Abstract
Management of Graves’ orbitopathy (GO) short of surgery is based on three pillars: (1) to refrain from smoking as smoking is associated with more severe GO and less favorable response to immunosuppressive treatment of GO; (2) restoration and maintenance of euthyroid function as both hypothyroidism and hyperthyroidism affect eye changes unfavorably; and (3) treatment of GO itself: (a) local measures as required (liberal use of artificial tears; dark glasses; botulinum toxin; prisms; (b) wait-and-see policy or selenium for mild GO; (c) intravenous methylprednisolone pulses (± mycophenolate) for active moderate-to-severe GO; in case of partial or no response, several options are available for second-line treatment (low-dose oral prednisone + either retrobulbar irradiation or cyclosporin; rituximab). Teprotumumab appears very effective, already approved in the USA; and (d) urgent high-dose intravenous methylprednisolone pulses for very severe GO (dysthyroid optic neuropathy); in case of partial or no response within 2 weeks, surgical orbital decompression.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Graves’ orbitopathy
- Treatment
- Selenium
- Steroids
- Teprotumumab
- Antithyroid drugs
- Radioactive iodine
- Smoking cessation
-
Relevance of smoking cessation
-
Relevance of restoration and maintenance of euthyroid function
-
Utility of local nonspecific measures
-
Mild GO: wait-and-see policy or selenium
-
Moderate-to-severe GO: steroids, if insufficient response, consider retrobulbar irradiation, cyclosporin, rituximab, or teprotumumab
-
Very severe GO (DON, dysthyroid optic neuropathy): high-dose steroids, if insufficient response, urgent orbital decompression
Introduction
The overall management of GO is based on three pillars: (1) smoking cessation, (2) restoration and maintenance of euthyroid function, and (3) management of the orbital disease itself. Here, we distinguish between (a) local measures to be applied in any stage of GO, (b) medical interventions to be applied in the active stage of GO, and (c) surgical interventions to be applied in the inactive stage of GO (like orbital decompression, eye muscle surgery, and eyelid surgery) (Fig. 17.1). GO guidelines are available [1]. One of the recommendations is to refer GO patients—except for the mildest cases- to combined thyroid-eye clinics or specialized centers providing endocrinological and ophthalmological expertise. There is some evidence that in doing so, outcomes are improved [2,3,4]. Patients often need moral and psychological support. Thyroid and/or GO patient associations and contact with fellow patients could be very important in helping patients to cope with their disease [4].
Smoking Cessation
To convince people to stop smoking is rarely successful. However, the deleterious effects of smoking on thyroid eye disease in particular may increase the likelihood that GO patients are susceptible to take the advice to stop smoking [5]. Current smoking increases the risk of Graves’ hyperthyroidism about twofold and of Graves’ ophthalmopathy about threefold. Smokers tend to have more severe GO. Smoking increases the risk of developing or worsening of eye changes about fourfold after 131I therapy of Graves’ hyperthyroidism. The outcome of GO treatment with glucocorticoids or retrobulbar irradiation is less favorable in smokers relative to nonsmokers. Refraining from smoking might reduce the risk of developing exophthalmos and diplopia. The regarding guideline reads “We recommend that physicians urge all patients with Graves’ hyperthyroidism, irrespective of the presence or absence of GO, to refrain from smoking, if necessary with the help of specialized smoking cessation programs or clinics” [1].
Restoration and Maintenance of Euthyroidism
GO occurs mostly in the presence of Graves’ hyperthyroidism, whereas a minority (about 5–10%) presents with euthyroidism or hypothyroidism. Both hyperthyroidism and hypothyroidism have a negative impact on GO, and prompt restoration and maintenance of euthyroidism is indicated [1, 6]. Hypothyroidism should be treated with levothyroxine tablets, but making the appropriate choice how to treat Graves’ hyperthyroidism under these circumstances is more complicated. This is caused by worsening of GO after radioactive iodine treatment in about 15% [7]. Risk factors for such worsening are recent onset GO, active GO, severe hyperthyroidism, high TSHR-Ab (TSH-receptor antibodies), and smoking. Worsening can be prevented with oral prednisone starting with a daily dose of 0.3–0.5 mg/kg (or lower doses in low-risk patients) for 6–12 weeks. Consequently, 131I therapy is not preferred in active moderate-to-severe GO and DON. 131I therapy in active mild GO can be used with steroid prophylaxis. Patients with inactive GO can safely receive radioiodine without steroid cover, as long as post-radioiodine hypothyroidism is avoided and other risk factors (particularly smoking) are absent. Antithyroid drugs can be used irrespective of active or inactive GO. Some centers continue antithyroid drugs as long as medical or surgical treatment of GO is required, and discontinue antithyroid drugs never or only after 2–4 years when GO has arrived in its late inactive stages [8, 9]. If recurrent hyperthyroidism occurs after stopping antithyroid drugs, radioiodine might be given successfully [8]. Total thyroidectomy could be effective treatment of GO at least theoretically by removing all thyroid antigens and thyroid-infiltrating lymphocytes, especially when done at a rather early stage of the disease. Trials investigating early thyroid ablation in GO patients (131I therapy + thyroidectomy vs 131I therapy alone) have not shown convincingly the superiority of thyroidectomy [10].
Local Measures
GO patients may benefit from simple local measures [11]. Sunglasses are helpful against photophobia and reflex tearing in response to wind when outdoors. Dark glasses may be also comfortable for patients who are self-conscious of their changed appearance and prefer to hide their eyes. Ocular surface symptoms are common, related to dry eyes secondary to increased lid aperture, exophthalmos, and reduced blink rate. Artificial tears help to control surface symptoms during the day, while gels protect the corneal surface during the night. It is recommended that all GO patients are treated extensively with non-preserved artificial tears with osmoprotective properties. Raising the head of the bed and diuretics have been advocated as a mean to reduce periorbital oedema, but good evidence for their efficacy in this respect is lacking. Botulinum toxin type A has been used to reduce lid retraction by one or more injections of 5 IU into the levator complex of the upper lid. Botulinum toxin has also been used successfully for chemical denervation of the overactive supercilii muscle to address mid-brow frowning and glabellar lines in GO patients. Botulinum toxin might be applied to reduce diplopia by injecting the drug into the appropriate extraocular muscle; the effect lasts for about 12 weeks but nevertheless is often appreciated by patients to bridge the time until GO has become inactive and corrective eye muscle surgery can be done. Prisms might be considered as an alternative to manage troublesome diplopia in the active stage of GO.
Mild Graves’ Orbitopathy
Mild GO is characterized by ophthalmological features which have only a minor impact on daily life, insufficient to justify immunosuppressive or surgical treatment. Usually, there exists one or more of the following features: minor lid retraction (<2 mm), mild soft-tissue involvement, exophthalmos <3 mm above normal for race and gender, no or intermittent diplopia, and corneal exposure responsive to lubricants [1]. In most patients with mild GO, a “wait-and-see” strategy is sufficient. To refrain from smoking, restoration of euthyroid function and appropriate local measures are usually sufficient. It also helps that there exists a tendency to spontaneous improvement in the natural history of GO. Selenium supplementation has been formally recommended in mild GO [1]. In a randomized double-blind placebo-controlled study, patients with mild GO received either 100 μg selenium twice daily or placebo for 6 months [12]. At 6 months, quality-of-life as assessed by the disease-specific GO-QoL and overall ocular involvement had improved more often in the selenium group compared to the placebo group (61% vs 36%, p < 0.001); after withdrawal of selenium, the improvement was maintained at 12 months. The progression of GO to more severe forms was significantly lower in the selenium group than in the placebo group (7% vs 26%). No selenium-related adverse events were observed. Enrolled patients mostly came from marginally selenium-deficient areas in Europe [13]. It is unclear whether selenium supplementation is beneficial and safe in selenium-sufficient areas. In longstanding inactive mild GO, there is no evidence that selenium is effective. Selenium is incorporated as selenocysteine in selenoproteins, which play a major role in the maintenance of the cellular redox state [1]. Increased generation of reactive oxygen species may be involved in the pathogenesis of GO, providing a biologic rationale for the application of selenium [13, 14]. Occasionally, objectively mild GO has such a profound impact on quality-of-life that these cases might be considered not mild but moderate-to-severe: it would qualify for immunosuppressive treatment if active, or rehabilitative surgery if inactive [1].
Moderate-to-Severe Graves’ Orbitopathy
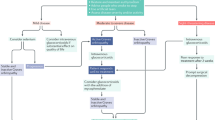
Moderate-to-severe GO is characterized by ophthalmological features which have sufficient impact on daily life to justify the risks of immunosuppression (if active) or surgical intervention (if inactive). Usually, there exists two or more of the following features: lid retraction ≥2 mm, moderate or severe soft tissue involvement, exophthalmos ≥3 mm above normal for race and gender, inconstant or constant diplopia. Sight-threatening GO should be absent [1]. Essential for an appropriate choice of treatment is the assessment of the disease activity of GO. This can be done by the clinical activity score (CAS), based on the classical signs of inflammation (dolor, rubor, and tumor): a CAS <3 likely indicates inactive GO and a CAS ≥3 active GO [15]. Ever since the MRC report of Sir Brain in 1955, glucocorticoids have been the mainstay of medical treatment for advanced forms of GO [16]. Glucocorticoids are more effective than placebo (response rates 83% vs 11%) [17], intravenous methylprednisolone is more effective and better tolerated than oral prednisone (response rates 77% vs 51%) [18], and intravenous methylprednisolone + mycophenolate is more effective than iv methylprednisolone alone (response rate 71% vs 53%) [19], as demonstrated in successive RCTs [1]. A commonly used dosing schedule for oral prednisone is 60 mg for 2 weeks, 40 mg for 2 weeks, 30 mg for 4 weeks, 20 mg for 4 weeks, and then tapering down to zero dose in 8 weeks. The optimal dose of intravenous methylprednisolone pulse therapy has been investigated in a RCT [20]. The preferred scheme was 500 mg iv once weekly for 6 weeks followed by 250 mg once weekly for another 6 weeks, a cumulative dose of 4.5 g [20]. A lower cumulative dose of 2.25 g was less effective, but a higher cumulative dose of 7.5 g might be used in the most severe cases. Mycophenolate can be added to the iv methylprednisolone pulse therapy (cumulative dose 4.5 g given in 12 weeks) in a daily dose of 360 mg twice daily for 24 weeks [19]. Steroids are effective especially against soft tissue involvement (pain, redness, and oedema) and double vision, but their effect on exophthalmos reduction is often disappointing. The side effects of steroids are well known, but in the early days of intravenous pulses of methylprednisolone, rare but very severe side effects occurred (like liver failure and cardiovascular events) [21]. Current recommendations therefore are that iv pulses of steroids should not be used in patients with recent viral hepatitis, significant liver dysfunction, severe cardiovascular morbidity, or psychiatric disorders [1, 22]. Cumulative doses exceeding 8 g should be avoided, and daily doses should be restricted to ≤1 g and infused slowly. Diabetes and hypertension should be controlled. Using this strategy, steroids will inactivate GO in most patients, allowing rehabilitative surgery as required in the late inactive fibrotic stages of the disease. Not all patients with active moderate-to-severe GO respond to GO, and a flare-up of the ophthalmopathy after an initial response to steroids is frequently seen. It could be seen that combining iv steroids with orbital irradiation or mycophenolate reduces the relapse rate. In case of a partial or no response to steroids, shared decision-making is recommended to select a second-line therapy [1]. Several options are available if GO is still active (Fig. 17.2). (1) A second course of iv methylprednisolone pulses if the patient tolerates it, and a cumulative dose of >8 g is avoided. (2) Low-dose oral prednisone (e.g. 20 mg daily) + orbital irradiation (administered e.g. in 10 daily doses of 2 Gy over 2 weeks). Retrobulbar irradiation in GO is more effective than sham irradiation according to two RCTs [23, 24]. (3) Low-dose oral prednisone + cyclosporine (e.g. starting dose 5 mg/kg), which has shown to be effective in two RCTs [25, 26]. (4) Rituximab in a dose of 1000 mg given twice over 2 weeks, causes depletion of B-cells (also in the orbit) and modulation of all B-cell functions. The results of two RCTs in active moderate-to-severe GO have been conflicting: one study compared rituximab with placebo and found no difference [27] and the other study compared rituximab with iv methylprednisolone pulses and found rituximab slightly better in improving CAS, ocular motility and QoL [28]. The discrepancy is incompletely understood [29], but on the other hand, the number of positive responses on even single doses of 500 mg or even 100 mg rituximab in open studies cannot be denied. It appears to justify rituximab therapy in case of steroid failure. A serious but fortunately rare adverse event of rituximab is a cytokine storm leading to the subacute development of dysthyroid optic neuropathy (DON) [30]. Monoclonal antibodies against tumor necrosis factor-alpha (anti-TNFα) have been tried in active moderate-to-severe GO with disappointing results [1]. Monoclonal antibodies against interleukin-6 receptors (anti-IL-6R, tocilizumab) have been given with remarkable success in GO patients not responding to steroids [31]; these results await confirmation by independent groups. A major breakthrough has been the recent development of monoclonal antibodies blocking the IGF-1 receptors on orbital fibroblasts and lymphocytes. Teprotumumab (anti-IGF-1R monoclonal antibody) in a dose of 10 mg/kg for the first infusion and 20 mg/kg for the next seven infusions or placebo was administered intravenously once every 3 weeks for 24 weeks in 170 patients with active moderate-to-severe GO [32, 33]. Responders were 73% in the teprotumumab and 14% in the placebo group. The response involved improvement in CAS, proptosis, double vision, and GO-QoL. Especially, the reduction in exophthalmos was impressive: the mean reduction in proptosis by week 24 ranged from 2.95 to 3.32 mm. That extent of proptosis reduction is not often seen upon steroid treatment. The safety profile of the drug is reassuring. It is not known whether or not teprotumumab will have the same great effect on proptosis reduction if given during the late inactive fibrotic stage of GO. Teprotumumab has been approved for adult GO by the FDA in the USA. Application in Europe awaits head-to-head comparison with iv steroids, as well as cost-effectiveness studies.
Very Severe Graves’ Orbitopathy
Very severe GO or sight-threatening GO includes patients with dysthyroid optic neuropathy (DON) and/or corneal breakdown. There has been just one RCT on the treatment of DON, comparing high-dose steroid pulses and immediate orbital decompression surgery [34]. About 83% of the surgically treated patients failed to improve visual acuity within 2 weeks vs 44% in the medically treated patients; all patients improved after switching to the other treatment modality. Consequently, the recommendation is to start immediately with very high doses of methylprednisolone: 1000 mg iv daily for 3 consecutive days during the first week, to be repeated in the second week. If response is absent or poor at day 14, urgent orbital decompression is required. Severe corneal exposure should be treated medically or by means of progressively more invasive surgeries in order to avoid progression to corneal breakdown [1].
Conclusion
The outcome of Graves’ orbitopathy has improved greatly over the last few decades. Improvement has been caused not only by earlier diagnosis and treatment of associated Graves’ hyperthyroidism, but also by early referral to specialized thyroid/eye clinics and a structured approach in the diagnosis and management of the ophthalmopathy itself. Further improvement is to be expected as Graves’ orbitopathy is to a certain extent a preventable disease (stop smoking!), and a real causal therapy of Graves’ disease will be available in the next few years directed at blocking signaling via the TSH receptor.
References
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves’ Ophthalmopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5:9–26.
Estcourt S, Hickey J, Perros P, Dayan C, Vaidya B. The patient experiences of services for thyroid eye disease in the United Kingdom: results of a nationwide survey. Eur J Endocrinol. 2009;161:483–7.
Perros P, Dayan CM, Ezra D, Estcourt S, Kickey J, Lazarus JH, et al. Management of patients with Graves’ orbitopathy: initial assessment, management outside specialized centres and referral pathways. Clin Med. 2015;151:173–8.
Perros P, Zarkovic M, Azzolini C, Ayvaz G, Baldeschi L, Bartalena L, et al. PREGO (presentation of Graves’ orbitopathy) study: changes in referral patterns to European Group on Graves’ Orbitopathy (EUGOGO) centres over the period from 2000 to 2012. Br J Ophthalmol. 2015;99:1531–5.
Wiersinga WM. Smoking and thyroid. Clin Endocrinol. 2013;79:145–51.
Prummel MF, Wiersinga W, Mourits M, Koornneef L, Berghout A, van der Gaag R. Amelioration of eye changes of Graves’ ophthalmopathy by achieving euthyroidism. Acta Endocrinol. 1989;121(Suppl. 2):185–9.
Traisk E, Tallstedt L, Abraham-Nordling M, Andersson T, Berg G, Calissendorff J, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700–7.
Elbers L, Mourits M, Wiersinga W. Outcome of very long-term treatment with antithyroid drugs in Graves’ hyperthyroidism associated with Graves’ orbitopathy. Thyroid. 2011;21:279–83.
Laurberg P, Berman DC, Andersen S, Bulow-Pedersen I. Sustained control of Graves’ hyperthyroidism during long-term low-dose antithyroid drug therapy in patients with severe Graves’ orbitopathy. Thyroid. 2011;21:951–6.
Menconi F, Leo M, Vitti P, Marcocci C, Marino M. Total thyroid ablation in Graves’ orbitopathy. J Endocrinol Investig. 2015;38:809–15.
Boboridis K, Anagnostis P. Local treatment modalities. In: Wiersinga WM, Kahaly GJ, editors. Graves’ orbitopathy. A multidisciplinary approach—questions and answers. 3rd ed. Basel: Karger; 2017. p. 202–6.
Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, e t al. Selenium and the course of mild Graves’ orbitopathy. N Engl J Med. 2011;364:1920–31.
Rayman MP. The importance of selenium in human health. Lancet. 2000;356:233–41.
Marcocci C, Bartalena L. Role of oxidative stress and selenium in Graves’ hyperthyroidism and orbitopathy. J Endocrinol Investig. 2013;36(Suppl):15–20.
Terwee CB, Dekker FW, Mourits MP, Gerding MN, Baldeschi L, Kalmann R, et al. Interpretation and validity of changes in scores on the Graves’ ophthalmopathy quality of life questionnaire (GO-QoL) after different treatments. Clin Endocrinol. 2005;54:391–8.
Brain SR. Cortisone in exophthalmos: report on therapeutic trial of cortisone and corticotrophin (ACTH) in exophthalmos and exophthalmic ophthalmoplegia by a panel appointed by the Medical Research Council. Lancet. 1955;268:6–9.
Van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, Mourits MP. Methylprednisolone pulse therapy for patients with moderately severe Graves’ ophthalmopathy: prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008;15(8):229–37.
Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single-blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40.
Kahaly GJ, Riedl M, Konig J, et al. Mycophenolate plus methyl prednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diab Endocrinol. 2018;6:287–98.
Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–63.
Marino M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marcocci C. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid. 2004;14:403–6.
Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, et al. Fatal and nonfatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol. 2012;166:247–53.
Mourits MP, van Kempen-Harteveld ML, Garcia MB, Koppeschaar HP, Tick L, Terwee CB. Radiotherapy for Graves’ orbitopathy: randomised placebo-controlled study. Lancet. 2000;355:1505–9.
Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2004;89:15–20.
Kahaly G, Schrezenmeir J, Krause U, Schweikert B, Meuer S, Muller W, et al. Ciclosporin and prednisone v. prednisone in the treatment of Graves’ ophthalmopathy: a controlled, randomized and prospective study. Eur J Clin Investig. 1986;16:415–22.
Prummel MF, Mourits MP, Berghout A, Krenning EP, van der Gaag R, Koornneef L, Wiersinga WM. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321:1353–9.
Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100:432–41.
Salvi M, Vannucchi G, Campi I, Curro N, Dazzi D, Simonetta S, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100:422–31.
Stan MN, Salvi M. Rituximab therapy for Graves’ orbitopathy—lessons from randomized control trials. Eur J Endocrinol. 2017;176:R101–9.
Krassas GE, Stafilidou A, Boboridis KG. Failure of rituximab treatment in a case of severe thyroid ophthalmopathy unresponsive to steroids. Clin Endocrinol. 2010;72:853–5.
Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodriguez Alvarez FM, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant Graves’ orbitopathy: a randomized clinical trial. Am J Ophthalmol. 2018;195:181–90.
Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–61.
Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–52.
Wakelkamp IM, Baldeschi L, Saeed P, Mourits MP, Prummel MF, Wiersinga WM. Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves’ ophthalmopathy? A randomized controlled trial. Clin Endocrinol. 2005;63:323–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Wiersinga, W.M. (2023). Medical Management of Graves’ Orbitopathy. In: Gooris, P.J., Mourits, M.P., Bergsma, J. (eds) Surgery in and around the Orbit. Springer, Cham. https://doi.org/10.1007/978-3-031-40697-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-031-40697-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40696-6
Online ISBN: 978-3-031-40697-3
eBook Packages: MedicineMedicine (R0)