and salt reabsorption. The term fluid accumulation is preferred over fluid overload and describes a pathologic state of overhydration associated with a clinical impact which may vary by age, comorbidity and phase of illness. It may occur with concomitant intravascular hypovolemia, euvolemia and hypervolemia and may or may not be associated with clinical or imaging signs of oedema. It describes a continuum. No specific threshold of fluid balance alone can define fluid accumulation across all individuals. Fluid overload, volume overload and fluid accumulation generally refer to the expansion of the extracellular fluid volume and usually indicate water and sodium retention. The risk for fluid accumulation is dependent on the presence of fluid unresponsiveness in combination with fluid intolerance (Fig. 1.6).
Relationship between fluid responsiveness and fluid tolerance and the risk of fluid accumulation. (Adapted with permission from Monnet et al. [19])
Fluid accumulation syndrome: The term to describe the presence of any degree of fluid accumulation or fluid overload with a negative impact on end-organ function which may or may not be associated with global increased permeability syndrome.
Fluid administration: The administration of fluids (or infusion) to a (critically ill) patient either via the oral, enteral or parenteral route. The rate or speed of fluid administration is usually described in mL/kg/min (for fluid bolus or fluid challenge) or mL/kg/h (for maintenance or nutrition solutions). Fluid rates described in mL/h or mL/min are meaningless unless they are referenced to body weight. The dose, duration and de-escalation of fluid administration should be stated and prescribed.
Fluid balance: See under daily and cumulative fluid balance.
Fluid bolus: A fluid bolus is the rapid infusion of fluids over a short period of time. In clinical practice, a fluid bolus is usually given to correct hypovolemia, hypotension, inadequate blood flow or impaired microcirculatory perfusion. A fluid bolus typically includes the infusion of 4–6 mL/kg of IV fluid over a maximum of 10–20 min.
Fluid challenge: A fluid challenge is a dynamic functional test to assess a patient’s fluid responsiveness by giving a fluid bolus of at least 4 mL/kg over 5–10 min and simultaneously monitoring the evolution of the hemodynamic status to be able to identify fluid responsive state. Recently, it has been shown that in clinical practice there is a marked variability in how fluid challenge tests are performed [20].
Fluid compartments: Describes the distribution of total body water (TBW) within several well-defined spaces separated from each other by cell membranes. Together, the intravascular and interstitial fluid compartments comprise the ECW (see under extracellular water), and contain approximately one-third of the TBW, while ICW (see under intracellular water) contains approximately two-thirds of the TBW. See also under the four spaces and the four compartments.
Fluid creep: A term that refers to the unintentional and unmeasured fluid volumes administered in the process of delivering medication (antibiotics, sedatives, painkillers, etc.) and nutrition through enteral and parenteral routes. Fluid creep can also be described as the administration of fluids in excess of the requirements calculated by the Parkland Formula [not only in severe burns] [21]. It is also a term that refers to the unintentional and unmeasured fluid volumes administered in the process of delivering medication (antibiotics, sedatives, painkillers, etc.) and nutrition through enteral and parenteral routes. It may sum up to 33% of all fluids administered, compared to maintenance (25%), nutrition (33%) and resuscitation (6%) type of fluids [22].
Fluid infusion: See fluid administration.
Fluid loss and gain: Fluid loss is defined as a negative fluid balance, regardless of intravascular status. Fluid gain is the opposite of fluid loss (Table 1.2).
Fluid overload (see overhydration): An increase in total body fluid (both water and electrolytes) in excess of physiologic requirements. Traditionally it is defined as excess fluid buildup in the body and has a negative impact on end-organ function. Some publications identify a threshold of 10% or more cumulative fluid balance for increased risk of adverse effects. There is an ambiguity in this term, as it is interchangeably used in situations of volume overload, which refers to excess fluid in the intravascular fluid compartment, and overhydration, which describes increased total body water but does not necessarily reflect intravascular volume overload. While volume overload usually leads to hypervolemia and hyperhydration resulting in peripheral oedema, the opposite is not always true as oedema can be present in the absence of volume overload. Therefore, some colleagues suggest avoiding the misleading term fluid overload (all or nothing) and using fluid accumulation (graded phenomenon) instead [3].
Fluid refill rate: In stable patients undergoing intermittent haemodialysis, the fluid refill rate is 2–6 mL/kg/h but may exceed 10 mL/kg/h at high rates of ultrafiltration [23]. Since transcapillary refill rate depends on oncotic pressure, vascular integrity and blood pressure, it is reduced during critical illness [24]. A recent study showed that in critically ill adults receiving continuous venovenous hemodiafiltration for acute kidney injury, the rate of net ultrafiltration (UF) at which mortality is increased seemed to be from 1.75 mL/kg/h and certainly from 2.8 mL/kg/h upwards [25].
Fluid responsiveness: Fluid responsiveness indicates a condition in which a patient will respond to fluid administration by a significant increase in stroke volume and/or cardiac output or their surrogates. A threshold of 15% is commonly used for this definition. Physiologically, fluid responsiveness means that a linear relationship exists between cardiac output and cardiac preload, i.e. the steep portion of the slope on the Frank–Starling relationship. However, this may not be true at all times (in euvolemia or hypervolemia) and in all patients (pre-existing cardiac dysfunction). Many studies have shown that fluid responsiveness, which is a normal physiologic condition, exists in only half of the patients receiving a fluid challenge in intensive care units. Different techniques for assessing fluid responsiveness and the thresholds used are shown in Fig. 1.7.
Illustration of the concept of preload dependence. (a) Fluid responsiveness illustrated by a greater increase in mean systemic filling pressure with 7 mmHg (from 22 to 29 mmHg) compared to the 2 mmHg increase in CVP (from 6 to 8 mmHg) resulting in a 15% increase in cardiac output from 5.4 to 6.2 L/min. (b) Fluid unresponsiveness illustrated by an equal increase in mean systemic filling pressure with 4 mmHg (from 24 to 28 mmHg) and a 3 mmHg increase in CVP (from 8 to 11 mmHg) not resulting in a significant increase in cardiac output (from 5.9 to 6.0 L/min)
Fluid resuscitation: See under resuscitation solutions and resuscitation phase.
Fluid or water retention: Non-specific term used for describing the accumulation of excess fluid in body tissues resulting in clinical oedema. See under fluid accumulation.
Fluid space: See under four spaces.
Fluid stewardship: Fluid stewardship is a series of coordinated interventions, introduced to select the optimal fluid, dose and duration of therapy that results in the best clinical outcome, prevention of adverse events and cost reduction with a focus on value-based healthcare.
Fluid therapy: The process of administering fluids as a medical treatment or preventative measure to maintain or restore normal body fluid balance. There are four indications for fluid therapy: resuscitation, maintenance, replacement, and nutrition.
Fluid titration: Adjustment of IV fluid based on choice, type, rate, speed, dose, volume and timing in order to improve hemodynamic stability and optimize tissue perfusion (microcirculation), oxygen delivery and uptake.
Fluid tolerance: Can be defined as the degree to which a patient can tolerate the administration of fluids without causation of organ dysfunction [26]. Fluid tolerance comes to fill in the continuum between fluid responsiveness and fluid overload or accumulation and overcome their inherent limitations (Table 1.3). It balances the impact of fluids during the resuscitation phase from downstream (i.e. organ perfusion) to upstream (i.e. venous congestion). This may allow clinicians to potentially modify their strategy and provide a more harmonic resuscitation.
Fluid underload: Decrease in total body fluid, resulting in a fluid deficit of the extracellular and/or intracellular fluid. Similar to dehydration and the opposite of fluid overload.
Global increased permeability syndrome (GIPS): The term used to describe the ongoing fluid accumulation due to increased vascular permeability; often referred to as “the third hit of shock”. Some patients will not transgress to the “flow” phase spontaneously and will remain in a persistent state of global increased permeability syndrome (GIPS) and ongoing fluid accumulation. It typically has a positive cumulative fluid balance with ongoing capillary leak and organ failure.
Goal-directed therapy (GDT): See under early goal-directed (fluid) therapy (EGDT).
Hyperchloremic metabolic acidosis: Metabolic acidosis caused by hyperchloremia, accompanied by a decrease in bicarbonate levels. Bicarbonate loss or dilution is a possible explanation (Henderson–Hasselbalch approach) for metabolic acidosis. However, according to Stewart’s approach, a decrease in SID caused by an increase in chloride (e.g. after infusion of large amounts of (ab)normal saline 0.9% NaCl) is responsible for metabolic acidosis. Synonym: normal anion gap acidosis.
Hyperdynamic state: Hemodynamic status characterized by supraphysiologic blood flow and cardiac output to the tissues (e.g. thyrotoxicosis, liver cirrhosis, severe burns, severe pancreatitis, morbus Paget, thiamine deficiency (Beri-Beri), multiple myeloma and plasmocytoma, chronic anaemia or polycythemia, AV fistula. Synonym: hyperperfusion.
Hyperhydration (see overhydration).
Hyperoncotic solution: A colloid solution with an oncotic pressure above that of plasma (e.g. 10% hydroxyethyl starch, 20% human albumin).
Hypertonicity: Plasma hypertonicity is accompanied by cell shrinkage (dehydration). The water balance is regulated via antidiuretic hormone (ADH), thirst and the renin–angiotensin–aldosterone system (RAAS).
Hypertonic solution: An IV crystalloid solution with a higher effective osmolality than plasma (e.g. 3% sodium chloride has an osmolality of 1027 mOsm/L). Hypertonic saline is a sterile hypertonic intravenous crystalloid composed of water, sodium and chloride. Available in multiple concentrations including 3%, 5% and 7.2%.
Hypertonic–hyperoncotic solution: A solution containing a hypertonic crystalloid (>310 mOsm/L) in combination with a hyperoncotic (>5%) colloid that is used as an alternative strategy during small-volume fluid resuscitation (e.g. 7.5% saline and 6% Dextran-70, however this solution is no longer available).
Hypervolemia: Hypervolemia is the opposite of hypovolemia and is defined by intravascular overfilling. This can be monitored in different ways: the absence of fluid responsiveness, increased barometric or volumetric preload indicators, and ultrasound findings.
Hypo-oncotic solution: A colloid solution with an oncotic pressure below that of plasma (e.g. 4% human albumin).
Hypoperfusion: Inadequate blood flow to the tissues, resulting in decreased oxygen delivery. End-organ hypoperfusion can clinically manifest as cool extremities, reduced pulse quality (pulsus filiformis), increased capillary refill time, tachycardia and oliguria [27].
Hypotonicity: Plasma hypotonicity is accompanied by cellular oedema (hyperhydration). The water balance is regulated via antidiuretic hormone (ADH) and thirst and the renin–angiotensin–aldosterone system (RAAS).
Hypotonic solution: An IV solution with a lower effective osmolality than plasma (e.g. 0.45% sodium chloride: 154 mOsm/L). It has to be noted that glucose-containing solutions like 5% dextrose in water is also classified as hypotonic solution despite having a normal osmolality (278 mOsm/L) since the dextrose is rapidly taken up into cells and metabolized following infusion, leaving water behind.
Hypovolemia: Hypovolemia is the term used to describe a patient with insufficient total intravascular circulating blood volume. It does not refer to total body fluid but rather refers solely to the intravascular compartment. Absolute hypovolemia can be caused by dehydration (i.e. water and electrolyte loss) or the loss of blood from the body or into a body cavity (e.g. abdomen) [6]. Total body fluid comprises approximately 60% of the body weight of men and 50% of women. Blood volume can be estimated according to Gilcher’s rule of fives at 70 mL/kg for men and 65 mL/kg for women. Blood loss is frequently followed by the recruitment of interstitial fluid and the movement of fluid from the interstitium to the intravascular compartment. Vasoconstriction of the splanchnic mesenteric vasculature is one of the first physiologic responses. Sodium and water retention results from the activation of the RAAS which replenishes the interstitial reserves and maintains trans-capillary perfusion. As a result, the body may lose up to 30% of blood volume before hypovolemia becomes clinically apparent. Therefore, undiagnosed hypovolemia may be present long before clinical signs and symptoms occur. Hypovolemia can also occur in oedematous patients, where total body water is increased, but intravascular volume is reduced (e.g. eclamptic patients). Finally, some patients are fluid responsive but not necessarily hypovolemic. Even the most basic of paradigms, such as the description of early sepsis and distributive shock being a hypovolemic state needing aggressive fluid resuscitation, has recently been called into question, with data suggesting improved outcomes with less or even no administered intravenous fluid. Greater focus on the health and function of the microcirculation and the endothelial glycocalyx, potential new treatment paradigms calling for less fluids and earlier vasopressor use has become the focus. These elements make an accurate assessment of fluid status in the critically ill a challenging task.
Ineffective osmoles: Small dissolved particles in solution that contribute to total osmolality but do not exert an osmotic pressure because they freely cross and equilibrate across cell membranes (e.g. urea, dextrose).
Insensible water loss: Body fluid losses that cannot be easily measured, such as evaporative losses from the skin and respiratory tract, and the water content of the stool. The daily amount of insensible losses can be calculated with Dubois’ formula: 550 mL/body surface area, where body surface area = 71.84 × (body weight in kg)0.425 × (height in cm)0.725. In the case of mechanical ventilation or active humidification, this value can be divided by 2. Temperature corrections can also be made (for each 1 °C increase of temperature above 37 °C, a 13% increase in insensible losses).
Interstitial dehydration: A negative sodium balance leads to a decrease in extracellular volume.
Interstitial fluid: The total volume of extracellular water contained within the interstitial tissues surrounding cells (12–15% of total body weight), or thus the fluid in which cells are bathed.
Interstitial hyperhydration: A positive sodium balance leads to an increase in extracellular volume (interstitial fluid overload).
Intra-abdominal hypertension (IAH): IAH is defined by a sustained or repeated pathologic elevation of IAP ≥12 mmHg. IAH is graded as follows: Grade I: IAP 12–15 mmHg, Grade II: IAP 16–20 mmHg, Grade III: IAP 21–25 mmHg and Grade IV: IAP >25 mmHg [11]. IAH has a tremendous impact on organ function within and outside the abdominal cavity (Fig. 1.8) [28].
Summary of the most important pathophysiologic effects of increased intra-abdominal pressure on end-organ function within and outside the abdominal cavity. AKI acute kidney injury, APP abdominal perfusion pressure, Cdyn dynamic respiratory compliance, CO cardiac output, CPP cerebral perfusion pressure, CVP central venous pressure, EVLW extravascular lung water, GFR glomerular filtration rate, GRV gastric residual volume, HR heart rate, IAP intra-abdominal pressure, ICP intra-cranial pressure, ITP intra-thoracic pressure, MAP mean arterial pressure, PIP peak inspiratory pressure, Paw airway pressures, PCWP pulmonary capillary wedge pressure, pHi intra-mucosal gastric pH, PPV pulse pressure variation, Qs/Qt shunt fraction, RVP renal venous pressure, RVR renal vascular resistance, SMA superior mesenteric artery, SPV systolic pressure variation, SVR systemic vascular resistance, SVV stroke volume variation, Vd/Vt dead-space ventilation
Intra-abdominal pressure (IAP): Intra-abdominal pressure (IAP) is the pressure concealed within the abdominal cavity. IAP should be expressed in mmHg and measured at end-expiration in the complete supine position after ensuring that abdominal muscle contractions are absent and with the transducer zeroed at the level of the mid-axillary line. The reference standard for intermittent IAP measurement is via the bladder with a maximal instillation volume of 20–25 mL of sterile saline. Normal IAP is approximately 5–7 mmHg and around 10 mmHg in critically ill adults [11].
Intracellular volume (ICV): See intracellular water.
Intracellular water (ICW): Intracellular water is the body water that exists inside the cell membrane, or thus the fluid of all body cells, and comprises 60% of total body water or 40% of total body weight. Water balance is regulated via ADH, thirst and the RAAS.
Intravascular fluid (IVF): Fluids that exist inside the intravascular space (arteries, veins and capillaries) and account for up to 6–8% of total body weight.
Isooncotic solution: A colloid solution with an oncotic pressure similar to that of plasma (e.g. 6% hydroxyethyl starch, 5% human albumin).
Isotonic solution: An IV solution with an effective osmolality close to that of normal plasma (278 mOsm/L). The osmolality of intravenous solutions containing sodium (the main driver for osmolality) is approximately 0.93 × the osmolarity, due to the fact that sodium chloride is not 100% disassociated in solution and plasma (but rather 93%). Therefore, the osmolality of both plasma and 0.9% saline is approximately 287 mOsm/kg (308 mOsm/L × 0.93) [29].
Late conservative fluid management (LCFM): LCFM describes a moderate fluid management strategy following the initial EAFM in order to avoid (or reverse) fluid overload. Recent studies showed that LCFM, defined as two consecutive days of negative fluid balance within the first week of the ICU stay is a strong and independent predictor of survival [30]. LCFM must be adapted according to the variable clinical course of septic shock during the first days of ICU treatment, e.g. patients with persistent systemic inflammation maintain trans-capillary albumin leakage and do not reach the flow phase mounting up positive fluid balances.
Late goal-directed fluid removal (LGFR): LGFR describes that in some patients more aggressive and active fluid removal by means of diuretics or renal replacement therapy with net ultrafiltration being needed either or not in combination with hypertonic solutions to mobilize the excess interstitial oedema. This is referred to as de-resuscitation, a term that was coined for the first time in 2014 [1].
Liberal vs. restrictive fluid therapy: Term applied to studies looking at the effect on morbidity and mortality of a conservative (restrictive) fluid strategy, compared to a standard (liberal) fluid regimen [31, 32]. Most standard and liberal fluid regimens are more likely to result in a positive fluid balance [33, 34]. The fluid strategy is probably more important than the fluid itself (Fig. 1.9).
The fluid strategy, protocol or guidelines are more important than the type of fluid itself in relation to morbidity and mortality. In view of some recent results and insights, early liberal should probably be replaced by early adequate fluid management. EL early liberal, ER early restrictive, LL late liberal, LR late restrictive
Macrocirculation: Large and medium-sized arteries and veins that serve as conduit vessels, transporting blood and oxygen to and from organs.
Maintenance solutions: These IV fluids are given to cover the daily needs for water (1 mL/kg/h), glucose (1–1.5 g/kg/day) and electrolytes, mainly potassium (0.75–1.25 mmol/kg/day), sodium (1–1.5 mmol/kg/day), phosphate (0.1–0.5 mmol/kg/day), chloride (1 mmol/kg/day), calcium (0.1–0.2 mmol/kg/day) and magnesium (0.1–0.2 mmol/kg/day).
Mean systemic or circulatory filling pressure (Pmsf): Pmsf is the blood pressure throughout the vascular system at zero flow and offers information on vascular compliance, volume responsiveness and it allows the calculation of (un)stressed volume. For the determination of mean circulatory filling pressure, two bedside methods are available, either based on inspiratory hold-derived venous return curves (Pmsf hold) or on arterial and venous pressure equilibration (Pmsf arm) [35]. Pmsf hold is based on the linear relation between CVP and venous return (VR): VR = (𝑃𝑚𝑠𝑓−𝐶𝑉𝑃)/𝑅𝑉𝑅, where RVR is the resistance to VR. Hereby, the CVP is increased by performing a series of end-inspiratory hold manoeuvres and CO is measured in the last 3 s of the 12 s inspiratory hold. After 7–10 s, a steady state occurs when VR = CO. By plotting the CVP and CO values, a VR curve is constructed and the zero-flow pressure (Pmsf) is extrapolated (Fig. 1.10). As Pmsf is defined as the steady-state blood pressure during no-flow conditions, the arm is used to estimate the Pmsf arm. The upper arm is occluded to 50 mmHg above systolic blood pressure, using a rapid cuff inflator or a pneumatic tourniquet. Measurements of arterial and venous pressures through a radial artery catheter and a peripheral venous cannula in the forearm are performed. When these two pressures equalize, Pmsf arm values are obtained.
Integrated venous return curve (a) and cardiac function/output curve (b). The intersection of these two curves (c) is the working point of the circulation. The central venous pressure (CVP) when venous return equals zero is the Pmsf (d). The slope of the VR is determined by the resistance to venous return. (Adapted with permission from Wijnberge et al. under the Open Access CC BY License 4.0 [35])
Microcirculation: Blood vessels <200–300 μm in diameter, consisting of small arteries, arterioles, capillaries and venules where oxygen diffuses to the tissues.
Mini fluid challenge: A mini fluid challenge is a dynamic functional test to assess a patient’s fluid responsiveness by giving a fluid bolus of 1 mL/kg over 1–5 min to predict the fluid responsiveness (10% increase in VTI) of a full fluid challenge.
Nutrition solutions: These solutions come in different types and can be given either orally (total enteral nutrition, TEN) or intravenously (total parenteral nutrition, TPN). When given instead of maintenance solutions they should cover the daily needs for water as well as the daily caloric needs for glucose, lipids, protein, including essential amino-acids, vitamins and trace elements.
Oncoticity: Refers to the oncotic pressure, also called the colloid osmotic pressure (COP), which is a form of osmotic pressure (see tonicity) exerted by the amount of proteins in the intravascular space. Oncotic pressure shifts fluids from the extravascular space, or interstitial tissues, into the intravascular space. Hence, depleted COP increases the risk of interstitial and generalized oedema. A higher COP of a fluid prolongs the intravascular half-life and consequently results in prolonged effects on blood pressure and perfusion. When given 1 L of fluids, the intravascular volume will be increased after 1 h with 83 mL for dextrose 5% in water, 250 mL for saline or balanced crystalloids and 1000 mL for a colloid solution.
Ongoing fluid losses: See replacement fluids.
Optimization phase: The optimization phase focuses on organ rescue (maintenance) and avoiding fluid overload (fluid creep). Aiming for neutral fluid balance.
Osmolality: A measure of the concentration of osmotically active particles per unit volume of solution, measured in milliosmoles per litre of solution (mOsm/L). In clinical practice, osmolarity and osmolality are similar enough to be used interchangeably. It determines the tolerability of a solution. Serum osmolality can be calculated by the simplified formula: 2 × serum sodium (mmol/l) + BUN (blood urea nitrogen)/2.8 (mg/dL) + glucose/18 (mg/dL) or 2 × Na + BUN + glucose (all in mmol/L) [36].
Osmolal gap (serum): measured serum osmolality—calculated serum osmolality.
Osmolarity: A measure of the concentration of osmotically active particles per unit mass of solution, measured in milliosmoles per kilogram of solution (mOsm/kg). Figure 1.11 shows the regulation of vasopressin secretion by plasma osmolarity.
Control of vasopressin (ADH) secretion by plasma osmolarity and circulating blood volume in a shock state. Hypovolemia results in increased plasma osmolarity and decreased arterial pressure that both will increase vasopressin release. Negative and positive feedback loops are indicated with (−) and (+), respectively. ADH antidiuretic hormone, AT-II angiotensin II, CO cardiac output, CVP central venous pressure, ECW extracellular water, GEDVI global end-diastolic volume index, IVF intravascular fluid, MAP mean arterial pressure, RAAS renin–angiotensin–aldosterone system, RPP renal perfusion pressure, VR venous return
Osmosis: Osmosis or diffusion is the movement of water across a semi-permeable membrane from a less concentrated solution into a more concentrated solution.
Overhydration (see also fluid overload and fluid accumulation): A state of having a positive fluid balance or description of a state where there is excess water in the body. Dividing the cumulative fluid balance in litres by the patient’s baseline body weight and multiplying by 100 defines the percentage of fluid accumulation. Overhydration at any stage is the opposite of dehydration and can be classified as mild (5%), moderate (5–10%) or severe (>10%) fluid accumulation. Overhydration is also associated with worse outcomes. Fluid administration potentially induces a vicious cycle, where interstitial oedema induces organ dysfunction that contributes to fluid accumulation.
Oxygen consumption (VO2): The amount of oxygen consumed by the cells. Can be calculated as the difference between oxygen delivery (DO2) measured at the arterial side versus the mixed venous side. Simplified formula: VO2 = (SaO2 − SvO2) × CO × Hgb; with SaO2: arterial oxygen satuation, SvO2: mixed venous oxygen saturation, Hgb: Haemoglobin.
Oxygen delivery (DO2): The amount of oxygen delivered to the cells can be calculated as follows: cardiac output × total arterial oxygen content. With cardiac output equal to heart rate multiplied by stroke volume. And total oxygen content is defined by the oxygen bound to haemoglobin (Hgb × Sat × 1.36) plus the oxygen freely dissolved in the plasma (pO2 × 0.0036). Simplified formula: DO2 = SaO2 × CO × Hgb. The DO2 vs VO2 relationship is illustrated in Fig. 1.12.
Simplified oxygen delivery and oxygen consumption (DO2-VO2) relationship in normal conditions (black line) and sepsis (red line). The initial part shows supply dependency from the critical DO2 (red dot) value onwards. At this point ScvO2 (mixed venous oxygen saturation) will drop and lactate will increase illustrating DO2-VO2 imbalance and anaerobic metabolism
Parenteral: Administration of food or medication through a non-enteral (e.g. non-oral) route, such as intravenous, subcutaneous, intramuscular and intradermal.
Parkland formula: A fluid resuscitation protocol for burn patients which suggests administration of a balanced crystalloid solution (e.g. Ringer’s lactate) dosed at 4 mL/kg/% of the total body surface area burned (TBSA) [37, 38]. Half of the volume is then delivered over the first 8 h and the remainder over the next 16 h.
Passive leg raising test: The passive leg raising test is aimed at evidencing fluid responsiveness. It consists of moving a patient from the semi-recumbent position to a position where the legs are lifted at 45° and the trunk is horizontal. The transfer of venous blood from the inferior limbs and the splanchnic compartment towards the cardiac cavities mimics the increase in cardiac preload induced by fluid [39]. In general, the threshold to define fluid responsiveness with the passive leg raising test is a threshold of 10% increase in stroke volume and/or cardiac output.
Perfusion: The passage of fluid through the circulatory system to organs and tissues.
Permissive hypotension: Permissive hypotension involves keeping the blood pressure low enough (systolic pressures <90 mmHg) to avoid exacerbating uncontrolled haemorrhage while maintaining perfusion to vital end-organs. The potential detrimental mechanisms of early, aggressive crystalloid resuscitation are well known and the limitation of fluid intake by using colloids, hypertonic saline or hyperoncotic albumin solutions has been associated with favourable effects [40]. Hypertonic saline allows not only for rapid restoration of circulating intravascular volume with less administered fluid but also attenuates post-injury oedema at the microcirculatory level and may improve microvascular perfusion.
Pharmacodynamics: Pharmacodynamics relates the drug concentrations to its specific effect. For fluids, the Frank–Starling relationship between cardiac output and cardiac preload is the equivalent of the dose–effect curve for standard medications. Because of the shape of the Frank–Starling relationship, the response of cardiac output to the fluid-induced increase in cardiac preload is not constant (Fig. 1.13).
Frank–Starling curve adapted from the original paper by Patterson and Starling where central venous pressure (CVP) was on the Y-axis and cardiac output on the X-axis [41]. The dark lines represent the individual curves obtained per dog in the experiment. The blue line is the mean interpolation. The light blue arrows indicate fluid loading: (1) great increase in stroke volume (SV) in a state of low preload; (2) moderate increase in SV in case of intermediate preload and (3) absent increase in SV after fluid bolus in case of high preload. This reflects the dose–response or dose–effect curve or thus the pharmacodynamics for fluids. The dotted red lines indicate the separation between low–intermediate–high preload
Pharmacokinetics: Describes how the body affects a drug resulting in a particular plasma and effect site concentration. Pharmacokinetics of intravenous fluids depend on distribution volume, osmolality, tonicity, oncoticity and kidney function. Eventually, the half-time depends on the type of fluid and also on the patient’s condition and the clinical context.
Plasma: The portion of blood that remains after the cells are removed. Plasma is retrieved by centrifugation of an anticoagulated blood sample, and unlike serum, it contains fibrinogen and clotting factors.
Prediction of fluid responsiveness: A process that consists of predicting before fluid administration whether or not fluid administration will increase cardiac output. It avoids unnecessary fluid administration and contributes to reduce the cumulative fluid balance. It also allows one to undertake fluid removal being sure that it will not result in a hemodynamic impairment. Prediction of fluid responsiveness cannot be achieved with static markers of cardiac preload, such as the central venous pressure, the pulmonary artery occlusion pressure and its echocardiographic estimates or the left ventricular end-diastolic dimensions. It is based on a dynamic assessment of the cardiac output/preload relationship. The classic fluid challenge predicts fluid responsiveness but is inherently associated with fluid boluses that do not increase cardiac output. The respiratory variations of stroke volume and its surrogates (arterial pulse pressure, aortic blood flow, maximal velocity in the left ventricular outflow tract, amplitude of the plethysmographic signal) in patients under mechanical ventilation are reliable predictors of fluid responsiveness but are not reliable in some conditions, the most common being spontaneous breathing, cardiac arrhythmias, ventilation at low tidal volume and low lung compliance. The respiratory variation in the diameter of the inferior and superior venae cavae shares the same limitations, except cardiac arrhythmias. The passive leg raising (see below) and the end-expiratory occlusion test are reliable in these circumstances. The threshold to define fluid responsiveness depends on the change in cardiac preload induced by the test (e.g. 15% for the fluid challenge, 10% for the PLR test and 5% for the end-expiratory occlusion test) (Fig. 1.14).
Tests and indices of preload responsiveness with proposed timeline. The principle of the dynamic assessment of preload responsiveness is to observe spontaneous or induced changes in cardiac preload, and the resulting change in cardiac output, stroke volume or their surrogates. Some tests or indices use heart–lung interactions in mechanically ventilated patients, while some others mimic a classical fluid challenge. Diagnostic threshold and year of description are indicated. CO cardiac output, PPV pulse pressure variation. (Adapted with permission from Monnet et al. [19])
Plasma volume: See volemia.
Preload: From a theoretical point of view preload is the initial stretch on a single myocyte prior to contraction. From a practical point, preload is the LVEDV and corresponding LVEDP that stretches the left ventricle to its greatest dimensions under variable physiologic demand. Ideally, preload is a (combination of) parameter(s) that tell(s) the clinician if fluids are needed and can be given safely. In real life, preload is also affected by the afterload and contractility as illustrated schematically in Fig. 1.15.
Pulmonary West zones: West zones describe areas of the lung based upon variations in pulmonary arterial pressure (pa), pulmonary venous pressure (pv) and alveolar pressure (pA). These differences result from a 20 mmHg increase in blood flow found in the base of the lung relative to the apex as a result of gravity in an upright patient. The lung can be divided into discrete regions according to the interplay between pA, pa and pv. These regions are zone 1, where alveolar pressure is higher than arterial or venous pressure; zone 2, where the alveolar pressure is lower than the arterial but higher than the venous pressure and zone 3, where both arterial pressure and venous pressure are higher than alveolar. This is illustrated in Fig. 1.16.
Evolution of West zones during hypovolemia and PEEP. Left panel shows normal West zone distribution. The middle panel shows the situation in a patient under mechanical ventilation and hypovolemia where zone 1 conditions (pA > pa > pv) expand to zone 2 (pa > pA > pv) and zone 2 conditions to zone 3 (pa > pv > pA). With pa arterial capillary pressure, pA > alveolar pressure and pv venous capillary pressure. The right panel shows the situation in a hypovolemic patient with excessive PEEP causing further excursion of zone 1 conditions to zone 3 resulting in a right-to-left shunt explaining the premature hump seen on the transpulmonary thermodilution curve. (Adapted with permission from Hofkens et al. [42])
Pulse pressure (PP): The difference between arterial systolic and diastolic blood pressure measured in millimetres of mercury (mmHg) as illustrated in Fig. 1.17.
Schematic representation of the waterfall-effect. CO cardiac output, represented by the pump. CVP central venous pressure, MAP mean arterial pressure, represented by the height of the water tower, MSFP mean systemic filling pressure, PP pulse pressure, RVR resistance to venous return, SVR systemic vascular resistance, VR venous return
Pulse pressure variation (PPV): The mean difference between the maximum (PPmax) and minimum (PPmin) arterial pulse pressures during a series of respiratory cycles, expressed as a percentage [42]. PPV is a functional hemodynamic parameter that predicts fluid responsiveness in mechanically ventilated patients.
Redistribution: When a fluid is infused into the body via an intravenous (IV) route, it is introduced directly into the bloodstream. As a result, there can be an initial change in the volume and distribution of body fluids as the body responds to the extra influx of fluids. Intravenous infusion can cause a temporary increase in the volume of fluid in the circulatory system, which can result in an increase in blood pressure and/or stroke volume. This can trigger a response from the kidneys, which attempt to compensate by excreting more fluid and electrolytes. The net result of this process is a redistribution of fluid from the circulatory system to other body compartments, such as the interstitial spaces (the spaces between cells) and the intracellular spaces (the fluid within cells). The speed and extent of fluid redistribution can be influenced by a variety of factors, including the rate of infusion, the characteristics of the fluid being infused, and the overall health status and comorbidities of the patient receiving the infusion. In some cases, fluid redistribution can lead to unwanted side effects, such as swelling, oedema or electrolyte imbalances. However, in many cases, the body is able to adjust to the changes in fluid volume and distribution and return to a state of equilibrium relatively quickly. One must bear in mind that every ml of fluid that is infused will be lost at some point to the extravascular space (Fig. 1.18).
Theoretical redistribution of intravenous fluids after infusion for a 70 kg man. (Adapted with permission from Frost [43])
Relative hypovolemia: See vasoplegia.
Replacement solutions: These IV fluids are given to replace ongoing fluid losses (insensible losses, diarrhoea, vomiting, during fever, burns, etc.). As such they must resemble as close as possible to the fluid that is lost. Ongoing fluid losses refer to the continuous loss of fluids from the body over time. Fluid losses can occur through a variety of mechanisms, including urine output, sweating, breathing and digestive processes. In order to maintain proper hydration and prevent dehydration, it is important to replenish fluid losses through drinking fluids and consuming foods that contain water. The amount of fluids needed will vary depending on factors such as age, activity level and environmental conditions. In cases of excessive fluid loss, such as due to illness or exercise, it may be necessary to increase fluid intake to avoid dehydration. Figure 1.19 gives an overview of the different potential ongoing fluid losses per day.
Diagram of ongoing losses. (Adapted from National Clinical Guideline Centre “Intravenous fluid therapy in adults in hospital”, NICE clinical guideline 174 (December 2013) © National Institute for Health and Care Excellence 2013. All rights reserved. https://www.nice.org.uk/guidance/cg174/resources/diagram-of-ongoing-losses-pdf-191664109)
Resuscitation phase: Life-saving resuscitation phase with focus on patient rescue and early adequate fluid management (EAFM), e.g. 30 mL/kg/1 h according to surviving sepsis campaign guidelines or a fluid challenge/bolus of 4 mL/kg given in 5–10 min.
Resuscitation solutions: These IV fluids are given to restore and stabilize hemodynamic status in order to save lives in patients with shock and imbalance between oxygen delivery and oxygen consumption illustrated by an increase in serum lactate levels beyond 3 mmol/L.
Revised Starling equation: An updated version of the traditional Starling equation that incorporates current understanding of the role of the endothelial glycocalyx in transvascular fluid filtration, also known as the Starling Principle.
ROSE: A conceptual framework that describes four different stages of fluid resuscitation, beginning with initial rapid fluid administration to treat life-threatening shock (Rescue), continued fluid therapy until adequate perfusion is restored (Optimization), followed by ongoing maintenance fluids (Stabilization) and gradual discontinuation of fluid support (i.e. evacuation or de-escalation) [1, 5, 44, 45]. This is illustrated in Fig. 1.20.
Graph showing the four-hit model of shock with evolution of patients’ cumulative fluid volume status over time during the five distinct phases of resuscitation: Resuscitation (R), Optimization (O), Stabilization (S) and Evacuation (E) (ROSE), followed by a possible risk of hypoperfusion in case of too aggressive de-resuscitation. On admission patients are often hypovolemic, followed by normovolemia after fluid resuscitation (escalation or EAFM, early adequate fluid management), and possible fluid overload, again followed by a phase returning to normovolemia with de-escalation via achieving zero fluid balance or late conservative fluid management (LCFM) and followed by late goal directed fluid removal (LGFR) or de-resuscitation. In the case of hypovolemia, O2 cannot get into the tissue because of convective problems, in the case of hypervolemia O2 cannot get into the tissue because of diffusion problems related to interstitial and pulmonary oedema and gut oedema (ileus and abdominal hypertension). Adapted from Malbrain et al. with permission, according to the Open Access CC BY License 4.0 [5]. * Volumetric preload indicators such as GEDVI, LVEDAI or RVEDVI are preferred over barometric ones like CVP or PAOP. ** Vasopressor can be started or increased to maintain MAP/APP above 55/45 during the de-resuscitation phase. # can only be measured via the Swan-Ganz pulmonary artery catheter (PAC) and became obsolete. APP abdominal perfusion pressure (APP = MAP-IAP), BIA bio-electrical impedance analysis, CI cardiac index, CLI capillary leak index (serum CRP divided by serum albumin), COP colloid oncotic pressure, CVP central venous pressure, EAFM early adequate fluid management, ECW/ICW extracellular/intracellular water, EVLWI extravascular lung water index, FAS fluid accumulation syndrome, GEDVI global end-diastolic volume index, GIPS global increased permeability syndrome, IAP intra-abdominal pressure, ICG-PDR indocyanine green plasma disappearance rate, IVCCI inferior vena cava collapsibility index, LCFM late conservative fluid management, LGFR late goal-directed fluid removal, LVEDAI left ventricular end-diastolic area index, MAP mean arterial pressure, PAOP pulmonary artery occlusion pressure, PF PaO2 over FiO2 ratio, PLR passive leg raising, PPV pulse pressure variation, PVPI pulmonary vascular permeability index, RVEDVI right ventricular end-diastolic volume index, ScvO2 central venous oxygen saturation, SSCG surviving sepsis campaign guidelines, SvO2 mixed venous oxygen saturation, SVV stroke volume variation, VE volume excess (from baseline body weight), VExUS venous congestion by ultrasound
Saline: Normal saline, also known as 0.9% sodium chloride (NaCl) solution, is a type of intravenous infusion fluid that is commonly used in medical settings. It is a sterile solution of water and sodium chloride, with a concentration of 9 g of salt per litre of water and 3.5 g of sodium (the daily dietary requirement being 2.3 g). Normal saline is used for a variety of purposes, including to restore fluid and electrolyte balance, to flush intravenous lines, to dilute medications or to maintain intravenous access. Normal saline is generally considered safe and well-tolerated, however there are substantial side effects. Excessive infusion of normal saline can lead to salt and fluid accumulation, electrolyte (increased sodium, chloride and potassium levels) and acid–base disturbances (hyperchloremic metabolic acidosis), increased vasopressor and RRT need and AKI (Fig. 1.21). A recent meta-analysis showed that normal saline should also be used with caution in patients with sepsis, burns or diabetic ketoacidosis as well as those with heart failure, kidney disease or other conditions that can affect fluid and electrolyte balance [46]. The only indications for abnormal saline left are patients with traumatic brain injury and those with excessive gastro-intestinal losses.
Deleterious effects of excessive intravenous abnormal saline infusion. AKI acute kidney injury, APP abdominal perfusion pressure, Cl- chloride, CS compartment syndrome, EGL endothelial glycocalix layer, FAS fluid accumulation syndrome, GFR glomerular filtration rate, GI gastrointestinal, IAH intra-abdominal hypertension, IAP intra-abdominal pressure, IS interstitial, K+ potassium, MAP mean arterial pressure, Na+ sodium, RRT renal replacement therapy
Sensible water loss: Measurable macroscopic body fluid losses, such as urine production, vomiting and diarrhoea.
Serum: The portion of plasma that does not contain fibrinogen and clotting factors.
Shock state: A life-threatening, generalized form of acute circulatory failure associated with imbalance between oxygen delivery and oxygen consumption, resulting in inadequate anaerobic oxygen metabolism by the cells. Shock can be classified into four major classifications: hypovolemic shock refers to reduced effective circulating volume, from internal or external intravascular fluid loss; obstructive shock results from physical impairment to blood flow, such as from thromboembolic disease; distributive shock is caused by maldistribution of blood flow due to loss of vasomotor tone, such as during sepsis or anaphylaxis and cardiogenic shock describes cardiac pump dysfunction resulting in decreased forward flow.
Stabilization phase: Stabilization phase with focus on organ support (homeostasis). Late conservative fluid management (LCFM) is defined as two consecutive days of negative fluid balance within the first week.
Standard base excess (SBE): Standard base excess or the base excess of the extracellular fluid is the amount of strong acid (millimoles per litre) that needs to be added in vitro to 1 L of fully oxygenated whole blood to return the sample to standard conditions (pH of 7.40, PCO2 of 40 mmHg, and temperature of 37 °C), at a haemoglobin concentration of ~50 g/L. Standard base excess has been adjusted to reflect the extracellular fluid buffering capacity of haemoglobin in vivo, which is not the case for traditional base excess. It is used clinically to determine the degree of metabolic acidosis.
Starling principle: Traditional principle describing the fluid passage across the semipermeable capillary membrane, which is determined by the net result between hydrostatic and oncotic pressures such that fluid leaves the capillary at the arterial end of the capillary and is absorbed at the venous end of capillary (Fig. 1.22).
Stewart’s approach to acid–base: In the late 1970s Peter Stewart, a Canadian biophysicist, described a quantitative approach to acid–base disorder. His approach was based upon fundamental physicochemical properties of a solution that include principles of electroneutrality, law of conservation of mass and dissociation equilibrium of all incompletely dissociated substances in a solution.
Stressed volume: The circulating blood volume that creates positive transmural pressure via the elastic recoil of the vessel wall is termed “stressed volume”. See also under vasoplegia.
Stroke volume variation (SVV): The mean difference between the maximum (SVmax) and minimum (SVmin) stroke volume during a series of respiratory cycles, expressed as a percentage [42]. SVV is a functional hemodynamic parameter that predicts fluid responsiveness in mechanically ventilated patients.
Strong ion: An anion or cation that is considered to be fully dissociated at physiologic pH. The major strong anions are sulphate, chloride and lactate, while the major strong cations in plasma are calcium, sodium and magnesium.
Strong ion difference (SID): The difference between the concentrations of strong cations and strong anions in plasma.
Strong ion gap (SIG): SIG is a predictor of morbidity and mortality and quantifies [unmeasured anions] − [unmeasured cations] of both strong and weak ions. It reflects the difference between the activity of all common cations (Na+, K+, Mg2+, Ca2+) and the common anions (Cl−, lactate, urate) and other measured non-volatile weak acids (A−). SIG is calculated as SIDa − SIDe, or more specifically, as [Na+] + [K+] + [Mg2+] + [Ca2+] − [Cl− corrected] − [lactate] − [A−] − [HCO3−], in milli-equivalents per litre; where SIDa is the apparent strong ion difference and SIDe is the effective strong ion difference.
Surviving sepsis campaign guidelines (SSCG): Sepsis and septic shock are leading causes of death worldwide. The international Surviving Sepsis Campaign (SSC) is a joint initiative of the European Society of Intensive Care Medicine (ESICM) and the Society of Critical Care Medicine (SCCM). The SSC is led by multidisciplinary international experts committed to improving time to recognition and treatment of sepsis and septic shock. Initiated in 2002 at the ESICM’s annual meeting with the Barcelona Declaration, the campaign progressed has several aims, including the development of guidelines for diagnosis, treatment and post-ICU care of sepsis and a reduction of mortality from sepsis. The latest update was done in 2021 [47, 48].
Sweating ongoing loss: Sweat is produced by the sweat glands in response to heat or exercise, and helps to regulate body temperature. The amount of sweat produced can vary depending on environmental conditions, activity level and individual factors such as age and fitness level. See also replacement fluids.
Therapeutic dilemma: A therapeutic conflict is a situation where each of the possible therapeutic decisions carries some potential harm. In high-risk patients, the decision about fluid administration should be made within the context of a therapeutic conflict. Therapeutic conflicts are the biggest challenge for protocolized cardiovascular management in anaesthetized and critically ill patients. A therapeutic conflict is where our decisions can make the most difference (Fig. 1.23).
Diagnostic and therapeutic options when confronted with a therapeutic dilemma: one must always outweigh the potential risks and benefits of fluid administration versus fluid removal. CVP central venous pressure, EVLW extravascular lung water, GEDVI global end-diastolic volume index, LAP left atrial pressure, LVEDAI left ventricular end-diastolic area index, PCWP pulmonary capillary wedge pressure, P/F PaO2 over FiO2 ratio, PLR passive leg raising test, PPV pulse pressure variation, PVPI pulmonary vascular permeability index, RVEDVI right ventricular end-diastolic volume index, SPV systolic pressure variation, SVV stroke volume variation, TEO tele-expirtatory occlusion test
Third space fluid: Fluid without a physiological function confined in a body fluid compartments or spaces that is anatomically separated from other compartments. Fluid movement to these spaces may occur following overzealous intravenous fluid administration, but need probably to be included in the interstitial fluids. Because third space fluids are reabsorbed into the central fluid compartment some consider them to be a myth.
Tonicity: The measurement of the effective osmolality of a solution (also referred to as osmotic pressure) is the concentration of a solution as described by total solutes per volume which corresponds to its ability to cause water to diffuse across a semi-permeable membrane, such as the cell membrane. The tonicity determines the distribution of the given solution. The cell will shrink when placed in a hypertonic solution, swell when placed in a hypotonic solution and the cell volume will remain unchanged when placed in an isotonic solution. Serum osmolality can be calculated by the simplified formula: 2 × serum sodium (in mmol/L).
Total body water (TBW): Total body water is the body water that exists in- and outside of the cell membrane: TBW = ICW + ECW. Accounts for 55% (female) up to 65% (man) of total body weight (Fig. 1.24).
Body water composition for an adult 80 kg male. ECW extracellular water, ICW intracellular water, ISF interstitial fluid, IVF intravascular fluid, TBW total body water, TCF transcellular fluid. These fluids are contained within epithelial lined spaces. Examples of this fluid are cerebrospinal fluid, pleural fluid, pericardial fluid, peritoneal fluid, bladder urine, eye fluid, joint fluid, etc.
Unstressed volume: The volume inside a vessel at near zero transmural pressure is termed “ unstressed volume” . This volume fills the system without exerting tension on the vessel wall. The sum of the stressed (~30% of total volume) and unstressed (~70% of total volume) volumes is the total blood volume within the venous system. More than 70% of total circulating blood volume (CBV) is located in the large veins, and increased vascular compliance (vasoplegia) like in sepsis or induced by anesthesia can cause a substantial increase in CBV (with up to 80%). This increased venous compliance and increased CBV in early sepsis may represent a recruitable source of preload, as veins are very sensitive to low doses of vasopressor. These drugs directly convert unstressed blood volume to stressed blood volume while maintaining nearly normal venous elastance (Fig. 1.25).
Effect of fluid loading and venoconstriction on volume. (a) Effect of volume loading on mean systemic filling pressure (Pmsf) and (un)stressed volume. Administration of a fluid bolus increases Pmsf (from Pmsf1 to Pmsf2, indicated respectively by position A (red dot) to B (green dot) on the pressure/volume curve). Unstressed volume remains constant while stressed volume increases. Total volume = unstressed + stressed increases, carrying a risk for fluid overload. (b) Effect of venoconstriction and venodilation on mean systemic filling pressure (Pmsf) and (un)stressed volume. Venoconstriction increases Pmsf (from Pmsf1 to Pmsf2, indicated respectively by position A (red dot) to B (green dot) on the pressure/volume curve). Unstressed volume decreases while stressed volume increases. Total volume = unstressed + stressed remains constant, resulting in an auto-transfusion effect. Venodilation as seen in sepsis (vasoplegia) decreases Pmsf (from Pmsf1 to Pmsf3, indicated respectively by position A (red dot) to C (blue dot) on the pressure/volume curve). Unstressed volume increases while stressed volume decreases. Total volume = unstressed + stressed remains constant, resulting in an intravascular underfilling effect. (Adapted according to the Open Access CC BY License 4.0 with permission from Jacobs et al. [49])
Urine output: The kidneys filter waste products and excess fluids from the blood, and these are excreted from the body as urine. The amount of urine produced can be affected by factors such as hydration status, medications and underlying medical conditions. Urine output is normally between 1 and 2 mL/kg/h and is sometimes used as a resuscitation target in severely burned patients. Oliguria <0.3–0.5 mL/kg/h and anuria are part of the KDGO definition of AKI. See also replacement fluids.
Vascular volume: See circulating blood volume.
Vasoplegia: Condition characterized by a low systemic vascular resistance in conjunction with profound hypotension and a normal to increased cardiac output. Also known as vasoplegic, distributive or hyperdynamic shock. Results in relative hypovolemia with a reduction in the effective circulating blood volume due to venodilation and increased venous capacitance. The early use of vasopressors may cause an autotransfusion effect with an increase in mean systemic filling pressure and redistribution and recruitment of preload from the venous side, thereby increasing the stressed volume towards the central circulation [49].
Volemia (plasma volume): Anatomically defined as the volume limited by the vascular endothelium. The effective arterial blood volume that really perfuses organs; cannot be really measured. Interstitial and plasma volume can be uncoupled = most frequently interstitial fluid accumulation associated with hypovolemia (plasma dehydration). Euvolema normal volemia, hypovolemia low volemia, hypervolemia high volemia.
Volume depletion: The loss of water and electrolytes from the extracellular fluid compartment.
Volume kinetics: This is an adaptation of pharmacokinetic theory that makes it possible to analyse and simulate the distribution and elimination following an infusion of intravenous fluids. Applying this concept, it is possible, by simulation, to determine the infusion rate that is required to reach a predetermined plasma volume expansion.
Volume overload: See fluid overload and fluid accumulation.
Water: Because water contains a lot of small water molecules (with a molar mass of 18 Da and a molecular weight of 18 g/mol), the molar concentration of water is about 55.3 mol/L at 37 °C. There are much less hydrogen ions than there are water molecules in a glass of water. In reality water is only slightly dissociated and a virtually inexhaustible source of protons (H+). The concentration of [H+] is therefore solely determined by the dissociation of water and not by the amount of [H+] that is added or removed. According to Stewart’s approach, the three independent variables that determine the dissociation of water and hence the amount of [H+] are the strong ion difference (SID), the amount of weak acids and the partial pressure of carbon dioxide (pCO2).
Weak ion: An anion or cation that is not fully dissociated at physiologic pH.
West zones (abdominal): See abdominal West zones.
West zones (pulmonary): See pulmonary West zones.
Worsening renal function (WRF): Recent interest has focused on worsening renal function (WRF), a situation strongly related to mortality, but seemingly only when heart failure status deteriorates [50]. Worsening renal function (WRF) is defined as a 0.3–0.5 mg/dL rise in serum creatinine or a decrease in glomerular filtration rate (GFR) of 9–15 mL/min during hospitalization for acute decompensated heart failure. From the patients with acute decompensated heart failure who develop WRF, 66% die within 1 year [51]. Previous data have shown that CVP may even be more important than CO to predict WRF especially in patients with ADHF as well as sepsis [52, 53] (Fig. 1.26).
Statistical model of nonparametric logistic regression showing the relationship between mean central venous pressure during the first 24 h after admission and the probability of new or persistent acute kidney injury. Note the plateau for the incidence of acute kidney injury (AKI) when the lower limit of central venous pressure (CVP) was between 8 and 12 mmHg. Over this limit, the rise in CVP was associated with a sharp increase in new or persistent AKI incidence. (Adapted from Legrand et al. [53])
Conclusions
We hereby would like to quote and thank Rosalind S. Chow [6]: “Despite the frequency with which fluids are administered to critically ill patients, developing an effective fluid management plan and strategy may at times be surprisingly complex. A thorough understanding of the physiology of body fluids, fluid administration routes, therapeutic delivery strategies, risks, and complications will help to optimize patient outcomes. National and multinational organizations, such as the International Fluid Academy (IFA, www.fluidacademy.org), provide opportunities for clinicians and researchers to promote research and education in the practice of fluid therapy. The use of clear and consistent terminology is a key component to fostering effective communication and collaboration within the veterinary and human healthcare fields.”
References
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–80.
Malbrain ML, Mythen M, Rice TW, Wuyts S. It is time for improved fluid stewardship. ICU Manag Pract. 2018;18(3):158–62.
Vincent JL, Pinsky MR. We should avoid the term “fluid overload”. Crit Care. 2018;22(1):214.
Malbrain M, Van Regenmortel N, Owczuk R. It is time to consider the four D’s of fluid management. Anaesthesiol Intensive Ther. 2015;47:S1–5.
Malbrain M, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, Teboul JL, Rice TW, Mythen M, Monnet X. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66.
Chow RS. Terms, definitions, nomenclature, and routes of fluid administration. Front Vet Sci. 2020;7:591218.
Malbrain M, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, De Laet I, Minini A, Wong A, Ince C, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020;10(1):64.
Hawkins WA, Smith SE, Newsome AS, Carr JR, Bland CM, Branan TN. Fluid stewardship during critical illness: a call to action. J Pharm Pract. 2020;33(6):863–73.
Benes J, Kirov M, Kuzkov V, Lainscak M, Molnar Z, Voga G, Monnet X. Fluid therapy: double-edged sword during critical care? Biomed Res Int. 2015;2015:729075.
Malbrain ML, Van Regenmortel N, Owczuk R. The debate on fluid management and haemodynamic monitoring continues: between Scylla and Charybdis, or faith and evidence. Anaesthesiol Intensive Ther. 2014;46(5):313–8.
Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–206.
Takata M, Wise RA, Robotham JL. Effects of abdominal pressure on venous return: abdominal vascular zone conditions. J Appl Physiol (1985). 1990;69(6):1961–72.
Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204.
Minini A, Rola P, Malbrain MLNG. Kidney failure associated with polycompartment syndrome. In: Coccolini F, Malbrain ML, Kirkpatrick AW, Gamberini E, editors. Compartment syndrome hot topics in acute care surgery and trauma. Cham: Springer; 2021.
Dabrowski W, Rola P, Malbrain M. Intra-abdominal pressure monitoring in cardiac surgery: is this the canary in the coalmine for kidney injury? J Clin Monit Comput. 2022;37(2):351–8.
Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8.
Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, Pinsky MR, Artigas A, Teboul JL, Reiss IKM, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018;44(3):281–99.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77.
Monnet X, Malbrain M, Pinsky MR. The prediction of fluid responsiveness. Intensive Care Med. 2022;49(1):83–6.
Toscani L, Aya HD, Antonakaki D, Bastoni D, Watson X, Arulkumaran N, Rhodes A, Cecconi M. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care. 2017;21(1):207.
Cartotto R, Zhou A. Fluid creep: the pendulum hasn’t swung back yet! J Burn Care Res. 2010;31(4):551–8.
Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409–17.
Mitsides N, Pietribiasi M, Waniewski J, Brenchley P, Mitra S. Transcapillary refilling rate and its determinants during haemodialysis with standard and high ultrafiltration rates. Am J Nephrol. 2019;50(2):133–43.
Dull RO, Hahn RG. Transcapillary refill: The physiology underlying fluid reabsorption. J Trauma Acute Care Surg. 2021;90(2):e31–9.
Murugan R, Kerti SJ, Chang CH, Gallagher M, Clermont G, Palevsky PM, Kellum JA, Bellomo R. Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the randomized evaluation of normal vs augmented level (RENAL) of renal replacement therapy trial. JAMA Netw Open. 2019;2(6):e195418.
Kattan E, Castro R, Miralles-Aguiar F, Hernandez G, Rola P. The emerging concept of fluid tolerance: a position paper. J Crit Care. 2022;71:154070.
Van der Mullen J, Wise R, Vermeulen G, Moonen PJ, Malbrain M. Assessment of hypovolaemia in the critically ill. Anaesthesiol Intensive Ther. 2018;50(2):141–9.
Regli A, Pelosi P, Malbrain M. Ventilation in patients with intra-abdominal hypertension: what every critical care physician needs to know. Ann Intensive Care. 2019;9(1):52.
Moritz ML. Why 0.9% saline is isotonic: understanding the aqueous phase of plasma and the difference between osmolarity and osmolality. Pediatr Nephrol. 2019;34(7):1299–300.
Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–9.
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, Blackwood B, Fan E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–70.
Silversides JA, Perner A, Malbrain M. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 2019;45(10):1440–2.
Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, Jakob SM, Cecconi M, Nalos M, Ostermann M, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 2022;386(26):2459–70.
Silversides JA, McMullan R, Emerson LM, Bradbury I, Bannard-Smith J, Szakmany T, Trinder J, Rostron AJ, Johnston P, Ferguson AJ, et al. Feasibility of conservative fluid administration and deresuscitation compared with usual care in critical illness: the Role of Active Deresuscitation After Resuscitation-2 (RADAR-2) randomised clinical trial. Intensive Care Med. 2022;48(2):190–200.
Wijnberge M, Sindhunata DP, Pinsky MR, Vlaar AP, Ouweneel E, Jansen JR, Veelo DP, Geerts BF. Estimating mean circulatory filling pressure in clinical practice: a systematic review comparing three bedside methods in the critically ill. Ann Intensive Care. 2018;8(1):73.
Faria DK, Mendes ME, Sumita NM. The measurement of serum osmolality and itsapplication to clinical practice and laboratory:literature review. J Bras Patol Med Lab. 2017;53(1):38–45.
Peeters Y, Lebeer M, Wise R, Malbrain M. An overview on fluid resuscitation and resuscitation endpoints in burns: past, present and future. Part 2—avoiding complications by using the right endpoints with a new personalized protocolized approach. Anaesthesiol Intensive Ther. 2015;47:S15–26.
Peeters Y, Vandervelden S, Wise R, Malbrain M. An overview on fluid resuscitation and resuscitation endpoints in burns: past, present and future. Part 1—historical background, resuscitation fluid and adjunctive treatment. Anaesthesiol Intensive Ther. 2015;47:S6–S14.
Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care. 2015;19:18.
Duchesne JC, Kaplan LJ, Balogh ZJ, Malbrain M. Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe haemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensive Ther. 2015;47(2):143–55.
Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. J Physiol. 1914;48(5):357–79.
Hofkens PJ, Verrijcken A, Merveille K, Neirynck S, Van Regenmortel N, De Laet I, Schoonheydt K, Dits H, Bein B, Huber W, et al. Common pitfalls and tips and tricks to get the most out of your transpulmonary thermodilution device: results of a survey and state-of-the-art review. Anaesthesiol Intensive Ther. 2015;47(2):89–116.
Frost P. Intravenous fluid therapy in adult inpatients. BMJ. 2015;350:g7620.
Hoste EA, Maitland K, Brudney CS, Mehta R, Vincent JL, Yates D, Kellum JA, Mythen MG, Shaw AD, Group AXI. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740–7.
Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–34.
Hammond NE, Zampieri FG, Tanna GLD, Garside T, Adigbli D, Cavalcanti AB, Machado FR, Micallef S, Myburgh J, Ramanan M, et al. Balanced crystalloids versus saline in critically ill adults: a systematic review with meta-analysis. NEJM Evid. 2022;1(2):EVIDoa2100010.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Executive summary: surviving sepsis campaign: international guidelines for the management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):1974–82.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–143.
Jacobs R, Lochy S, Malbrain M. Phenylephrine-induced recruitable preload from the venous side. J Clin Monit Comput. 2019;33(3):373–6.
Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36(23):1437–44.
Metra M, Ponikowski P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, et al. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9(6-7):684–94.
Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, Mullens W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62(6):485–95.
Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17(6):R278.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Malbrain, M.L.N.G., Wong, A., Malbrain, L., Nasa, P., Wilkinson, J. (2024). Terms and Definitions of Fluid Therapy. In: Malbrain, M.L., Wong, A., Nasa, P., Ghosh, S. (eds) Rational Use of Intravenous Fluids in Critically Ill Patients. Springer, Cham. https://doi.org/10.1007/978-3-031-42205-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-42205-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42204-1
Online ISBN: 978-3-031-42205-8
eBook Packages: MedicineMedicine (R0)