Abstract
The standard of care for differentiated thyroid carcinoma (DTC) includes surgery, risk-adapted postoperative radioiodine [iodine-131 (131I)] therapy, individualized thyroid hormone therapy, and follow-up for detection of patients with recurrent or persistent disease.
Recently, several international associations like ATA, EANM, and SNMMI developed specific guidelines for the management of these patients. They shared that an individualized risk-adapted approach should be suggested considering the main clinical, epidemiological, and histopathological features. The postoperative management of DTC is a challenge because several biomarkers and molecular imaging tools are available. The choice of execution and the timing of neck ultrasound, serum anti-thyroglobulin antibody and basal/stimulated thyroglobulin, the 131I/123I diagnostic whole-body scans integrated by single photon emission computed tomography/computed tomography (SPECT/CT) if indicated, and 18F-fluorodeoxyglucose ([18F]-FDG) positron emission tomography/CT (PET/CT) is directly related to the patients’ characteristics. In this chapter, we summarize the role of the main biomarker and molecular imaging examinations in the management of DTC patients in postoperative setting.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Differentiated thyroid carcinoma (DTC) is the most common endocrine cancer and its incidence has increased in the past decades. Generally, DTC has an indolent course with a favorable prognosis, except for cases with distant metastases at the time of diagnosis. For a long time, DTC was treated by thyroidectomy followed by radioiodine [iodine-131 (131I)] therapy, with the theoretical result of the absence of residual benign thyroid tissue, letting thyroglobulin (Tg) be an ideal marker of disease.
Nowadays, recent American Thyroid Association (ATA) guidelines [1] suggest an individualized risk-adapted approach where the type and extent of surgery (lobectomy or total thyroidectomy), the indication and the goal of 131I therapy (ablation, adjuvant, curative), replacement therapy with levothyroxine features (TSH suppressive or not) are individually personalized for each case [2].
For example, nowadays low to intermediate risk DTC patients are currently treated by thyroidectomy without radioiodine ablation or, in selected low risk, even by lobectomy alone. In these cases, the absolute value of Tg should be less significant for the follow-up due to the difficulties to discriminate between physiological and pathological Tg values, and the absence of a specific reference value [3, 4].
8.2 Biomarkers: Tg and TgAb
Postoperative risk stratification is based on the criteria reported by ATA guidelines [1].
Tg is a 660-kDa glycoprotein produced exclusively in the thyroid gland where it serves as the source for thyroxine (T4) and triiodothyronine (T3) production within the thyroid follicles. Small amounts of Tg are detected in the serum of healthy individuals as it is secreted alongside T4 and T3 [5]. Increased serum Tg levels are present during several disordered thyroid growths, increased thyroid activity, and glandular destruction such as in goiter, Graves’ disease, and thyroiditis, or in DTC cells. Thus, the measurement of Tg for the initial evaluation of suspicious thyroid nodules is not recommended, due to the overlap in Tg levels in patients with DTC and benign nodules [3, 4].
Absolute Tg concentrations are correlated with tumor load and are widely employed to assess the extension of the disease and evaluate the response to treatments. However, this single tumor marker measurement may not be exhaustive in the whole comprehension of disease status and treatment response, because it is not intrinsically inclusive of previous measurements and the overall trend.
During initial follow-up, the recommended interval for serum Tg measurement is about 6–12 months, unless in high-risk patients where more frequent measurements may be suggested.
Tg together with thyroglobulin autoantibodies (TgAb), neck ultrasound (US), and any additional imaging procedures (i.e., 131I whole-body scintigraphy [WBS], computed tomography [CT], positron emission tomography [PET], and magnetic resonance imaging [MRI]) are mandatory for the monitoring of patients in the postoperative field, aiding in the early detection of persistent or recurrent disease and guiding the evaluation of dynamic risk.
The main limitation of Tg is the potential interference of TgAb which makes Tg not perfectly evaluable. More rarely, also heterophilic antibodies (HAb) may interfere with Tg measurement in vitro and cause false-positive Tg results. In DTC patients, the presence of TgAb and HAb is not so rare, with a prevalence described in up to 15–25% for TgAb and 1% for HAb [6].
Historically, Tg measurement was first performed by competitive radioimmunological assays (RIAs). However, RIAs were replaced by direct immunometric assays (IMAs), which are more sensitive, have a shorter incubation, a more robust labeled antibody reagent and a larger working range. Tg-IMAs are based on a two-site reaction that involves Tg capture by a solid-phase antibody followed by the addition of a labeled antibody that targets different epitopes on the captured Tg. Over the years, Tg assays have evolved to achieve superior sensitivities and a number of commercially available Tg-IMAs have functional sensitivities of 0.1–0.2 mg/L, referred to as high-sensitive (hsTg) or second-generation Tg-IMas [7]. Before the introduction of hsTg, thyroid hormone withdrawal or recombinant human TSH stimulation was necessary to reach the best degree of diagnostic sensitivity. Now, these procedures can be skipped in most cases with a significant improvement in patient quality of life and a reduction in costs.
When possible it is fundamental that consecutive Tg measurements be performed in the same laboratory using the same assay each time. If any change is unavoidable, a new baseline should be established and proceed with the same method.
A big problem of different IMAs is the inter-method variability, despite the introduction of the Certified Reference Material (CRM 457, currently called BCR 457) has partially reduced this variability. For this reason, it would be desirable to have assay-specific Tg cutoffs instead of fixed thresholds.
Moreover, the susceptibility of Tg-IMAs to antibodies-based interference is another limitation, causing an underestimation of Tg. Otherwise, TgAb concentration may become a surrogate tumor marker and guide patient management. Tumor recurrence can be anticipated by a rise in Tg antibodies with or without an increase in serum Tg.
Serum Tg concentrations are further influenced and regulated by the degree of thyroid stimulating hormone (TSH) stimulation. Measurements of Tg can be TSH suppressed while patients remain on suppressive doses of thyroid hormone, or TSH stimulated after thyroid hormone withdrawal or administration of recombinant human TSH (rhTSH).
Minor fluctuations between Tg measurements are possible and not necessarily referable to the recurrence or progression of the disease. However, a progressive increase in circulating Tg measurement strongly suggests recurrence/progression and should lead to further imaging tests to identify the site of the disease. The dynamic changes of Tg over time may be an alternative or complementary tumor marker, aside from the absolute Tg value. Tg kinetics may be expressed as Tg doubling time (Tg-DT) or Tg velocity (Tg-vel). Tg doubling time (Tg-DT) was studied more and has been demonstrated as a valuable biomarker to predict loco-regional recurrences, distant metastases, and survival independently from the main prognostic variables (like gender, age, and TNM stage) [8]. In addition, Tg-DT may also help to select patients that will benefit from [18F]-FDG PET/CT [9]. About Tg-vel only initial evidences are available and further studies are mandatory [10].
8.2.1 Biomarkers Role in DTC Patients Treated by Total Thyroidectomy and 131I
As previously discussed, Tg is a pivotal sensitive tool used in monitoring patients with DTC for the presence of residual or recurrent disease. In particular, Tg values have the highest sensitivity and specificity for the detection of recurrent disease after total thyroidectomy and 131I ablation.
In the past, a measurement of stimulated Tg (after thyroid hormone withdrawal or rhTSH administration) every 6–12 months was suggested. Stimulated Tg < 1–2 ng/mL without evidence of structural disease (negative clinical examination, US, and/or other imaging modalities) predicted an excellent prognosis with a very low risk of recurrence and a normal life expectancy even in patients with high-risk disease [1]. More recently, with the development of Tg immunoassays more sensitive (hsTg), measurement of serum Tg concentrations of 0.1–0.2 ng/mL is possible. hsTg assays let to avoid the need for TSH stimulation, due to the fact that hsTg less than 0.1–0.2 ng/mL has a comparable negative predictive value (>95%) than sTg less than 1–2 ng/mL.
ATA guidelines classified response assessment after total thyroidectomy and radioiodine in different categories according to clinical, imaging, and serum results [1].
Response to therapy is assessed at each clinical appointment during surveillance. Careful clinical assessment and review of serial Tg and TgAb results allow us to follow patients during the course of the disease. An excellent response is defined in case of no evidence of disease on clinical exam and imaging and undetectable serum Tg measurements. A biochemical incomplete response is defined in a case of no clinical or imaging evidence of disease but with an elevated or rising Tg or TgAb concentration. Structural incomplete response is defined in case of evidence of disease in the thyroid bed, cervical nodes, or at distant sites in the presence of any Tg or TgAb value. Lastly, an indeterminate response category is for patients with non-specific or borderline biochemical or structural findings. Often patient surveillance with serial Tg and imaging will allow them to be recategorized into one of the above groups. Table 8.1 summarizes the response to therapy definitions according to ATA guidelines.
For patients undergoing total thyroidectomy and 131I therapy that achieved an excellent response, an undetectable Tg during the follow-up may avoid performing imaging procedures. Instead in case of increasing Tg or TgAb levels, further investigations, like neck US and radioiodine WBS, should be performed [11].
8.2.2 Biomarkers Role in DTC Patients Treated by Total Thyroidectomy
A total or near-total thyroidectomy without 131I ablation is now suggested in selected low- to intermediate-risk DTC. In this scenario, Tg is significantly influenced by the amount of residual thyroid remnants and the TSH level at the time of Tg measurement [12]. Thus, Tg potentially obscures possible tumor-related Tg levels and reduces the accuracy of dynamic risk stratification. Spencer et al. [13] speculated that Tg values under TSH suppression remain in the 0.1–0.5 mg/L range during long-term follow-up of these patients. Moreover, the amount of residual remnant thyroid tissue is strictly surgeon-dependent and widely variable, and chronic TSH suppression is no longer recommended in these patients. Tg reference intervals mathematically normalized to TSH level and residual thyroid tissue are needed to be validated. However, to guarantee reproducible results, stable TSH values are desirable or, at least, extremely low lot-to-lot variability and extremely good reproducibility are needed over a long time to guarantee reproducible results [3, 4, 14]. Dynamic evaluation of circulating Tg concentration may still provide useful information in such circumstances. A trend of decreasing Tg after surgery is usually reassuring, but general interpretation criteria for Tg in non-ablated DTC are lacking.
8.2.3 Biomarkers Role in DTC Patients Treated by Lobectomy
In DTC patients treated only by lobectomy, measuring Tg is not so useful as Tg levels will not depend on the presence or absence of tumor foci, but rather on the mass of the remaining thyroid lobe, TSH concentration, and current iodine status.
A recent meta-analysis based upon 7 studies for a total of 2455 patients demonstrated that circulating Tg was non-reliable in detecting early response and predicting recurrence in patients treated with lobectomy/hemithyroidectomy, especially those with a low initial ATA classification [15].
Thus, the benefit of Tg concentration in this setting is questionable. If performed, the results should be carefully interpreted, taking into account both the corresponding TSH value and the imaging findings (such as neck US). The options for follow-up of DTC patients treated by lobectomy are to perform periodic neck US and, if recurrence or metastasis is suspected, to confirm the diagnosis through a fine-needle biopsy or further examinations.
8.2.4 Patients with Positive TgAb
TgAb are present in approximately 10% of the general population and in up to 25–30% of patients with DTC [5]. Serum levels of TgAb are not correlated with the tumor load of the patient, but rather indicate the activity of the immune system [16]. However, TgAb interference may result in false low results in Tg-IMAs. TgAb interferences are variable in different patients and different IMAs and are independent of TgAb levels [17].
Tg RIAs are reported to be more resistant to TgAb interference; however, a significant number of falsely low and falsely high results have been described; moreover, the functional sensitivities of these assays are suboptimal in comparison with IMAs.
Recently, tandem mass spectrometry-liquid chromatography (MS/MS-LC) and Tg (mini)-recovery test has emerged as a promising method to overcome interferences in Tg measurement, but the current generation of MS/MS-LC assays had suboptimal functional sensitivity and yield false-negative results in a significant number of patients with evidence of structural disease and slightly detectable Tg concentrations in high-sensitive assays (0.1–0.5 ng/mL).
Thus, hsTg assays remain the mainstay of monitoring TgAb-negative patients, and also in patients with TgAb-positive with detectable Tg levels can be indicated.
Serum TgAb had an average disappearance time of 3 years after thyroid ablation for DTC, indicating that TgAb can be used as a “surrogate tumor marker.” Similarly to Tg, the use of serial TgAb measurements as a surrogate tumor marker needs continuity of the laboratory method to perform an accurate comparison.
A consistent reduction in the serum TgAb level (especially when the reduction is more than 50% in the year after operation) indicates that the patient is likely to be free of disease, whereas a consistent rise or de novo appearance of serum TgAb raises suspicion of recurrence and prompts additional investigations; instead, an unchanged serum TgAb concentrations should be regarded as indeterminate and carefully monitored over time [6].
In summary, undetectable high-sensitive Tg and declining TgAb levels are both highly reassuring and predict favorable outcomes in TgAb-positive DTC patients after complete thyroid ablation and the lower, but still detectable Tg levels can be followed over time by high-sensitive Tg assay. No data are currently available to properly inform the management of TgAb-positive DTC patients treated by surgery alone without radioiodine ablation.
8.3 Molecular Imaging
8.3.1 Whole-Body Scintigraphy and SPECT/CT
8.3.1.1 Postoperative Setting
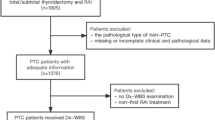
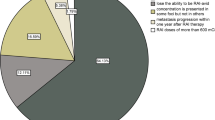
The last ATA guidelines [1] suggest that postoperative planar whole-body scintigraphy (WBS), after the administration of a diagnostic activity (1–5 mCi) of 131I, may be useful for the assessment of differentiated thyroid cancer (DTC) when the extent of thyroid remnants or the presence of residual disease could not be accurately ascertained from surgical report or ultrasonography (US). In this setting, the specific choice of the dose of 131I used to perform 131I therapy can be made empirically or can be guided by the integration of postoperative WBS. This latter method is theoretically able to improve risk stratification and staging of DTC patients, influencing the clinical management of the patients in up to 25–50% of the cases [11, 18, 19]. However, there are no clear evidences to suggest the superiority of one method over the other.
One of the issues that need to be considered with the use of postoperative WBS is the so-called “stunning effect,” resulting in a temporary suppression of iodine trapping function of the thyrocytes and thyroid cancer cells, as a result of the radiation given off by 131I. In this setting, several studies have reported that the use of 5 mCi of 131I before RAI treatment was independently associated with an increased risk of remnants ablation failure. However, these findings were not confirmed in other studies, resulting in heterogeneous insights. Recent improvements in technology, imaging acquisition, and imaging processing enable to use of lower 131I doses, resulting in reduced risk of the stunning effect. In this setting, the possible negative impact of postoperative WBS on RAI therapeutic efficacy and on the success of remnant ablations may be reduced or avoided with the use of low-activity of 131I (1–3 mCi at least 72 h before the therapeutic dose) or the use of alternative isotopes such as 123I [20].
The use of single photon emission computed tomography/computed tomography (SPECT/CT) is one of the main factors that allow the reduction of 131I dose and it has also demonstrated significant clinical benefit in terms of staging, risk stratification, and follow-up of patients with DTC, influencing the choice of the therapeutic dose to use. Notably, SPECT/CT is able to improve the diagnostic accuracy of planar WBS, reducing the number of equivocal foci interpretations. In this contest, it has demonstrated the ability to detect residual or unsuspected regional metastasis in about one-third of cases and distant metastases in about 10%. When coupled with the presence of high stimulated Tg values, postoperative diagnostic SPECT/CT underlined the presence of unsuspected nodal and distant metastasis resulting in a change in the estimated risk of recurrence and management. In addition, SPECT/CT is also able to perform three-dimensional imaging, enabling the execution of dosimetric evaluation in selected cases [21, 22].
8.3.1.2 Posttherapy Setting
Planar WBS, usually obtained 3–10 days after 131I therapy, is considered as an essential diagnostic tool in order to complete the staging, the risk stratification, the assessment of residual disease, the therapeutic planning, and the detection of recurrence in patients affected by DTC. Recent ATA guidelines [1] suggest that a posttherapy WBS (with or without the use of SPECT/CT) is recommended after 131I treatment to complete the disease staging and document radioiodine avidity of any structural disease. The same guidelines suggest that WBS integrated by SPECT/CT after therapeutic radioiodine, and pre-ablation stimulated Tg measurement, remain the most accurate tools for the restaging of postoperative DTC and are fundamental elements of the risk stratification system. In practice, WBS allows the detection of possible unknown loco-regional and distant metastases, resulting in changing risk stratification that is able to customize additional therapy and subsequent follow-up. Notably, the presence of a negative diagnostic WBS is pivotal to underline the absence of persistent disease, to fully reassure the patients, and therefore to monitor them periodically simply by clinical examination and basal Tg measurement [11, 18].
The evaluation of the biodistribution of 131I can be usually well defined with planar WBS imaging, however, this modality lacks anatomical information and has poor image resolution. As previously mentioned, the use of SPECT/CT can improve the diagnostic accuracy of WBS, resulting in accurate anatomic localization, reduction of the number of indeterminate findings, and correct assessment of size, localization, and avidity of metastatic lesions. These data are therefore able to guide further management decisions and, in particular, it has been reported that SPECT/CT was able to change patients’ management in 25% of the cases, in particular for what concerns the frequency and intensity of follow-up studies. Interestingly, the incremental value of SPECT/CT in influencing the therapeutic approach appears to be greater in studies where its role has been reserved in situations with diagnostic uncertainty at posttherapy WBS or in advanced diseases with inconclusive WBS findings. In this field, the combination of posttherapy planar WBS and SPECT/CT of the neck and thorax had a sensitivity of 78% and a specificity of 100% for the assessment of DTC and furthermore the use of SPECT/CT can reduce the need for additional cross-sectional imaging. Interestingly, it was reported that a positive finding on SPECT/CT was more predictive of treatment failure than a positive finding on WBS [18].
The assessment of nodal localization of disease is mandatory for the correct staging of DTC. In this setting, WBS is mandatory for lymph node assessment and SPECT/CT has been demonstrated to be more accurate than WBS when evaluating nodal metastases, resulting again in a change in risk stratification [21, 22].
SPECT/CT has, however, some limitations, such as the presence of additional radiation exposure to the patient derived from the CT component (low-dose CT usually delivers to the patients a dose of 2–5 mSv.), the need for additional imaging time and the increased costs.
8.3.1.3 Response Assessment, Disease Monitoring, and Long-Term Follow-Up
The initial risk assessment of DTC patients is continuously modified and refined by the evaluation of response to treatment. Tg measurements, neck US and WBS (with both 123I or 131I) are used as primary tools for the follow-up of such subjects, having the potential to impact prognosis and risk stratification. However, at present no shared consensus on the routine use of 131I WBS during the follow-up of DTC patients is available. In this setting, the aforementioned ATA guidelines suggest performing WBS in patients with high- or intermediate-risk of persistent disease, while it should not be routinely performed for the follow-up of other patients [1]. The use of WBS in low-risk patients should be strongly discouraged, especially in the presence of negative Tg values and neck US [11, 23].
A diagnostic WBS, performed 6–12 months after RAI therapy, can be useful for the follow-up of patients with high- or intermediate-risk and should be performed with 123I or 131I. There is a strong agreement on the relevant role of WBS in patients with positive TgAb that reduce the evaluation of Tg, even in presence of negative US imaging, with extra-thyroid uptake at postoperative WBS, with large thyroid remnants precluding the execution of postoperative WBS and in selected cases based on individual risk profile [11, 24].
Moreover, WBS has been reported as a useful tool to select patients with a high risk of persistence/recurrence of disease, to assess patients with metastases and for the clear evaluation of patients with rising markers (Tg or TgAb). Notably, 131I WBS performed after primary treatment of DTC has been reported as the only imaging modality associated with improved disease-specific survival. Furthermore, the use of rhTSH has shown to give reduce patients’ discomfort and significantly improve the diagnostic performances of planar WBS [25,26,27].
As previously mentioned, also in the follow-up setting, SPECT/CT after WBS is usually recommended due to the incremental diagnostic value over planar imaging. SPECT/CT is associated with an increased number of patients with a diagnosis of metastatic lymph nodes and a decreased frequency of equivocal findings. In this setting, by providing precise localization and characterization of the residual thyroid tissue and 131I-avid metastases, it strongly impacts the treatment approach for DTC patients, leading to a decrease in unnecessary 131I treatment in 20% of patients without disease [22].
WBS is also crucial to define the presence of iodine refractory disease, the condition when DTC has lost fully or partially the ability to concentrate 131I despite the presence of disease.
8.3.2 PET/CT Imaging
8.3.2.1 Postoperative Setting
The effective role of 18F-fluorodeoxyglucose [18F]-FDG) positron-emission tomography/CT (PET/CT) for the assessment of DTC remnants at the time of postoperative evaluation is yet unclear, with low evidences available in literature.
[18F]-FDG PET/CT might be useful, especially in high-risk patients, such as those with aggressive variants and poorly differentiated carcinoma, or in case of positive findings on other imaging modalities. In this setting, it has been reported that [18F]-FDG PET/CT can be very effective to search for distant metastases. Particularly, it can be sensitive for the evaluation of neck and mediastinal involvement and it may also be considered a prognostic tool in patients with metastatic disease, in order to identify subjects at higher risk for rapid disease progression and poor survival [1, 28].
8.3.2.2 Suspicious Relapse
In general, high-quality evidences about the role of [18F]-FDG PET/CT in studying DTC suspected relapse have been present. In particular, a pooled sensitivity ranging from 80% to 88% and a pooled specificity ranging from 84% to 90% were reported in the literature. In this setting, several factors may influence the sensitivity of [18F]-FDG PET/CT, such as tumor dedifferentiation, larger tumor burden and, with less evidences, TSH stimulation [29, 30].
The diagnostic performances of [18F]-FDG PET/CT may improve after TSH stimulation, however, it has been reported that sensitivity can be only marginally improved with such intervention and more studies are required to clearly identify the clinical benefit of this stimulation, in particular in patients with low Tg values. In this field, it has been described that [18F]-FDG PET/CT after TSH stimulation is able to detect more lesions than imaging performed on thyroid hormone treatment. However, the sensitivity to detect patients with at least one pathological site was not different in these two conditions and again the clinical benefit related to the identification of focal uptake at PET/CT scan remains to be proven. As a consequence, there are still no clear evidences that TSH stimulation improves the prognostic values of [18F]-FDG PET/CT [28, 31].
The last ATA guidelines recommend the use of [18F]-FDG PET/CT in order to assess the possible presence of DTC relapse in patients with increasing Tg levels, negative US, and negative WBS imaging. Also in cases of patients with negative WBS and US but increasing levels of TgAb [18F]-FDG PET/CT has been proposed [1].
Interestingly, it has been reported that [18F]-FDG PET/CT is more sensitive than neck US in the detection of relapse in the retropharyngeal or retro-clavicular regions [28].
The best Tg cutoff able to define whether [18F]-FDG PET/CT has been indicated to be performed is still under debate. The aforementioned ATA guidelines suggest that PET/CT should be performed when Tg levels are higher than 10 ng/mL and concomitant negative 131I imaging. But, it has been reported that true-positive findings are present in 10–20% of DTC patients with Tg levels lower than this threshold [32]. Recently, it has emerged the potential role of Tg kinetics (expressed as Tg doubling time and/or Tg velocity) to independently predict a positive [18F]-FDG PET/CT scan in patients with biochemical relapse of disease. In particular, the accuracy of [18F]-FDG PET/CT imaging significantly improves when the Tg doubling time is less than 1 year, irrespective of the absolute value of Tg [9, 33]. Further studies are needed to confirm or controvert these results.
Another potential field of application of [18F]-FDG PET/CT is in the presence of high TgAb levels, where Tg values cannot be reliably assessed; in presence of elevated TgAb values and a negative 131I WBS a pooled sensitivity of 84% and a pooled specificity of 78% of [18F]-FDG PET/CT was reported [34].
Moreover, it was also described that a second empiric session of 131I therapy and subsequent WBS were not diagnostically or therapeutically useful in patients with negative [18F]-FDG PET/CT scan but elevated Tg levels. This evidence suggests that the correct use of PET/CT imaging could be able to reduce unnecessary administrations of high 131I activities. In this scenario, the correct integration of 131I imaging and [18F]-FDG PET/CT may optimize additional administrations of high 131I activities and inform alternative strategies such as surgery or external beam radiation. Furthermore, the presence of 18F-FDG uptake on PET/CT imaging in metastatic patients is a major negative predictive factor for response to 131I treatment and an independent prognostic factor for survival [18].
The presence of positive findings on [18F]-FDG PET/CT may change the clinical management of DTC patients in 20–40% of the cases. In this setting, in the presence of 18F-FDG positive lesions, alternative procedures instead of 131I therapy may be considered [28].
8.3.2.3 Prognostic Role of PET Imaging
In general, more aggressive and high-grade DTC are characterized by higher [18F]-FDG uptake compared to low-grade and less aggressive tumors. The glycolytic rate of the most active lesion (sometimes expressed as maximum standardized uptake value, SUVmax) and the number of FDG-avid lesions are strongly associated with survival, even better than RAI uptake, histology, or immunohistochemical pattern [35,36,37].
On the other hand, a negative [18F]-FDG PET/CT scan is able to predict a favorable prognosis because it is associated with the absence of active DTC and the disappearance of TgAb over time. On the opposite, the presence of residual FDG-avid lesions is associated with more aggressive disease and persistently increased levels of TgAb [38, 39].
Recent studies showed a prognostic role of metabolic tumor volume (MTV) and total lesion glycolysis (TLG) in predicting overall survival and progression-free survival [40].
8.3.2.4 Assessment of Iodine Refractory Disease
In general, [18F]-FDG PET/CT helps address disease aggressiveness, detect distant metastases, and risk-stratify patients with iodine-refractory DTC and anaplastic cancers. It is known that while more differentiated thyroid cells tend to retain iodine and have lower glucose metabolism, undifferentiated cells tend to present with a lower ability to retain iodine but higher glucose metabolism. In this context, [18F]-FDG PET/CT is able to identify nodal localization or distant metastases of DTC patients that are not or only partially detected with 131I WBS and as a consequence, the need to perform further 131I therapy should be reconsidered and avoided in these situations, given its low probability to alter the outcome of such patients. [18F]-FDG PET/CT may detect new iodine-negative localization of disease in patients with advanced DTC with stable 131I WBS and rising Tg levels. In this scenario, more appropriate treatments other than RAI should be considered [28, 41, 42].
About the role of [18F]-FDG PET/CT for the prediction of systemic therapy in iodine refractory DTC, a significant association between average percent change in SUV and the response evaluation criteria in solid tumors (RECIST) response criteria were reported. Early [18F]-FDG PET/CT in patients on tyrosine kinase inhibitors (TKI) treatment could be an early indicator of response and could identify patients that are unlikely to respond to therapy. It has also been reported that [18F]-FDG imaging assessment at the baseline is able to predict radiological response but not clinical outcomes [43].
8.3.2.5 124I
124I is an iodine isotope able to emit positron and it is, therefore, a suitable tracer to assess iodine metabolism with PET/CT imaging. The role of 124I in the assessment of DTC is based on the assumption that this tracer is able to overcome some intrinsic limits of both 131I and 123I such as the reduced spatial resolution of SPECT/CT, poor image quality, and dose exposure. Theoretically, 124I PET/CT could allow the selection of patients with rising Tg levels but negative neck US and [18F]-FDG PET/CT imaging, that will benefit from subsequent radioiodine in order to avoid inappropriate therapies. A high level of agreement between 124I PET and 131I WBS scan was reported in the literature, suggesting that 124I PET/CT could be used for individualized treatment planning and staging in DTC patients. In this setting, data in the literature are however still controversial [1, 36].
It was reported that 124I PET/CT is able to predict the response to high dose 131I and would be a good diagnostic tool to support clinical decisions with high diagnostic accuracy. In particular, this imaging modality was able to reveal previously unknown lymph nodes and distant metastases [30, 44].
A negative 124I PET/CT scan could suggest avoiding 131I and performing further imaging to detect the localization of the non-iodine-avid disease. In contrast, it has also been reported that 124I imaging had low sensitivity in detecting non-iodine avid metastases that were subsequently identified by post-therapeutic 131I WBS. In this field, the high false-negative rate of rhTSH-stimulated 124I PET/CT could preclude its use as a scouting procedure to prevent futile 131I therapy. It is worth underlining that low diagnostic sensitivity of 124I imaging was reported in the conditions of rhTSH stimulation, while in studies with high sensitivity the patients were on thyroid hormone withdrawal [45,46,47].
124I PET/CT could also be useful for the assessment of post-operative DTC with high sensitivity, providing the detection of unknown metastases of disease and guiding subsequent 131I [48].
8.3.2.6 Other PET Tracers
Somatostatin Analogs
DTC expresses somatostatin receptors 2, 3, and 5; thus, as a consequence PET/CT with labeled somatostatin analogs has been proposed as a diagnostic tool in such disease. In this setting, the comparison between 68Ga-DOTATOC or 68Ga-DOTANOC and [18F]-FDG PET/CT revealed similar sensitivity in a patient-based analysis. However, lesion-based analyses in multiple studies revealed a higher sensitivity for [18F]-FDG. Interestingly, it was reported that the accuracy of both modalities was not related to serum Tg levels, without significant differences in terms of accuracy between patients with low and high Tg [49,50,51].
When using 68Ga-DOTANOC in patients with both negative 131I WBS and [18F]-FDG PET/CT, it was reported that the presence of positive findings was significantly higher in poorly differentiated and oxyphilic carcinomas than in papillary or follicular tumors. In summary, these insights suggest that the diagnostic role of radiolabeled somatostatin analogs PET/CT in DTC is characterized by conflicting results and therefore it should not be considered in clinical practice [1].
More recently peptide receptor radionuclide therapy has been proposed as an alternative for the treatment of DTC and therefore 68Ga-DOTATOC PET/CT was suggested as a guide to select patients for treatment. These insights are however in an embryonal phase and need therefore to be confirmed by other data [52].
Choline
It has been reported that PET/CT with radiolabeled choline may be useful in patients with metastatic DTC and negative [18F]-FDG PET/CT. This imaging modality can also be considered complementary to [18F]-FDG PET/CT, thereby increasing information about the status of the disease. Data in literature are, however, still in the early phases, and therefore this imaging modality should not be considered in clinical practice [53].
PSMA
There are only a few evidences in the literature on the role of labeled prostate-specific membrane antigen (PSMA) PET/CT for the assessment of DTC, underlying its potential usefulness for the assessment of metastatic radioiodine negative subjects. However, these insights need to be verified by further evidences [54].
NIS Imaging
18F-tetrafluoroborate (18F-FTB) and 18F-fluorosulfate (18F-FSO3) are iodine analogs that are recently emerging as potential candidates to assess DTC given their ability to visualize sodium/iodide symporter (NIS) in preclinical and preliminary clinical applications. Even if in small samples, they revealed high sensitivity in some cases but their applications in DTC need, however, to be verified with further studies [55, 56].
8.4 Future Perspectives: Artificial Intelligence and Radiomics
In the last years, new knowledge and better technologies expanded the applications of artificial intelligence (AI) and extended it to medical problems: management of the malignant tumor represents one of the most important and promising fields of application of AI, particularly in the diagnosis of malignancy, in the prognostication and in the management [57,58,59].
Moreover, a rising interest in quantitative image analysis using techniques such as texture analysis has been developed. This has led to the introduction of radiomics, which has come to define large radiological image-derived feature sets, primed for exploration and analysis with data mining or machine learning approaches. Radiomics is a method that extracts a large number of features from radiographic medical images using data-characterization algorithms. The goal of both radiomics and texture analysis is to go beyond the size or human eye-based semantic descriptors, to enable the non-invasive extraction of quantitative radiological data to correlate them with clinical outcomes or pathological characteristics [59, 60].
But, there is great uncertainty about the actual clinical value of information derived from radiomic features as questions are raised on their reproducibility and interpretability in biological terms, beyond ethical concerns.
Regarding the postoperative field, a little number of studies that investigate the potential role of IA and/or radiomics are available.
The artificial neural network showed to have optimal accuracy in identifying factors that predict the presence of central nodal metastases [61], even better than traditional logistic regression analysis [62]. In a recent study [63], the application of machine learning algorithms showed to be superior to the classical US and clinical features in predicting central nodal metastases and after multivariate analysis and feature selection, the combination of young age, male gender, low serum thyroid peroxidase antibody, and US characteristics (like the presence suspected lymph nodes, size>1.1 cm and microcalcifications) were the most contributing predictors.
Furthermore, it has been reported that machine learning techniques could predict the presence of lymph node metastases with high sensitivity and specificity using only visual histopathological data derived from the primary tumor [64, 65].
The possible presence of skip metastases (a nodal disease in laterocervical levels without the involvement of the VI level), was also assessed reporting a model with high accuracy, specificity, and NPV. In this setting, the possibilities to better predict the presence of undetectable central node metastases and exclusion of skip metastases may help to choose when prophylactic lymphadenectomy of the VI level could be necessary [66].
Another promising application seems to be the prediction of disease recurrence: recent studies currently reported an accuracy of about 70–90% in the prediction of disease recurrence with an optimal NPV. According to the main international guidelines, thyroglobulin level after thyroidectomy, tumor size, and presence of contralateral nodal disease apparently are the most significant parameters derived by Machine Learning systems. More studies are needed to establish the best family of parameters or the combination of them for predicting the risk of recurrence. The possibility to predict DCT metastases and local recurrence could help in defining therapeutic and follow-up strategies.
It is worth underlining that despite a large number of works in the literature about the characterization of thyroid nodules and preoperative management of thyroid malignancy, no studies about the possible clinical usefulness of the analysis of radiomics features, a specific field of AI based on the extraction radiological and nuclear medicine imaging, in the postoperative management of DTC are actually available.
The application of promising machine learning techniques for the assessment of DTC has just started and more studies are necessary in order to create better models able to guide clinical practice.
References
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. https://doi.org/10.1089/thy.2015.0020.
Petranović Ovčariček P, Kreissl MC, Campenni A, de Keizer B, Tuncel M, Vrachimis A, Deandreis D, Giovanella L. SNMMI/EANM practice guideline vs. ETA consensus statement: differences and similarities in approaching differentiated thyroid cancer management-the EANM perspective. Eur J Nucl Med Mol Imaging. 2022; https://doi.org/10.1007/s00259-022-05935-1.
Knappe L, Giovanella L. Life after thyroid cancer: the role of thyroglobulin and thyroglobulin antibodies for postoperative follow-up. Expert Rev Endocrinol Metab. 2021;16(6):273–9. https://doi.org/10.1080/17446651.2021.1993060.
Giovanella L. Circulating biomarkers for the detection of tumor recurrence in the postsurgical follow-up of differentiated thyroid carcinoma. Curr Opin Oncol. 2020;32(1):7–12. https://doi.org/10.1097/CCO.0000000000000588.
Algeciras-Schimnich A. Thyroglobulin measurement in the management of patients with differentiated thyroid cancer. Crit Rev Clin Lab Sci. 2018;55(3):205–18. https://doi.org/10.1080/10408363.2018.1450830.
Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, Rimmele H, Seregni E, Smit JW, Theimer C, Giovanella L. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23(10):1211–25. https://doi.org/10.1089/thy.2012.0606.
Giovanella L, Clark PM, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, Leenhardt L, Luster M, Schalin-Jäntti C, Schott M, Seregni E, Rimmele H, Smit J, Verburg FA. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur J Endocrinol. 2014;171(2):R33–46. https://doi.org/10.1530/EJE-14-0148.
Giovanella L, Garo ML, Albano D, Görges R, Ceriani L. The role of thyroglobulin doubling time in differentiated thyroid cancer: a meta-analysis. Endocr Connect. 2022;11(4):e210648. https://doi.org/10.1530/EC-21-0648.
Albano D, Tulchinsky M, Dondi F, Mazzoletti A, Lombardi D, Bertagna F, Giubbini R. Thyroglobulin doubling time offers a better threshold than thyroglobulin level for selecting optimal candidates to undergo localizing [18F]FDG PET/CT in non-iodine avid differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2021;48(2):461–8. https://doi.org/10.1007/s00259-020-04992-8.
Albano D, Tulchinsky M, Dondi F, Mazzoletti A, Bertagna F, Giubbini R. The role of Tg kinetics in predicting 2-[18F]-FDG PET/CT results and overall survival in patients affected by differentiated thyroid carcinoma with detectable Tg and negative 131I-scan. Endocrine. 2021;74(2):332–9. https://doi.org/10.1007/s12020-021-02755-5.
Campennì A, Barbaro D, Guzzo M, Capoccetti F, Giovanella L. Personalized management of differentiated thyroid cancer in real life - practical guidance from a multidisciplinary panel of experts. Endocrine. 2020;70(2):280–91. https://doi.org/10.1007/s12020-020-02418-x.
Giovanella L, Avram AM, Clerc J, Hindié E, Taïeb D, Verburg FA. Postoperative serum thyroglobulin and neck ultrasound to drive decisions about iodine-131 therapy in patients with differentiated thyroid carcinoma: an evidence-based strategy? Eur J Nucl Med Mol Imaging. 2018;45(12):2155–8. https://doi.org/10.1007/s00259-018-4110-4.
Spencer C, LoPresti J, Fatemi S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):394–404. https://doi.org/10.1097/MED.0000000000000092.
Wang W, Chang J, Jia B, Liu J. The blood biomarkers of thyroid cancer. Cancer Manag Res. 2020;6(12):5431–8. https://doi.org/10.2147/CMAR.S261170. Erratum in: Cancer Manag Res. 2022 Jan 05;14:49-50.
Giovanella L, Ceriani L, Garo ML. Is thyroglobulin a reliable biomarker of differentiated thyroid cancer in patients treated by lobectomy? A systematic review and meta-analysis. Clin Chem Lab Med. 2022;60(7):1091–100. https://doi.org/10.1515/cclm-2022-0154.
Feldt-Rasmussen U, Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, Rimmele H, Seregni E, Smit JW, Theimer C, Giovanella L. Thyroglobulin autoantibodies as surrogate biomarkers in the management of patients with differentiated thyroid carcinoma. Curr Med Chem. 2014;21(32):3687–92. https://doi.org/10.2174/0929867321666140826120844.
Giovanella L, Feldt-Rasmussen U, Verburg FA, Grebe SK, Plebani M, Clark PM. Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clin Chem Lab Med. 2015;53(9):1301–14. https://doi.org/10.1515/cclm-2014-0813.
Giovanella L, Deandreis D, Vrachimis A, Campenni A, Ovcaricek PP. Molecular imaging and theragnostics of thyroid cancers. Cancers (Basel). 2022;14(5):1272. https://doi.org/10.3390/cancers14051272.
Avram AM, Esfandiari NH, Wong KK. Preablation 131-I scans with SPECT/CT contribute to thyroid cancer risk stratification and 131-I therapy planning. J Clin Endocrinol Metab. 2015;100(5):1895–902. https://doi.org/10.1210/jc.2014-4043.
Chen MK, Yasrebi M, Samii J, Staib LH, Doddamane I, Cheng DW. The utility of I-123 pre-therapy scan in I-131 radioiodine therapy for thyroid cancer. Thyroid. 2012;22:304–9.
Avram AM. Radioiodine scintigraphy with SPECT/CT: an important diagnostic tool for thyroid cancer staging and risk stratification. J Nucl Med. 2012;53:754–64.
Lee SW. SPECT/CT in the treatment of differentiated thyroid cancer. Nucl Med Mol Imaging. 2017;51(4):297–303. https://doi.org/10.1007/s13139-017-0473-x.
Lamartina L, Deandreis D, Durante C, Filetti S. ENDOCRINE TUMOURS: imaging in the follow-up of differentiated thyroid cancer: current evidence and future perspectives for a risk-adapted approach. Eur J Endocrinol. 2016;175(5):R185–202. https://doi.org/10.1530/EJE-16-0088.
Verburg FA, Mäder U, Reiners C, Hänscheid H. Long-term survival in differentiated thyroid cancer is worse after low-activity initial post-surgical 131I therapy in both high- and low-risk patients. J Clin Endocrinol Metab. 2014;99(12):4487–96. https://doi.org/10.1210/jc.2014-1631.
Alzahrani AS, AlShaikh O, Tuli M, Al-Sugair A, Alamawi R, Al-Rasheed MM. Diagnostic value of recombinant human thyrotropin-stimulated 123I whole-body scintigraphy in the follow-up of patients with differentiated thyroid cancer. Clin Nucl Med. 2012;37(3):229–34. https://doi.org/10.1097/RLU.0b013e31823ea463.
Barwick T, Murray I, Megadmi H, Drake WM, Plowman PN, Akker SA, Chew SL, Grossman AB, Avril N. Single photon emission computed tomography (SPECT)/computed tomography using Iodine-123 in patients with differentiated thyroid cancer: additional value over whole body planar imaging and SPECT. Eur J Endocrinol. 2010;162(6):1131–9. https://doi.org/10.1530/EJE-09-1023.
Spanu A, Solinas ME, Chessa F, Sanna D, Nuvoli S, Madeddu G. 131I SPECT/CT in the follow-up of differentiated thyroid carcinoma: incremental value versus planar imaging. J Nucl Med. 2009;50(2):184–90. https://doi.org/10.2967/jnumed.108.056572.
Piccardo A, Trimboli P, Foppiani L, Treglia G, Ferrarazzo G, Massollo M, Bottoni G, Giovanella L. PET/CT in thyroid nodule and differentiated thyroid cancer patients. The evidence-based state of the art. Rev Endocr Metab Disord. 2019;20(1):47–64. https://doi.org/10.1007/s11154-019-09491-2.
Schütz F, Lautenschläger C, Lorenz K, Haerting J. Positron emission tomography (PET) and PET/CT in thyroid cancer: a systematic review and meta-analysis. Eur Thyroid J. 2018;7:13–20.
Zampella E, Klain M, Pace L, Cuocolo A. PET/CT in the management of differentiated thyroid cancer. Diagn Interv Imaging. 2021;102(9):515–23. https://doi.org/10.1016/j.diii.2021.04.004.
Ma C, Xie J, Lou Y, Gao Y, Zuo S, Wang X. The role of TSH for 18F-FDG-PET in the diagnosis of recurrence and metastases of differentiated thyroid carcinoma with elevated Tg and negative scan: a meta-analysis. Eur J Endocrinol. 2010;163:177–83.
Bertagna F, Albano D, Bosio G, Piccardo A, Dib B, Giubbini R. 18F-FDG-PET/CT in patients affected by differentiated thyroid carcinoma with positive thyroglobulin level and negative 131I whole body scan. It’s value confirmed by a bicentric experience. Curr Radiopharm. 2016;9(3):228–34. https://doi.org/10.2174/1874471009666160523145005.
Giovanella L, Trimboli P, Verburg FA, Treglia G, Piccardo A, Foppiani L, et al. Tg levels and Tg doubling time independently predict a positive (18) F-FDG PET/CT scan in patients with biochemical recurrence of differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:874–80.
Kim SJ, Lee SW, Pak K, Shim SR. Diagnostic performance of PET in thyroid cancer with elevated anti-Tg Ab. Endocr Relat Cancer. 2018;25:643–52.
Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505.
Treglia G, Giovanella L. Prognostic role of FDG-PET/CT in differentiated thyroid carcinoma: where are we now? J Med Imaging Radiat Oncol. 2015;59:278–80.
Deandreis D, Al Ghuzlan A, Leboulleux S, Lacroix L, Garsi JP, Talbot M, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr Relat Cancer. 2011;13(18):159–69.
Marcus C, Antoniou A, Rahmim A, Ladenson P, Subramaniam RM. Fluorodeoxyglucose positron emission tomography/computerized tomography in differentiated thyroid cancer management: importance of clinical justification and value in predicting survival. J Med Imaging Radiat Oncol. 2015;59:281–8.
Bogsrud TV, Hay ID, Karantanis D, Nathan MA, Mullan BP, Wiseman GA, et al. Prognostic value of 18 F-fluorodeoxyglucose-positron emission tomography in patients with differentiated thyroid carcinoma and circulating antiTg auto-antibodies. Nucl Med Commun. 2011;32:245–51.
Albano D, Dondi F, Mazzoletti A, Bellini P, Rodella C, Bertagna F. Prognostic role of 2-[18F]FDG PET/CT metabolic volume parameters in patients affected by differentiated thyroid carcinoma with high thyroglobulin level, negative 131I WBS and positive 2-[18F]-FDG PET/CT. Diagnostics (Basel). 2021;11(12):2189. https://doi.org/10.3390/diagnostics11122189.
Iwano S, Kato K, Ito S, Tsuchiya K, Naganawa S. FDG PET performed concurrently with initial I-131 ablation for differentiated thyroid cancer. Ann Nucl Med. 2012;26:207–13.
Piccardo A, Foppiani L, Morbelli S, Bianchi P, Barbera F, Biscaldi E, Altrinetti V, Villavecchia G, Cabria M. Could [18]F-fluorodeoxyglucose PET/CT change the therapeutic management of stage IV thyroid cancer with positive (131)I whole body scan? Q J Nucl Med Mol Imaging. 2011;55(1):57–65.
Marotta V, Ramundo V, Camera L, Del Prete M, Fonti R, Esposito R, Palmieri G, Salvatore M, Vitale M, Colao A, Faggiano A. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin Endocrinol. 2013;78(5):760–7. https://doi.org/10.1111/cen.12057.
Capoccetti F, Criscuoli B, Rossi G, Ferretti F, Manni C, Brianzoni E. The effectiveness of 124I PET/CT in patients with differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2009;53:536–45.
Freudenberg LS, Jentzen W, Müller SP, Bockisch A. Disseminated iodine-avid lung metastases in differentiated thyroid cancer: a challenge to 124I PET. Eur J Nucl Med Mol Imaging. 2008;35:502–8.
Kist JW, de Keizer B, van der Vlies M, Brouwers AH, Huysmans DA, van der Zant FM, THYROPET study group; other members of the THYROPET study group are John M.H. de Klerk, et al. 124I PET/CT to predict the outcome of blind 131I treatment in patients with biochemical recurrence of differentiated thyroid cancer: results of a multicenter diagnostic cohort study (THYROPET). J Nucl Med. 2016;57:701–7.
Khorjekar GR, Van Nostrand D, Garcia C, O'Neil J, Moreau S, Atkins FB, et al. Do negative 124I pretherapy positron emission tomography scans in patients with elevated serum Tg levels predict negative 131I posttherapy scans? Thyroid. 2014;24:1394–9.
Santhanam P, Taieb D, Solnes L, Marashdeh W, Ladenson PW. Utility of I-124 PET/CT in identifying radioiodine avid lesions in differentiated thyroid cancer: a systematic review and meta-analysis. Clin Endocrinol. 2017;86:645–51.
Middendorp M, Selkinski I, Happel C, Kranert WT, Grünwald F. Comparison of positron emission tomography with [(18)F] FDG and [(68)Ga] DOTATOC in recurrent differentiated thyroid cancer: preliminary data. Q J Nucl Med Mol Imaging. 2010;54:76–83.
Traub-Weidinger T, Putzer D, von Guggenberg E, Dobrozemsky G, Nilica B, Kendler D, et al. Multiparametric PET imaging in thyroid malignancy characterizing tumour heterogeneity: somatostatin receptors and glucose metabolism. Eur J Nucl Med Mol Imaging. 2015;42:1995–2001.
Kundu P, Lata S, Sharma P, Singh H, Malhotra A, Bal C. Prospective evaluation of (68)Ga-DOTANOC PET-CT in differentiated thyroid cancer patients with raised thyroglobulin and negative (131)I-whole body scan: comparison with (18)F-FDG PETCT. Eur J Nucl Med Mol Imaging. 2014;41:1354–62.
Versari A, Sollini M, Frasoldati A, Fraternali A, Filice A, Froio A, Asti M, Fioroni F, Cremonini N, Putzer D, et al. Differentiated thyroid cancer: a new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid. 2014;24:715–26.
Piccardo A, Trimboli P, Puntoni M, Foppiani L, Treglia G, Naseri M, Bottoni GL, Massollo M, Sola S, Ferrarazzo G, Bruzzone M, Catrambone U, Arlandini A, Paone G, Ceriani L, Cabria M, Giovanella L. Role of 18F-choline positron emission tomography/computed tomography to detect structural relapse in high-risk differentiated thyroid cancer patients. Thyroid. 2019;29(4):549–56. https://doi.org/10.1089/thy.2018.0552.
Verburg FA, Krohn T, Heinzel A, Mottaghy FM, Behrendt FF. First evidence of PSMA expression in differentiated thyroid cancer using 68GaPSMA-HBED-CC PET/CT. Eur J Nucl Med Mol Imaging. 2015;42:1622–3.
Jiang H, DeGrado TR. [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics. 2018;8(14):3918–31. https://doi.org/10.7150/thno.24997.
Jiang H, Schmit NR, Koenen AR, Bansal A, Pandey MK, Glynn RB, Kemp BJ, Delaney KL, Dispenzieri A, Bakkum-Gamez JN, Peng KW, Russell SJ, Gunderson TM, Lowe VJ, DeGrado TR. Safety, pharmacokinetics, metabolism and radiation dosimetry of 18F-tetrafluoroborate (18F-TFB) in healthy human subjects. EJNMMI Res. 2017;7(1):90. https://doi.org/10.1186/s13550-017-0337-5.
Liew C. The future of radiology augmented with artificial intelligence: a strategy for success. Eur J Radiol. 2018;102:152–6. https://doi.org/10.1016/j.ejrad.2018.03.019.
Nakata N. Recent technical development of artificial intelligence for diagnostic medical imaging. Jpn J Radiol. 2019;37(2):103–8. https://doi.org/10.1007/s11604-018-0804-6.
Rizzo S, Botta F, Raimondi S, Origgi D, Fanciullo C, Morganti AG, Bellomi M. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp. 2018;2(1):36. https://doi.org/10.1186/s41747-018-0068-z.
Corrias G, Micheletti G, Barberini L, Suri JS, Saba L. Texture analysis imaging “what a clinical radiologist needs to know”. Eur J Radiol. 2022;146:110055. https://doi.org/10.1016/j.ejrad.2021.110055.
Esce AR, Redemann JP, Sanchez AC, Olson GT, Hanson JA, Agarwal S, Boyd NH, Martin DR. Predicting nodal metastases in papillary thyroid carcinoma using artificial intelligence. Am J Surg. 2021;222(5):952–8. https://doi.org/10.1016/j.amjsurg.2021.05.002.
Ozden S, Er S, Saylam B, Yildiz BD, Senol K, Tez M. A comparison of logistic regression and artificial neural networks in predicting central lymph node metastases in papillary thyroid microcarcinoma. Ann Ital Chir. 2018;89:193–8.
Wu Y, Rao K, Liu J, Han C, Gong L, Chong Y, Liu Z, Xu X. Machine learning algorithms for the prediction of central lymph node metastasis in patients with papillary thyroid cancer. Front Endocrinol (Lausanne). 2020;21(11):577537. https://doi.org/10.3389/fendo.2020.577537.
Kim SY, Kim YI, Kim HJ, Chang H, Kim SM, Lee YS, Kwon SS, Shin H, Chang HS, Park CS. New approach of prediction of recurrence in thyroid cancer patients using machine learning. Medicine (Baltimore). 2021;100(42):e27493. https://doi.org/10.1097/MD.0000000000027493.
Park YM, Lee BJ. Machine learning-based prediction model using clinico-pathologic factors for papillary thyroid carcinoma recurrence. Sci Rep. 2021;11(1):4948. https://doi.org/10.1038/s41598-021-84504-2.
Zhu S, Wang Q, Zheng D, Zhu L, Zhou Z, Xu S, Shi B, Jin C, Zheng G, Cai Y. A novel and effective model to predict skip metastasis in papillary thyroid carcinoma based on a support vector machine. Front Endocrinol (Lausanne). 2022;5(13):916121. https://doi.org/10.3389/fendo.2022.916121.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Albano, D., Dondi, F., Bellini, P., Bertagna, F. (2023). Biomarkers and Molecular Imaging in Postoperative DTC Management. In: Giovanella, L. (eds) Integrated Diagnostics and Theranostics of Thyroid Diseases. Springer, Cham. https://doi.org/10.1007/978-3-031-35213-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-35213-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35212-6
Online ISBN: 978-3-031-35213-3
eBook Packages: MedicineMedicine (R0)