Abstract
Two species of pilot whales are globally distributed, the long-finned (Globicephala melas) in cold-temperate waters and the short-finned (G. macrorhynchus) in tropical and warm-temperate latitudes. Two subspecies of the long-finned pilot whale are recognized, G. m. melas in the North Atlantic and G. m. edwardii in the Southern Hemisphere. In addition, three types have been proposed in short-finned pilot whales. In general, it is assumed that pilot whales live in matrilineal societies composed of stable units/pods displaying bisexual natal philopatry, but inter- and intraspecific variabilities in the sociality of these units have been described worldwide. Moreover, there is inter- and intraspecific heterogeneity in life history and reproductive parameters, which supports geographic variation. To investigate life history parameters, sociobiology, and reproductive strategies within different populations of pilot whales, we reviewed the current literature and compiled novel data. We cover populations from both hemispheres and combine life history characteristics from strandings with field-/behavioral-based information such as long-term photographic-identification, social analysis with molecular sexing, and drone technology. This chapter contributes to improving our knowledge of the life history parameters between sexes and populations, interactions between animals of different sexes within units, social structures, and reproductive strategies in pilot whales. We explore pilot whales’ sexual group dynamics and social system and discuss whether they are strictly matrilineal in comparison with other “matrilineal” species.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
There are two species of pilot whales currently recognized (Fig. 15.1), the temperately distributed long-finned pilot whale (Globicephala melas; herein LFPW) as well as the tropically and subtropically distributed short-finned pilot whale (G. macrorhynchus; herein SFPW). LFPWs are split into two subspecies—one in the North Atlantic (Globicephala melas melas; herein North Atlantic LFPW) and the other in the Southern Hemisphere (G. melas edwardii; herein Southern Hemisphere LFPW) (Olson 2018). Recent genomic work suggests three SFPW types within the species: (1) an Atlantic Ocean (Atlantic Naisa) type, (2) a western/central Pacific and Indian Ocean (Pacific Naisa) type, and (3) an eastern Pacific Ocean and northern Japan (Shiho) type (Van Cise et al. 2019). There is evidence of interbreeding between these species, including post-F1 hybrids recorded in studies of both Iberian Peninsula and Faroe Islands genetic samples (Miralles et al. 2013, 2016).
Both pilot whale species have pronounced sexual dimorphism. Males grow to around 1.3 times the length of females and have taller dorsal fins, longer pectoral fins, more pronounced melons, and wider flukes than adult females of similar body lengths (Fig. 15.1, Table 15.1; Kasuya 2017; Betty et al. 2022a). The biological significance of sexual differences in adult male dorsal fin shape and size is not well understood, but they may serve a thermoregulatory function and/or act as a visual signal in mating interactions, while the longer and broader flukes and pectoral fins may function to give more propulsion compared to females (Mesnick and Ralls 2018). There are differences in the relative degree of sexual shape dimorphism of the dorsal fin between species, subspecies, and types, which is likely due to variations in ecology and sociality—with immature individuals having proportionally smaller fins (and lighter coloration) than mature pilot whales (Fig. 15.2). In LFPWs, sexes can be distinguished by distinctive urogenital markings from a young age; the light gray ventral stripe on females flares out to encompass the mammary slits before truncating off rather abruptly, while in males there is no distinctive flare and the light gray patch tapers off gradually before the caudal end of the genital slit (Fig. 15.3). While pilot whales share several reproductive characteristics with other large odontocetes (e.g., long lifespan, delayed maturity, bimaturism, sexual dimorphism, extended calving intervals, etc.), many of these differ significantly between species, subspecies, and types (Tables 15.1 and 15.2).
15.2 Social Structure and Reproductive Strategies
Pilot whales are well-known for their multilevel and highly cohesive social structure, which is a contributing factor toward their tendency to strand en masse. Often referred to as one of the most gregarious cetaceans, pilot whales are commonly found in temporary aggregations of up to several hundred individuals. The average reported group size is around 20 whales (Jefferson et al. 2008), though this varies by population. There is also some evidence that pilot whale “groups” are comprised of several smaller “units” of constant companions (Heimlich-Boran 1993; Ottensmeyer and Whitehead 2003; de Stephanis et al. 2008; Alves et al. 2013; Mahaffy et al. 2015; Augusto et al. 2017). Pilot whales are one of the few mammals that appear to have a matrilineal social structure (Amos et al. 1993a; Alves et al. 2013), along with sperm whales (Physeter macrocephalus; Cantor et al. 2015), killer whales (Orcinus orca; Bigg et al. 1990), elephants (Elephas maximus; Berger et al. 2021), some primates (Greenwood 1980), and humans (Behar et al. 2008). There are unique differences in how matrilines are structured, such as the lack of sex-biased dispersal from the natal groups in some ecotypes of killer whales (Ford 2019), male dispersal before sexual maturity to live primarily solitarily or with other males in sperm whales (Best 1979), and a possible mixture in pilot whales that is not fully understood (Amos et al. 1993a; Hill et al. 2019). Both pilot whale species have been reported to form long-term stable units consisting of several generations of maternally related individuals (with an increase in local relatedness with age), as well as strong mother-offspring associations with long periods of dependency (Marsh and Kasuya 1990; Brent et al. 2015; Croft et al. 2017; Nichols et al. 2020). There is some genetic evidence that groups of both LFPWs and SFPWs can contain multiple matrilines (Alves et al. 2013; Oremus et al. 2013; Nichols et al. 2020; Ball et al. 2021). It has been suggested that both pilot whale species form temporary associations comprising multiple matrilineal units, as supported by studies of SFPWs (Alves et al. 2013; Mahaffy et al. 2015) and North Atlantic LFPWs (Ottensmeyer and Whitehead 2003; de Stephanis et al. 2008; Augusto et al. 2017) where long-term stable units are smaller than the average observed group size.
The mating strategies and tactics of pilot whales are not well understood. Both LFPWs and SFPWs are assumed to have a polygynous mating system due to their sexual dimorphism. However, there is a lack of evidence for male combat in pilot whales. North Atlantic LFPWs have the fourth largest residual testes-to-body mass ratio when compared to 30 other cetacean species (MacLeod 2010). The lack of a trade-off with testis size indicates that male pilot whales (1) are not able to monopolize access to females to the same extent as those who compete by combat and (2) may invest in postcopulatory sperm competition (MacLeod 2010; Dines et al. 2015). For both pilot whale species, there is agreement that males mature about 7–9 years later than the females (Table 15.2), which concurs with the general delphinid pattern of bimaturism (Perrin and Reilly 1984). Male and female North Atlantic LFPWs have been documented engaging in sociosexual behavior from a very young age (see Case Study 3.1).
Pilot whale groups are mainly stable, with the young growing to maturity in their natal group and most remaining there for life. In a few populations, it has been suggested that young male pilot whales might disperse from their natal unit/pod to aggregate in other matrilines and/or form male-only groups (Kasuya and Marsh 1984; Desportes et al. 1992). Genetic and long-term photographic-identification studies suggest that males breed outside their family group and that they can remain with their group for decades (Kasuya and Marsh 1984; Amos et al. 1993b; de Stephanis et al. 2008; Alves et al. 2013; Mahaffy et al. 2015; Augusto et al. 2017; Van Cise et al. 2017; Boran and Heimlich 2019; Hill et al. 2019; Nichols et al. 2020). Therefore, mating must occur when two or more pods meet or when adult males visit other groups. This type of social structure where adult males stay with their female kin and mate elsewhere is unusual among mammals. Studies on males’ stability have covered only specific populations, such as the North Atlantic LFPW (Nova Scotia and Gibraltar; de Stephanis et al. 2008; Augusto et al. 2017) and the Pacific Naisa SFPW (Hawai’i and Mariana archipelagos; Mahaffy et al. 2015; Hill et al. 2019). There are still gaps in our understanding of the role of male pilot whales within the social structure of other subspecies/types; see Case Study 3.2 with discussion on male-only groups and natal philopatry in the Atlantic Naisa SFPW.
In general, fertility and reproductive success are low in newly mature female cetaceans, reaching a peak in young mature animals, followed by a plateau until they (often) decline with age (Best et al. 1984; Martin and Rothery 1993; Boyd et al. 1999). Females are defined as reproductively senescent, or post-reproductive, if conceiving or sustaining a successful pregnancy is no longer possible because of age-related changes to the reproductive system (Marsh and Kasuya 1986). The occurrence of reproductive senescence is contradictory to classical life history theory (Ellis et al. 2018a) and has been observed in females of several odontocete species including sperm whales (Best 1980), killer whales (Foster et al. 2012), false killer whales (Pseudorca crassidens; Photopoulou et al. 2017), beluga whales (Ferguson et al. 2020), narwhal (Monodon monoceros; Garde et al. 2015), and SFPWs (Marsh and Kasuya 1984). A detailed examination of ovarian aging in the Pacific Naisa SFPW showed an age-specific decline in the pregnancy rate, paralleled by a decline in the ovulation rate and a high incidence of infertile ovulations (atresia) in old females (Marsh and Kasuya 1984). Approximately 25% of mature female SFPWs examined (n = 298) had senescent ovaries, and it was concluded that SFPWs appear to cease ovulating before 40 years of age but may live up to 30 years (14 years on average) after the birth of their last calf (Marsh and Kasuya 1984). Curiously, post-reproductive females were observed much less frequently in the North Atlantic LFPW (< 5% of mature females; Sergeant 1962; Kasuya et al. 1988; Martin and Rothery 1993), and it has been reported that LFPWs do not appear to have a significant post-reproductive lifespan (Ellis et al. 2018a, Betty 2019, Nichols et al. 2020; see Case Study 3.3). Potential explanations for post-reproductive lifespan include the mother and grandmother hypotheses, where old nonreproductive mothers avoid reproductive competition with their daughters, and instead maximize their inclusive fitness, by aiding and enhancing the survival of their offspring (Johnstone and Cant 2010; Foster et al. 2012; Brent et al. 2015; Croft et al. 2017; Nichols et al. 2020). For reproductively active females of both pilot whale species, the calving interval is estimated to be about 4–5 years (Table 15.2). However, the calving interval and duration of lactation increase with maternal age, which may mean (1) higher calf survival, (2) milk is provided to calves other than the mother’s own, and (3) increased investment in calves with advancing age of the mother (Marsh and Kasuya 1984, Martin and Rothery 1993). Overall, there remains much to learn about pilot whale social structure, reproductive strategies, and life history.
15.3 Case Studies
This chapter presents three case studies from one Atlantic Naisa SFPW and two LFPW (both North Atlantic and Southern Hemisphere) populations, utilizing both strandings-based and field-/behavioral-based data. The case studies provide novel insights that further our understanding of (1) sociosexual behavior in immature North Atlantic LFPWs, (2) male natal group philopatry in Atlantic Naisa SFPWs, and (3) reproductive senescence in female Southern Hemisphere LFPWs.
15.3.1 Sociosexual Behavior in Immature North Atlantic LFPWs
LFPWs are thought to be matrilineal, with the Northern Hemisphere subspecies composed of mixed-sex social units that include females with their offspring (Augusto et al. 2017). These social units frequently associate with other matrilines and form large groups, suggesting that there are often both related and unrelated sexually immature pilot whales near each other. The sociosexual behaviors of LFPWs have not yet been studied nor formally described. Here we provide the first description of non-conceptive sexual behavior for immature North Atlantic LFPWs from a population that summers off Cape Breton Island, Nova Scotia, Canada.
While on research surveys for a study on North Atlantic LFPW behavioral ecology, we noticed several clusters of sexually immature individuals engaged in sociosexual behaviors within different groups that were followed for approximately 1 hour. Aerial footage was collected using a DJI Inspire 1 V2 drone fitted with a DJI X5 camera and Olympus Zuiko 25 mm f1.8 lens launched off a chartered vessel on August 17, 2020. Just over 21 minutes of video (from two different clusters of individuals) were analyzed frame-by-frame to categorize and count sociosexual behaviors based on an ethogram modified from Ham et al. (2022; Table 15.3).
Seven different types of sociosexual behavior were documented (Fig. 15.4). Age categories were assigned based on natural markings following Auger-Méthé and Whitehead (2007), with calves classified as newborns (nb; up to a couple months in age), fetal folds (ff; a few months to a year), or gray calves (gc; 1–3 years of age). The sexes of calves were determined by examination of the genital region when whales rolled over. Sociosexual behaviors were observed in LFPWs across ff and gc age cohorts and for both sexes (Table 15.4), as has been documented in other species of cetaceans (Ham et al. 2022; Lonati et al. 2022; Sanvito and Galimberti 2022; Ham et al. 2023, this book). As ff were involved in some of the sociosexual interactions, sexual play begins relatively early in the development of LFPWs. We did not observe any sexually mature LFPWs engaging in sociosexual behaviors during these encounters, though they were often in close proximity to the individuals engaged in non-conceptive sexual play (two to six sexually mature LFPWs per cluster). While male calves of several age cohorts were involved in sociosexual play, only juvenile males exhibited riding behavior (Table 15.4). Each receiver of these behaviors displayed avoidance of the pursuing males’ efforts at some point during the encounters. Rates of sociosexual behaviors varied between the two clusters, but some behaviors were observed consistently more often than others (e.g., genital rubs vs. riding behaviors).
15.3.1.1 Functions of Sociosexual Behavior in LFPWs
There are several hypothesized reasons for non-conceptive sexual behaviors in cetaceans (reviewed by Ham et al. 2023, this book). Practice may increase success once individuals reach sexual maturity (Mann 2006; Furuichi et al. 2014). Sociosexual behaviors may establish and strengthen bonds between conspecifics within and across age cohorts (Connor et al. 2006; Lilley et al. 2020), which may be especially important when sexually immature individuals bond with more dominant or socially connected conspecifics (Lilley et al. 2020). Sociosexual behaviors could also be a by-product of sexual physiology and drives. The sole female filmed in these interactions did not display any sociosexual behaviors aside from avoidance, perhaps being an unwilling participant. Body rolling avoidance behavior has been observed in female dusky dolphins (Lagenorhynchus obscurus) to avoid copulatory attempts by pursuing males (Orbach et al. 2015; Markowitz et al. 2023, this book). While our observations of sociosexual play in sexually immature North Atlantic LFPWs do not give concrete evidence for a specific function, they likely train individuals in sexual and social skills (Ham et al. 2022).
This study provides some preliminary evidence for the development of behavior over time, as seen in immature beluga whales (Delphinapterus leucas) (Ham et al. 2022). Only juvenile North Atlantic LFPWs were documented displaying riding of the individuals they were pursuing, which could be because (1) this behavior develops later than other non-conceptive sexual behaviors, (2) riding is more frequently used by older individuals or (3) the drivers and function of sociosexual behaviors change as a pilot whale approaches sexual maturity. The development and accumulation of sociosexual behaviors over time have been well documented in both beluga whales and bottlenose dolphins (Mann 2006; Ham et al. 2022), providing a likely explanation for the subtle differences in behaviors seen across different age classes of North Atlantic LFPWs.
Further studies into the non-conceptive sexual play of both immature and mature LFPWs are needed, particularly to determine whether these change across behavioral contexts and whether adults are sometimes engaged in sociosexual behaviors with younger age cohorts, as is observed in other cetacean species (Lilley et al. 2020; Lonati et al. 2022; Sanvito and Galimberti 2022). Recent technological advances may soon help lead to discoveries of additional pilot whale reproductive and non-conceptive sexual behaviors, particularly for behaviors that occur at depth or are not as readily observable as the ones documented here.
15.3.2 Stability and Fluidity of Naisa SFPW Social Groups of Known Sex off Madeira Island
Current knowledge supports the theory that pilot whales have a stable matrilineal kin-based structure, but it is unknown how much variation there is between species, subspecies/types, or even populations. Here we provide information on the stability of Atlantic Naisa SFPW social structure, using animals with known sex in social groups off Madeira Island, Portugal, to infer sex-biased dispersal. These data improve our knowledge on the debated natal group philopatry of males, given that some studies suggest males can have a non-kin-based social structure or question the stability of male associations due to the existence of multiple matrilines in closely associated groups and/or individuals (Oremus et al. 2013; Hill et al. 2019).
Atlantic Naisa SFPWs with several different residency patterns, but no genetic differentiation, are found in the coastal waters off Madeira Island (32° N 017° W), including both nomadic animals passing through sporadically and island-associated whales (i.e., seasonal visitors, residents) that occasionally visit the neighboring archipelagos of the Azores and Canaries (Alves et al. 2013, 2019; Boran and Heimlich 2019; Servidio et al. 2019). There have been over 100 island-associated whales documented off Madeira encompassing several matrilineal pods (akin to units in LFPWs), each with a mean size of 15 individuals (SD = 9) (Alves et al. 2013). These pods are made up of individuals that share documented long-lasting relationships (on a scale of decades), and genetic relatedness has been shown to be higher within groups than between them (Alves et al. 2013; Esteban et al. 2022). Atlantic Naisa SFPWs off Madeira have a high survival rate and the population size is stable (Alves et al. 2015; Verborgh et al. 2022). Although some degree of natal philopatry has been proposed for Atlantic Naisa SFPWs, the analyses of long-term stability of sexed animals within pods were inferred from a limited dataset of whales of known sex (Alves et al. 2013).
We used long-term photographic-identification data of 1275 Atlantic Naisa SFPWs off Madeira, complemented with biopsies of 51 individuals to genetically determine sex following Alves et al. (2020). Hierarchical cluster analysis was used to classify and illustrate relationships between the genetically sexed and distinctive whales captured on ≥4 encounters with high or full coverage (e.g., when the proportion of captured individuals per encounter was ≥0.8; Alves et al. 2013) and where documented pods had >1 animal of known sex. The truncated dataset used in the analysis selected 42 sexed/distinctive whales (24 females, 18 males) and 362 encounters collected year-round between 2003 and 2020. We defined the sampling period (and associations) as all individuals grouped within an encounter. Permutation tests were performed to understand whether preferred associations existed (Bejder et al. 1998; Whitehead 1999). Associations were calculated using the average-linkage method due to presenting the highest cophenetic correlation coefficient (CCC = 0.986). The association index corresponding to the maximum modularity was used to define community division by clusters (Whitehead 2008), and all analyses were carried out in SOCPROG 2.9 (Whitehead 2009).
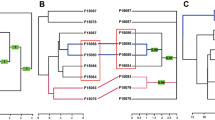
Significantly high coefficients of variation (observed CV = 3.223, random CV = 3.064, p < 0.001) of all association indices indicate (according to Whitehead 1999, 2009) that individuals have long-term preferred companions. A Mantel test showed no significant differences in association strength within or between sexes (p > 0.4). The cluster diagram divided the individuals into six pods of mixed sexes and two pods containing only females (males may have been present but were not photographed or biopsied; Fig. 15.5). Males first documented in 2003–2005 were repeatedly captured together in their respective pods over the entire duration of this study (e.g., ID0089, ID0112, and ID0271 were captured 67, 89, and 94 times, respectively). A presumed mother-calf pair first captured in 2005 (male calf ID0271 in association with adult female ID0088) has been documented together over the course of 16 years. Although the mean association for all individuals was low (0.05 ± 0.02), the maximum association index for each individual was relatively high (mean = 0.68, SD = 0.19, range = 0.12–0.92); 33% of individuals displayed a maximum association index >0.80, indicating strong dyadic associations. Only one male (ID0114) in this study, with a maximum association index of 0.12, was not assigned to a specific pod due to not being regularly captured (n = 6) associated with the same individuals.
Dendrogram constructed using average-weight linkage hierarchical cluster analysis for 42 genetically sexed and distinctive Atlantic Naisa short-finned pilot whales (Globicephala macrorhynchus) captured on ≥4 encounters with high or full coverage and > 1 animal with known sex, between 2003 and 2020, off Madeira Island. The sex, ID of the individual, and year of the first capture (and the total number of captures) are shown for each whale
15.3.2.1 Sex-Biased Dispersal and Social Dynamics
This study confirms that Atlantic Naisa SFPWs off Madeira exhibit long-lasting and stable groups of mixed sexes, as suggested by Alves et al. (2013) and Esteban et al. (2022). Such female and male natal group philopatry complements the positive correlation between association indices and genetic relatedness coefficients previously described for this population (Alves et al. 2013), thus supporting the hypothesis that SFPW social groups are primarily matrilineal. A lack of male-biased dispersal has also been described in a population of killer whales (Ford 2019) and is known to benefit the inclusive fitness of living with kin by improving access to resources that require coordination and provide alloparental care or defense from predators (Boran and Heimlich 2019).
Pods composed of only males were not recorded in the present case study, nor have they been observed at sea (following Yahn et al. 2023) during nearly two decades of intensive fieldwork off Madeira. Although other studies have mentioned the possibility of all-male pilot whale groups, such anecdotal records are based on (1) strandings or drive fisheries that may not reflect natural group stability, (2) in situ visual sex determination, or (3) molecular sexing that might be influenced by (statistical) unit division criteria (Desportes et al. 1992; Mahaffy et al. 2015). To avoid inbreeding, it is possible that males may temporarily leave their natal group to mate, and therefore previously reported male individual/group sightings could represent short-term disassociations from their matrilines. This could be the case of ID0114, who was captured in association with different stable pods throughout the course of our study. Although this information advances our knowledge of social structure in Atlantic Naisa SFPWs and sheds new light on pilot whale social organization in general, additional genetic analyses are needed to clarify whether associated individuals are mothers and offspring, or siblings, to confirm matrilineality.
15.3.3 Examination of Reproductive Senescence in Female Southern Hemisphere LFPWs off Aotearoa New Zealand
Prolonged post-reproductive lifespans are rare in mammalian species. In contrast with the closely related SFPW, female North Atlantic LFPWs do not appear to have a significant post-reproductive lifespan (Martin and Rothery 1993; Ellis et al. 2018a). Reproductive senescence has not been previously examined in female Southern Hemisphere LFPWs. However, given that population variability in life history parameters exists for this species (Tables 15.1 and 15.2), it is important to investigate the potential existence of a significant post-reproductive lifespan in the Southern Hemisphere LFPW, specifically. Here we present the first investigation of female reproductive senescence for the Southern Hemisphere LFPW, through examination of reproductive data opportunistically collected from stranding events on the Aotearoa New Zealand, coast.
As part of a study investigating the life history of the Southern Hemisphere LFPW (Betty 2019), postmortem reproductive data were collected from 166 females following 14 independent stranding events on the coast of Aotearoa New Zealand (2008–2017). Where possible, teeth were collected for age determination, and reproductive organs (ovaries and uteri) were removed in situ via standard postmortem procedures (Geraci and Lounsbury 2005). Age was estimated by examining decalcified and stained tooth sections and counting growth layer groups in the dentine (Betty et al. 2022a). Assessment of female reproductive status was determined through ovarian, uterine, and mammary gland examination (Betty 2019). Sexual maturity was determined by the presence of a least one corpus luteum (CL) or corpora albicantia (CA) on the ovary and/or evidence of pregnancy or lactation, with sexually mature females further classified into one of three reproductive states (i.e., pregnant, lactating, resting). To investigate evidence of reproductive senescence, ovaries were examined for absence of (1) a CL, (2) young or medium CAs, and (3) macroscopic follicles (Fig. 15.6) following Marsh and Kasuya (1984).
Examples of long-finned pilot whale (Globicephala melas edwardii) ovaries: (a) left ovary of sexually immature female with no ovarian corpora scars; (b) left ovary of a resting mature female with a large fluid-filled follicle and a young, medium, and an old corpora albicantia (CA) visible; (c) left ovary of a lactating female with one young and two medium CAs visible; (d) right ovary of a pregnant female with a corpus luteum (CL) of late pregnancy, a medium, and an old CA visible with a (D1) median slice through the CL; (e) median slice through the left ovary of a lactating female, multiple follicles visible; (f) median slice through a young CA on the left ovary of a resting female; (g) median slice through a medium CA on the left ovary of a lactating female; and (h) median slice through an old CA on the left ovary of a resting female. All ovaries formalin fixed. Scale bar = 1 cm
As reported by Betty (2019), none of the 114 sexually mature females for which both ovaries were examined showed evidence of being post-reproductive (i.e., would not ovulate again). Where both the age and full reproductive status were available for sexually mature individuals (n = 102), the proportion of pregnant, lactating, and resting mature individuals was determined for six age groups (5–10, 11–15, 16–20, 21–25, 26–30, 31–35 years) to identify any changes in reproductive status with increasing age (Fig. 15.7). For the age class 5–10 years, a very small proportion of sexually mature pilot whales were resting (14%), and the majority of individuals were either pregnant (50%) or lactating (36%). A decreased proportion of pregnant and an increased proportion of resting individuals were noted in age classes >10 years (compared to the 5–10 year age class), except the single female in the oldest age class (31–35 years), which was aged at 33 years and was pregnant with a 23.5 cm fetus.
15.3.3.1 Lack of Evidence for a Post-reproductive Lifespan in LFPWs
The observation that pregnancy rates decrease and the duration of the resting periods increases in Southern Hemisphere LFPWs older than 10 years suggests that there is a reduction in fecundity with age, as also reported for the North Atlantic LFPW (Martin and Rothery 1993). In contrast to the North Atlantic LFPW, reproductive senescence was not evident in Southern Hemisphere LFPWs. However, the maximum female age estimated for Southern Hemisphere LFPWs in this study (33 years) was much lower than that recorded for North Atlantic LFPWs in the Faroe Islands (59 years; Martin and Rothery 1993) and Newfoundland (56.5 years; Sergeant 1962; Kasuya et al. 1988), where longevity exceeded 50 years (Table 15.1). The smaller sample size in this study, compared with the availability of much larger datasets from North Atlantic drive fisheries (e.g., n = 1402; Martin and Rothery 1993), decreased our probability of sampling the rare old (possibly senescent) females. However, even if true reproductive senescence does occur in a small proportion of the oldest females, few live long enough to enter this phase (e.g., approximately 10% of females reach 40 years of age in Faroese studies; Bloch et al. 1993) that it is unlikely to represent a significant and functional part of the life history or social ecology of this species (Martin and Rothery 1993; Ellis et al. 2018a). Fewer than 5% of female North Atlantic LFPWs are reported to become reproductively senescent, and pregnancy can potentially continue throughout life (oldest pregnant female 55 years; Martin and Rothery 1993).
It has been suggested that the demographic consequences of certain life history characteristics are important in the evolution of post-reproductive lifespans (Johnstone and Cant 2010; Croft et al. 2015; Ellis et al. 2018a; Nichols et al. 2020). However, such characteristics do not appear to necessitate the evolution of post-reproductive lifespans (Ellis et al. 2018a). Available evidence suggests that LFPWs exhibit similar life history characteristics and social structure to SFPWs and the other three odontocete species for which a substantial post-reproductive lifespan has been identified (resident killer whales, beluga whales, and narwhal; Ellis et al. 2018a; Nichols et al. 2020). For example, these species are all sexually dimorphic and highly social, have low lifetime productivity, and are known or believed to exist in stable matrilineal groups of closely related females with increasing local relatedness as females age, strong mother-offspring associations, and a long period of dependency (Bigg 1982; Kasuya and Marsh 1984; Heimlich-Boran 1993; Palsbøll et al. 1997; Whitehead and Mann 2000; Marcoux et al. 2009; Colbeck et al. 2013; O’Corry-Crowe et al. 2018; Nichols et al. 2020). However, no significant post-reproductive lifespan has been observed in LFPWs, but instead an acceleration in mortality rate (Bloch et al. 1993; Martin and Rothery 1993; Ellis et al. 2018a, b; Betty 2019; Betty et al. 2022b).
Although the social structure is thought to be similar for both pilot whale species, the observed variation in post-reproductive life history strategies may be due in part to the social organization within stable social groups and the relative costs and benefits of cooperative foraging and intergenerational transfer of information. To have an evolutionary benefit, post-reproductive females must be able to contribute to increasing the fitness of relatives in their group. In both SPFWs and killer whales, inclusive fitness is increased by late-life helping and post-reproductive females fulfilling mother and grandmother roles within their group (Kasuya and Marsh 1984; Brent et al. 2015; Croft et al. 2017). Late-life helping has not been observed in LFPWs, though it is acknowledged that empirical data are very limited and difficult to collect. However, genetic studies of LFPWs from the North Atlantic (Faroe Island drive fishery) have revealed that the probability of pregnancy declines with the number of philopatric daughters (but not sons), implying females may refrain from breeding when they come into reproductive competition with their daughters (Nichols et al. 2020). It has been proposed that this apparent plasticity in the cessation of reproduction could represent a step toward the evolution of a post-reproductive lifespan or an alternative strategy to a fixed (and irreversible) post-reproductive lifespan (Nichols et al. 2020), though further investigation is required. Overall, there remains much to be discovered regarding the occurrence, evolution, and function of post-reproductive lifespans in pilot whales and other toothed whales.
15.4 Conclusions and Future Directions
This chapter summarizes what is currently known about the life history, sociobiology, and reproductive strategies of both LFPWs and SFPWs. We have added significantly to what is known of sex in pilot whales by documenting non-conceptive reproductive behaviors in sexually immature North Atlantic LFPWs, providing evidence for male natal philopatry in Atlantic Naisa SFPWs, and reporting an apparent absence of post-reproductive lifespan in Southern Hemisphere LFPWs. However, there is much that remains unknown; we understand very little about conceptive and non-conceptive sexual behavior in free-ranging pilot whales. Up and coming drone studies may help assist, along with the collection of in situ morphometric data. In addition, genomic analyses of stable units/pods within different populations are needed to confirm if strict matrilineality occurs broadly across both species. Biologging several individuals of known sex in the same unit/pod will be useful to study behavior, fine-scale movements, how both sexes with stable associations interact, and whether males disperse (even briefly) for mating. Further, the evolutionary reason for the apparent differences in post-reproductive lifespans and mortality rate acceleration between LFPWs and SFPWs has not been established and warrants further investigation. Empirical data needed to examine reproductive senescence are often difficult to gather for long-lived species such as cetaceans. Longitudinal data are required to test hypotheses about how a post-reproductive lifespan might increase inclusive population fitness. However, mass stranding events provide valuable opportunities to investigate the interplay between social structure and life history strategies (e.g., the existence of post-reproductive females) across populations of both pilot whale species.
References
Alves F, Quérouil S, Dinis A, Nicolau C, Ribeiro C, Freitas L, Kaufmann M, Fortuna C (2013) Population structure of shortfinned pilot whales in the oceanic archipelago of Madeira based on photo-identification and genetic analyses: implications for conservation. Aqua Conserv Mar Freshw Ecosyst 23:758–776. https://doi.org/10.1002/aqc.2332
Alves F, Dinis A, Nicolau C, Ribeiro C, Kaufmann M, Fortuna C, Freitas L (2015) Survival and abundance of short-finned pilot whales in the archipelago of Madeira, NE Atlantic. Mar Mamm Sci 31:106–121. https://doi.org/10.1111/mms.12137
Alves F, Alessandrini A, Servidio A, Mendonça AS, Hartman KL, Prieto R, Berrow S, Magalhães S, Steiner L, Santos R, Ferreira R (2019) Complex biogeographical patterns support an ecological connectivity network of a large marine predator in the northeast Atlantic. Divers Distrib 25:269–284. https://doi.org/10.1111/ddi.12848
Alves F, Dromby M, Baptista V, Ferreira R, Correia AM, Weyn M, Valente R, Froufe E, Rosso M, Sousa-Pinto I, Dinis A (2020) Ecophysiological traits of highly mobile large marine predators inferred from nucleic acid derived indices. Sci Rep 10:4752. https://doi.org/10.1038/s41598-020-61769-7
Amos B, Bloch D, Desportes G, Majerus TMO, Bancroft DR, Barrett JA, Dover GA (1993a) A review of molecular evidence relating to social organization and breeding system in the long-finned pilot whale. Rep Int Whal Commn 14:209–217
Amos B, Schlötterer C, Tautz D (1993b) Social structure of pilot whales revealed by analytical DNA profiling. Science 260:670–672. https://doi.org/10.1126/science.8480176
Auger-Méthé M, Whitehead H (2007) The use of natural markings in studies of long-finned pilot whales (Globicephala melas). Mar Mamm Sci 23:77–93. https://doi.org/10.1111/j.1748-7692.2006.00090.x
Augusto JF, Frasier TR, Whitehead H (2017) Social structure of long-finned pilot whales (Globicephala melas) off northern Cape Breton Island, Nova Scotia. Behaviour 154:509–540. https://doi.org/10.1163/1568539X-00003432
Ball RJ, Kitchiner A, Davison NJ, Brownlow A, Berrow S, McKeown NJ, IJsseldijk LL, Geary M, McDowall I, Muir AP (2021) New haplotypes found in stranded long-finned pilot whales (Globicephala melas) in the eastern North Atlantic and adjacent waters. Mar Mamm Sci 38:898–912. https://doi.org/10.1111/mms.12893
Behar DM, Villems R, Soodyall H, Blue-Smith J, Pereira L, Metspalu E, Scozzari R, Makkan H, Tzur S, Comas D, Bertranpetit J (2008) The dawn of human matrilineal diversity. Am J Hum Genet 82:1130–1140. https://doi.org/10.1016/j.ajhg.2008.04.002
Bejder L, Fletcher D, Bräger S (1998) A method for testing association patterns of social animals. Anim Behav 56:719–725. https://doi.org/10.1006/anbe.1998.0802
Berger V, Reichert S, Lahdenperä M, Jackson J, Htut W, Lummaa V (2021) The elephant in the family: costs and benefits of elder siblings on younger offspring life-history trajectory in a matrilineal mammal. J Anim Ecol 90:2663–2677. https://doi.org/10.1111/1365-2656.13573
Best PB (1979) Social organization in sperm whales, Physeter macrocephalus. In: Winn HE, Olla BL (eds) Behavior of marine animals. Springer, Boston, MA, pp 227–289
Best PB (1980) Pregnancy rates in sperm whales off Durban. Rep Int Whal Commn 2:93–97
Best PB, Canham P, MacLeod N (1984) Patterns of reproduction in sperm whales, Physeter macrocephalus. Rep Int Whal Commn 6:51–79
Betty EL (2019) Life history of the long-finned pilot whale (Globicephala melas edwardii); insights from strandings on the New Zealand coast. PhD thesis, Auckland University of Technology
Betty EL, Stockin KA, Smith AN, Bollard B, Orams MB, Murphy S (2019) Sexual maturation in male long-finned pilot whales (Globicephala melas edwardii): defining indicators of sexual maturity. J Mamm 100:1387–1402. https://doi.org/10.1093/jmammal/gyz086
Betty EL, Stockin KA, Hinton B, Bollard BA, Smith AN, Orams MB, Murphy S (2022a) Age, growth, and sexual dimorphism of the southern hemisphere long-finned pilot whale (Globicephala melas edwardii). J Mamm 103:560–575. https://doi.org/10.1093/jmammal/gyab165
Betty EL, Stockin KA, Hinton B, Bollard BA, Orams MB, Murphy S (2022b) Age- and sex-specific survivorship of the southern hemisphere long-finned pilot whale (Globicephala melas edwardii). J Mamm 103:560. https://doi.org/10.1093/jmammal/gyac085
Bigg MA (1982) An assessment of killer whale (Orcinus orca) stocks off Vancouver Island, British Columbia. Rep Int Whal Commn 32:655–666
Bigg MA, Olesiuk PF, Ellis GM (1990) Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington state. Rep Int Whal Commn 12:383–405
Bloch D, Lockyer C, Zachariassen M (1993) Age and growth parameters of the long-finned pilot whale off The Faroe Islands. Rep Int Whal Commn 14:163–208
Boran J, Heimlich S (2019) Pilot whales: delphinid matriarchies in deep seas. In: Würsig B (ed) Ethology and behavioral ecology of odontocetes. Springer Nature, Cham, pp 281–304. https://doi.org/10.1007/978-3-030-16663-2_13
Boyd I, Lockyer C, Marsh H (1999) Reproduction in marine mammals. In: Reynolds JI, Rommel SA (eds) Biology of marine mammals. Smithsonian Institution Press, Washington, DC, pp 218–286
Brent LJ, Franks DW, Foster EA, Balcomb KC, Cant MA, Croft DP (2015) Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr Biol 25:746–750. https://doi.org/10.1016/j.cub.2015.01.037
Cantor M, Shoemaker LG, Cabral RB, Flores CO, Varga M, Whitehead H (2015) Multilevel animal societies can emerge from cultural transmission. Nat Comm 6:8091. https://doi.org/10.1038/ncomms9091
Colbeck GJ, Duchesne P, Postma LD, Lesage V, Hammill MO, Turgeon J (2013) Groups of related belugas (Delphinapterus leucas) travel together during their seasonal migrations in and around Hudson Bay. Proc Biol Sci 280:20122552. https://doi.org/10.1098/rspb.2012.2552
Connor R, Smolker R, Bejder L (2006) Synchrony, social behavior and alliance affiliation in Indian Ocean bottlenose dolphins, Tursiops adunucus. Anim Behav 72:1371–1378
Crespo E, Pagnoni G, Pedraza S (1985) Structure of a long-finned pilot whale school stranded in Patagonia. Sci Rep Whales Res Inst 36:97–106
Croft DP, Brent LJ, Franks DW, Cant MA (2015) The evolution of prolonged life after reproduction. Trends Ecol Evol 30:407. https://doi.org/10.1016/j.tree.2015.04.011
Croft DP, Johnstone RA, Ellis S, Nattrass S, Franks DW, Brent LJ, Mazzi S, Balcomb KC, Ford JK, Cant MA (2017) Reproductive conflict and the evolution of menopause in killer whales. Curr Biol 27:298–304. https://doi.org/10.1016/j.cub.2016.12.015
de Stephanis R, Verborgh P, Pérez S, Esteban R, Minvielle-Sebastia L, Guinet C (2008) Long-term social structure of long-finned pilot whales (Globicephala melas) in the strait of Gibraltar. Acta Ethol 11:81–94. https://doi.org/10.1007/s10211-008-0045-2
Desportes G, Andersen LW, Aspholm PE, Bloch D, Mouritsen R (1992) A note about a male-only pilot whale school observed in Faroe Islands. Fróđskaparrit 40:31–37
Desportes G, Saboureau M, Lacroix A (1993) Reproductive maturity and seasonality of male long-finned pilot whales, off The Faroe Islands. Rep Int Whal Commn 14:233–262
Dines JP, Mesnick SL, Ralls K, May-Collado L, Agnarsson I, Dean MD (2015) A trade-off between precopulatory and postcopulatory trait investment in male cetaceans. Evolution 69:1560–1572. https://doi.org/10.1111/evo.12676
Ellis S, Franks DW, Nattrass S, Currie TE, Cant MA, Giles D, Balcomb KC, Croft DP (2018a) Analyses of ovarian activity reveal repeated evolution of post-reproductive lifespans in toothed whales. Sci Rep 8:12833. https://doi.org/10.1038/s41598-018-31047-8
Ellis S, Franks DW, Nattrass S, Cant MA, Bradley DL, Giles D, Balcomb KC, Croft DP (2018b) Postreproductive lifespans are rare in mammals. Ecol Evol 8:2482–2494. https://doi.org/10.1002/ece3.3856
Esteban R, Verborgh P, Freitas L (2022) Dynamics of short-finned pilot whales long-term social structure in Madeira. Mamm Biol 102:1315. https://doi.org/10.1007/s42991-022-00280-0
Ferguson SH, Willing C, Kelley TC, Boguski DA, Yurkowski DJ, Watt CA (2020) Reproductive parameters for female beluga whales (Delphinapterus leucas) of Baffin Bay and Hudson Bay, Canada. Arctic 73:405–420
Ford JKB (2019) Killer whales: behavior, social organization, and ecology of the oceans’ apex predators. In: Würsig B (ed) Ethology and behavioral ecology of odontocetes. Springer Nature, Cham, pp 239–259. https://doi.org/10.1007/978-3-030-16663-2_11
Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JK, Croft DP (2012) Adaptive prolonged postreproductive life span in killer whales. Science 337:1313. https://doi.org/10.1126/science.1224198
Furuichi T, Connor R, Hashimoto C (2014) Non-conceptive sexual interactions in monkeys, apes, and dolphins. In: Yamagiwa J, Karczmarski L (eds) Primates and cetaceans: field research and conservation of complex mammalian societies. Springer, Tokyo, pp 385–408
Garde E, Hansen SH, Ditlevsen S, Tvermosegaard KB, Hansen J, Harding KC, Heide-Jørgensen MP (2015) Life history parameters of narwhals (Monodon monoceros) from Greenland. J Mamm 96:866–879. https://doi.org/10.1093/jmammal/gyv110
Geraci JR, Lounsbury VJ (2005) Marine mammals ashore: a field guide for strandings, 2nd edn. National Aquarium in Baltimore, Baltimore, MD
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162. https://doi.org/10.1016/S0003-3472(80)80103-5
Ham JR, Lilley MK, Lelekach J, Miller MR, Robeck TR, Pellis SM, Hill HM (2022) The emergence and early development of socio-sexual behavior in beluga calves (Delphinapterus leucas). Behav Process 200:14695. https://doi.org/10.1016/j.beproc.2022.104695
Ham JR, Lilley MK, Hill HM (2023) Non-conceptive sexual behavior in cetaceans: comparison of form and function. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Heimlich-Boran J (1993) Social organization of the short-finned pilot whale, Globicephala macrorhynchus, with special reference to the comparative social ecology of delphinids. PhD thesis, University of Cambridge
Hill MC, Bendlin AR, Van Cise AM, Milette-Winfree A, Ligon AD, AC Ü, Deakos MH, Oleson EM (2019) Short-finned pilot whales (Globicephala macrorhynchus) of the Mariana archipelago: individual affiliations, movements, and spatial use. Mar Mamm Sci 35:797–824. https://doi.org/10.1111/mms.12567
Jefferson TA, Webber MA, Pitman RL (2008) Marine mammals of the world: a comprehensive guide to their identification. Academic Press, London
Johnstone R, Cant M (2010) The evolution of menopause in cetaceans and humans: the role of demography. Proc Biol Sci 277:3765. https://doi.org/10.1098/rspb.2010.0988
Kasuya T (2017) Small cetaceans of Japan: exploitation and biology. CRC Press, Taylor & Francis Group, Boca Raton, FL
Kasuya T, Marsh H (1984) Life history and reproductive biology of the short-finned pilot whale, Globicephala macrorhynchus, off the Pacific coast of Japan. Rep Int Whal Commn 6:259–309
Kasuya T, Matsui S (1984) Age determination and growth of the short-finned pilot whale off the Pacific coast of Japan. Sci Rep Whales Res Inst 35:57–91
Kasuya T, Tai S (1993) Life history of short-finned pilot whale stocks off Japan and a description of the fishery. Rep Int Whal Commn 14:439–473
Kasuya T, Sergeant DE, Tanaka K (1988) Re-examination of life history parameters of long-finned pilot whales in the Newfoundland waters. Sci Rep Whales Res Inst 39:103–119
Lilley MK, Ham JR, Hill HM (2020) The development of socio-sexual behavior in belugas (Delphinapterus leucas) under human care. Behav Process 171:104025
Lockyer C (1993) A report on patterns of deposition of dentine and cement in teeth of pilot whales, genus Globicephala. Rep Int Whal Commn 14:137–161
Lonati GL, Hynes NJ, Howe KR, Durette-Morin D, Brown MW, Davies KT (2022) Observations of adult–calf nonreproductive copulatory behavior in North Atlantic right whales (Eubalaena glacialis). Aqua Mamm 48:639–645. https://doi.org/10.1578/am.48.6.2022.639
MacLeod C (2010) The relationship between body mass and relative investment in testes mass in cetaceans: implications for inferring interspecific variations in the extent of sperm competition. Mar Mamm Sci 26:370–380
Mahaffy SD, Baird RW, McSweeney DJ, Webster DL, Schorr GS (2015) High site fidelity, strong associations, and long-term bonds: short-finned pilot whales off the Island of Hawai‘i. Mar Mamm Sci 31:1427–1451. https://doi.org/10.1111/mms.12234
Mann J (2006) Establishing trust: socio-sexual behaviour and the development of male-male bonds among Indian Ocean. In: Sommer V, Vasey PL (eds) Homosexual behaviour in animals: an evolutionary perspective. Cambridge University Press, Cambridge, pp 107–130
Marcoux M, Auger-Méthé M, Humphries MM (2009) Encounter frequencies and grouping patterns of narwhals in Koluktoo Bay, Baffin Island. Polar Biol 32:1705–1716. https://doi.org/10.1007/s00300-009-0670-x
Markowitz TM, Markowitz WJ, Würsig B, Orbach DN (2023) Sociosexual behavior of nocturnally foraging dusky and spinner dolphins. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Marsh H, Kasuya T (1984) Changes in the ovaries of the short-finned pilot whale, Globicephala macrorhynchus, with age and reproductive activity. Rep Int Whal Commn 6:311–335
Marsh H, Kasuya T (1986) Evidence for reproductive senescence in female cetaceans. Rep Int Whal Commn 8:57–74
Marsh H, Kasuya T (1990) An overview of the changes in the role of a female pilot whale with age. In: Pryor K, Norris KS (eds) Dolphin societies: discoveries and puzzles. University of California Press, Berkeley, CA, pp 281–284
Martin AR, Rothery P (1993) Reproductive parameters of female long-finned pilot whales (Globicephala melas) around he Faroe Islands. Rep Int Whal Commn 14:263–304
Martin AR, Reynolds P, Richardson MG (1987) Aspects of the biology of pilot whales (Globicephala melaena) in recent mass strandings on the British coast. J Zool 211:11–23
Mesnick S, Ralls K (2018) Sexual dimorphism. In: Würsig B, Thewissen JGM, Kovacs KM (eds) Encyclopedia of marine mammals, 3rd edn. Academic Press, London, pp 848–853. https://doi.org/10.1016/B978-0-12-804327-1.00226-0
Miralles L, Lens S, Rodriguez-Folgar A, Carrillo M, Martin V, Mikkelsen B, Garcia-Vazquez E (2013) Interspecific introgression in cetaceans: DNA markers reveal post-F1 status of a pilot whale. PLoS One 8:e69511. https://doi.org/10.1371/journal.pone.0069511
Miralles L, Oremus M, Silva MA, Planes S, Garcia-Vazquez E (2016) Interspecific hybridization in pilot whales and asymmetric genetic introgression in northern Globicephala melas under the scenario of global warming. PLoS One 11:e0160080. https://doi.org/10.1371/journal.pone.0160080
Nichols HJ, Arbuckle K, Fullard K, Amos W (2020) Why don’t long-finned pilot whales have a widespread postreproductive lifespan? Insights from genetic data. Behav Ecol 31:508–518. https://doi.org/10.1093/beheco/arz211
O’Corry-Crowe G, Suydam R, Quakenbush L, Potgieter B, Harwood L, Litovka D, Ferrer T, Citta J, Burkanov V, Frost K, Mahoney B (2018) Migratory culture, population structure and stock identity in North Pacific beluga whales (Delphinapterus leucas). PLoS One 13:e0194201. https://doi.org/10.1371/journal.pone.0194201
Olson PA (2018) Pilot whales Globicephala melas and G. macrorhynchus. In: Würsig B, Thewissen JGM, Kovacs KM (eds) Encyclopedia of marine mammals, 3rd edn. Academic Press, London, pp 701–705
Orbach DN, Packard JM, Kirchner T, Würsig B (2015) Evasive behaviours of female dusky dolphins (Lagenorhynchus obscurus) during exploitative scramble competition. Behaviour 152:1953–1977
Oremus M, Gales R, Kettles H, Baker CS (2013) Genetic evidence of multiple matrilines and spatial disruption of kinship bonds in mass strandings of long-finned pilot whales, Globicephala melas. J Hered 104:301–311. https://doi.org/10.1093/jhered/est007
Ottensmeyer C, Whitehead H (2003) Behavioural evidence for social units in long-finned pilot whales. Can J Zool 81:1327–1338
Palsbøll PJ, Heide-Jørgensen MP, Dietz R (1997) Population structure and seasonal movements of narwhals, Monodon monoceros, determined from mtDNA analysis. Heredity 78:284. https://doi.org/10.1038/hdy.1997.43
Perrin WF, Reilly SB (1984) Reproductive parameters of dolphins and small whales of the family Delphinidae. Rep Int Whal Commn 6:97–133
Photopoulou T, Ferreira IM, Best PB, Kasuya T, Marsh H (2017) Evidence for a postreproductive phase in female false killer whales Pseudorca crassidens. Front Zool 14:30. https://doi.org/10.1186/s12983-017-0208-y
Sanvito S, Galimberti F (2022) Male–male sexual interactions between an adult and a calf killer whale (Orcinus orca) of the Falkland Islands. Aqua Mamm 48:759–763. https://doi.org/10.1578/am.48.6.2022.759
Sergeant DE (1962) The biology of the pilot or pothead whale Globicephala meleana (Traill) in Newfoundland waters. J Fish Res 132:84
Servidio A, Pérez‐Gil E, Pérez‐Gil M, Cañadas A, Hammond PS, Martín V (2019) Site fidelity and movement patterns of short-finned pilot whales within the Canary Islands: evidence for resident and transient populations. Aqua Conserv Mar Freshw Ecosyst 29:227–241. https://doi.org/10.1002/aqc.3135
Sigurjonsson J, Vikingsson G, Lockyer C (1993) Two mass strandings of pilot whales Globicephala melas on the coast of Iceland. Rep Int Whal Commn 14:407–423
Soto FA, Grandi MF, García NA, Crespo EA, Dans SL (2017) Reproductive parameters of female long-finned pilot whales (Globicephala melas edwardii) from the southwestern Atlantic. Zool Stud 56:1–12
Van Cise AM, Martien KK, Mahaffy SD, Baird RW, Webster DL, Fowler JH, Oleson EM, Morin PA (2017) Familial social structure and socially driven genetic differentiation in Hawaiian short-finned pilot whales. Mol Ecol 26:6730–6741. https://doi.org/10.1111/mec.14397
Van Cise AM, Baird RW, Baker CS, Cerchio S, Claridge D, Fielding R, Hancock-Hanser B, Marrero J, Martien KK, Mignucci-Giannoni AA, Oleson EM (2019) Oceanographic barriers, divergence, and admixture: phylogeography and taxonomy of two putative subspecies of short-finned pilot whale. Mol Ecol 28:2886–2902. https://doi.org/10.1111/mec.15107
Verborgh P, Janssen EH, Esteban R, Gauffier P, Freitas L (2022) Proposing a framework for monitoring demographic parameters in local cetacean populations: the case of short-finned pilot whales in Madeira. Mamm Biol 102:1425. https://doi.org/10.1007/s42991-022-00266-y
Whitehead H (1999) Testing association patterns of social animals. Anim Behav 57:F26–F29. https://doi.org/10.1006/anbe.1999.1099
Whitehead H (2008) Analyzing animal societies: quantitative methods for vertebrate social analysis. University of Chicago Press, Chicago, IL
Whitehead H (2009) SOCPROG programs: analyzing animal social structures. Behav Ecol Sociobiol 63:765–778. https://doi.org/10.1007/s00265-008-0697-y
Whitehead H, Mann J (2000) Female reproductive strategies of cetaceans: life histories and calf care. In: Connor R, Mann J, Tyack P, Whitehead H (eds) Cetacean societies: field studies of dolphins and whales. University of Chicago Press, Chicago, IL
Yahn SN, Baird RW, Mahaffy SD, Robertson KM (2023) Sexually dimorphic characteristics of short-finned pilot whales, false killer whales, pygmy killer whales, and melon-headed whales assessed using fin and body morphometrics from photographs taken at sea. Mar Mamm Sci 39:98. https://doi.org/10.1111/mms.12963
Acknowledgments
ELB acknowledges Ngāti Kuri, Ngāi Tokato, Te Aupouri, Waikato, Manawhenua ki Mohua, and Ngāi Tahu of the Department of Conservation Te Papa Atawhai for providing access to stranded pilot whales on the coast of Aotearoa New Zealand for sampling and, in many cases, also providing logistical support in the field. Biological samples from Aotearoa New Zealand were collected under marine mammal research permit 39635-MAR issued to ELB by the Department of Conservation Te Papa Atawhai. ELB also thanks the following people for support with sample collection and/or processing: Tim Beatson, Mat Betty, Barbara Bollard, Barrack Carle, Sarah Dwyer, Jeanie Fay, Sarah Gardiner, Nik Hannam, Severine Hannam, Bethany Hinton, Jessica Hiscox, Shabana Honetana, Odette Howarth, Rebecca Jarvis, Emmanuelle Martinez, Sinéad Murphy, Steve O’Shea, Karen Stockin, Andrew Wallace, Lindsey White, and Jessie Williams. EZ acknowledges data collection assistance from Grace Guiney, Aaron Clausen, Natalie Colbourne, and Oshan Whale Watch. FA and MW acknowledge support in data collection by Ana Dinis, Rita Ferreira, Annalisa Sambolino, and Marc Fernandez. Biological samples from Madeira were collected under research permits 1.856/2017, 508/2018, and 10661/2018 issued to FA by the Instituto de Florestas e Conservação da Natureza (Instituto Português—Região Autónoma da Madeira). The preparation of this chapter was supported by funding from the Kate Edger Educational Charitable Trust (ELB), Natural Sciences and Engineering Research Council of Canada (6799-503466-2017) (EZ), and Portuguese Foundation for Science and Technology through the strategic projects/grants UIDB/04292/2020, UIDP/04292/2020, and UI/BD/151240/2021 awarded to MARE and through the project LA/P/0069/2020 granted to the Associate Laboratory ARNET (FA and MW). The authors acknowledge that some of the content of this chapter is also included in the PhD thesis of ELB (Betty 2019).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
15.1 Supplementary Material
Supplement 1
Video of sociosexual behavior in immature North Atlantic long-finned pilot whales (Globicephala melas melas) documented off Cape Breton, Nova Scotia, Canada (MOV 541453 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Betty, E.L., Zwamborn, E.M.J., Weyn, M., Luck, E., Alves, F. (2023). Life History Parameters, Sociobiology, and Reproductive Strategies of Pilot Whales. In: Würsig, B., Orbach, D.N. (eds) Sex in Cetaceans. Springer, Cham. https://doi.org/10.1007/978-3-031-35651-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-35651-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35650-6
Online ISBN: 978-3-031-35651-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)