Abstract
Persistent organic pollutants (POPs) were identified in humans who have not been dealing with these chemicals intentionally—from organochlorine pesticides towards industrial chemicals, brominated and fluorine containing POPs. This chapter provides a brief overview of major developments in POPs monitoring in human milk and depicts a gradual broadening of the knowledge underpinned by advances in the instrumentation for chemical analysis as well as expansion of range of analytes that warranted attention. The chapter also shows how, in the course of the past 70 years, human milk monitoring has become an efficient and cost-effective non-invasive biomonitoring tool to evaluate the internal human exposure to POPs and the resulting body burden.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Human milk survey
- Organochlorine pesticide
- Polychlorinated biphenyl
- Polychlorinated dibenzo-p-dioxin
- Polychlorinated dibenzofuran
- Brominated flame retardant
- Hexabromocyclododecane
- Chlorinated paraffin
- PFAS
- Global distribution
- Influence on levels
- Temporal trend
- Lessons learned
1 Introduction
Over the past 70 years, human milk has become an efficient and cost-effective non-invasive biomonitoring tool to evaluate the internal human exposure with persistent organic pollutants (POPs). Since the first finding of DDT in human milk in 1951, the constant improvement of analytical instrumentation, in particular in terms of separation power and sensitivity, enabled the determination of an increasing number of lipophilic and persistent chemicals in human fat tissues and human milk, and thus broadened the knowledge on human body burden with these compounds. Comprehensive monitoring programmes did not only investigate the global distribution and the parameters that have major impacts on the extent of body burden but also examined temporal trends in order to monitor whether the numerous national and international measures taken to reduce the human body burden with POPs show positive effects. This chapter gives an overview on the gradually increasing number of POPs that are analysed in human milk from a historical perspective. Special focus is put on those human milk surveys that have been milestones in initiating global efforts to investigate the spatial contamination with the respective POPs. Moreover, it shortly summarizes important parameters that have a potential impact on the contaminant concentration in human milk, and the requirements for a reliable comparison of POPs concentration in human milk.
2 POPs Analysed in Human Milk in the Course of Time
This chapter provides a brief overview on the gradually increasing number of persistent organic pollutants that were determined in human milk in the course of the past 70 years. Special focus is put on those POPs that were early identified in human milk and subsequently initiated extensive surveys on their occurrence in human milk. It also describes how the advances in analytical instrumentation enabled a better insight into the evaluation of the human exposure to POPs and resulting body burden.
2.1 Organochlorine Pesticides
Organochlorine pesticides of the first generation were found in human milk in some cases already as early as in the 1950s/1960s, such as DDT, hexachlorocyclohexane (HCH), chlordane, and others. They are now listed among the initial 12 POPs of the Stockholm Convention.
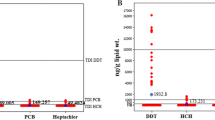
The discovery of the insecticidal properties of DDT in the late 1930s by Paul Müller was a milestone in the control of pests, such as malaria, typhus, and other insect-borne human diseases. DDT has also found widespread application on crops and livestock production. However, it soon turned out that the stability and long-lasting effects, originally considered as benefits were actually severe drawbacks as DDT was only poorly biodegradable, persistent, accumulated in the food chain and consequently was stored in the human body. Already in 1948, Howell reported a concentration of 17 ppmFootnote 1 in body fat of a person who was involved in DDT spaying for 4 years and “had consumed foods containing appreciable quantities of DDT” (Howell 1948; WHO 1979). After findings of DDT traces in crops, dairy and meat products by the US Food and Drug Administration in 1950, Laug et al. were the first to report on the occurrence of DDT in human fat and human milk samples of subjects who were not employed as pesticide workers (Laug et al. 1951). The survey was intended to set a baseline, i.e. to examine the background contamination of the general population who were not occupationally exposed. The authors analysed 75 samples of abdominal fat obtained at autopsy, biopsy, or abdominal surgery, and 32 human milk samples from women in Washington DC. In 60 out of the 75 abdominal fat samples, and 30 out of 32 human milk samples DDT could be determined. The concentrations were reported as 0–34 ppm (average: 5.3 ppm) and 0–0.77 ppm (average 0.13 ppm) for abdominal fat and human milk, respectively. In contrast to today’s convention, the DDT concentrations in human milk in those days were not expressed on a lipid but on a liquid basis. As the patients had never sprayed the insecticide, it was assumed that consumption of food besides inhalation of dust may be an important route of human exposure to DDT. In 1958, Hayes et al. confirmed the special importance of food for exposure of the general population to DDT. From their investigations they concluded that there is strong evidence that fat of animal origin in the diet is the main source from which DDT and its metabolite DDE are absorbed in those subjects who have no particular occupational exposure (Hayes et al. 1958).
The survey by Laug et al. demonstrated the special importance of the contamination of human milk, as it showed that DDT has reached the top of the food chain, i.e. the breastfed baby at a vulnerable period of life (Laug et al. 1951). Their results initiated further investigations into DDT levels in human fat and milk from the general population with no known occupational exposure. These surveys already often comprised besides p,p’-DDT, the major constituent of the technical product, also its isomer o,p’-DDT as well as the metabolite DDE (Egan et al. 1965; Quinby et al. 1965). Their concentrations found in human milk in the period between 1950 and the early 1970s were summarized in the Environmental Health Criteria No 9 “DDT and its Derivatives” published by WHO in 1979 (WHO 1979). The data illustrate the widespread distribution of DDT and its metabolites as well as the broad contamination of human milk with this organochlorine pesticide.
Initially, the analysis of DDT was performed by determination of organic chlorine (Carter 1947), colorimetric detection (Prickett et al. 1950), paper chromatography (Evans 1962), and thin-layer chromatography (Knoll and Jayaraman 1973). The introduction of gas chromatography with electron-capture detection (GC-ECD) by Goodwin et al. in 1961 facilitated a substantially improved separation of organochlorine pesticides in human samples (Goodwin et al. 1961). Besides better separation, the GC-ECD determination also enabled considerably lower limits of detection compared to the other analytical approaches applied until then. Nonetheless, higher concentrations of DDT and DDE analysed in human milk by gas chromatography on two columns of different polarity were sometimes still confirmed by paper chromatography (Egan et al. 1965).
The number of organochlorine pesticides analysed in human milk gradually increased over the years. While the first samples generally were only analysed for DDT and its major metabolite DDE, the improved analytical instrumentation, in particular offered by GC-ECD, enabled to widen the scope of analysis to further organochlorine pesticides that were commercially introduced in the 1940s/1950s because of their insecticidal properties similar to DDT, and then were also found to accumulate to some extent in the human body. This refers inter alia to the DDT analogue methoxychlor, HCH, chlordane, aldrin, dieldrin, and heptachlor. It was soon shown that aldrin and heptachlor are metabolized in mammals to dieldrin and heptachlor epoxide, respectively (WHO 1984a, 1989a). This is the reason that dieldrin and heptachlor epoxide rather than aldrin and heptachlor were mainly found in human milk samples. It was not possible to decide whether the occurrence of dieldrin in human milk was the result of the metabolization of aldrin or due to the exposure to the dieldrin pesticide itself.
In 1970, it was reported by Schwemmer et al. that oxychlordane is the most relevant mammalian metabolite of chlordane which is a complex technical mixture (Schwemmer et al. 1970). Oxychlordane is more persistent and toxic than the parent compound (WHO 1984b). Consequently, besides trans-nonachlor, a constituent of the technical mixture chlordane, oxychlordane became the primary analyte to examine the possible contamination of human milk with chlordane. The predominance of the metabolites oxychlordane and also heptachlor epoxide compared to their parent compounds in human milk was reported by Savage et al. in 1981 (Savage et al. 1981).
Comprehensive reports on the widespread regional contamination of the above-mentioned organochlorine pesticides and their metabolites determined in human milk until the 1970s are summarized in the WHO publications Environmental Health Criteria 9 (WHO 1979), 34 (WHO 1984b), 38 (WHO 1984a), and 91 (WHO 1989a).
HCH was first synthesized by Faraday in 1825 (Faraday 1825) and its insecticidal properties were discovered in the 1940s. Since the beginning of commercial production in the early 1950s, HCH became one of the most widely applied insecticides worldwide. Technical HCH mainly consists of five isomers, termed alpha-, beta-, gamma-, delta-, and epsilon-HCH (Hayes and Laws 1991). In older publications, HCH is often misleadingly named benzene hexachloride (BHC) which should not be confused with the fungicide hexachlorobenzene (HCB). Although only present in the technical product at 14–15%, the gamma-isomer was identified to be the active HCH constituent, but with relatively low persistence. In contrast, beta-HCH was found to be the isomer with the highest persistence, followed by alpha-HCH. Their fraction generally made up 7–10% and 65–70% of the technical product, respectively. Alpha- and beta-HCH both have no appreciable insecticidal activity. By purification of the technical product, the gamma-isomer was isolated and commercially marketed as lindane in honour to the chemist Van der Linden who described this isolation and purification already in 1912 (Van der Linden 1912). In agriculture, HCH was either applied as lindane or as the technical product, the latter in particular in developing countries. Lindane has also found use for wood and timber protection and in human medicine for treatment of head lice. The global technical HCH usage and its contamination consequences in the environment between 1948 and 1997 is summarized by Li (1999). The HCH pattern in human milk is generally dominated by the beta-isomer, followed by alpha- and gamma-HCH which are more rapidly metabolized. The ratio between beta-HCH and alpha-/gamma-HCH in human milk gives some indication on the type and phasing out of HCH application (whether technical HCH or lindane). Higher concentrations of alpha-HCH in human milk in addition to beta-HCH point to an application of technical HCH. With increasing time since termination of application, the detected concentration of alpha-HCH decreases. Details on the contamination of human milk by HCH between the 1950s and 1990 showing the pattern and widespread distribution of HCH isomers are given in the publications by WHO in 1991 (WHO 1991a, b).
Findings of hexachlorobenzene (HCB) in human fat and human milk were reported for the first time only in 1970 by Acker and Schulte (1970a). The analysis was performed by gas chromatographic separation and mass spectrometric identification (GC-MS). The mean value calculated from the analysis of 43 human milk samples was reported as 5.3 ppm on a lipid basis. This mean value was approximately 50% higher than the sum of DDT and its metabolite DDE. The authors had problems to interpret these findings as they could not imagine that the treatment of seeds, the main application area of this fungicide, could be the source of such high contamination. Several years later it was found that HCB can also be formed in combustion processes and occurs as an industrial waste product in the manufacture of a number of technical products (Courtney 1979). In general, concentrations of HCB in human milk in various countries or regions range widely and appear to be related to the degree of industrialization and/or urbanization within the survey area (WHO 1997).
The findings of organochlorine pesticides in the environment and human matrices as well as the increasing perception of adverse effects in biota and humans led to comprehensive regulations on the production and use of organochlorine pesticides in the 1970s. The regulations on organochlorine pesticides, in particular in the Western World have had positive effects on the extent of contamination in human milk as their concentrations were decreasing after the ban or restrictions (Van Haver et al. 1977; Jensen 1983, 1991; Smith 1999; Fång et al. 2015). However, in countries where DDT and similar lipophilic pesticides were applied longer and in some cases are still applied for malaria vector control today, the decrease observed is not as prominent. Even in developed countries of the Western World, due to their long half-life and persistence, several organochlorine pesticides and metabolites, such as DDE, beta-HCH, and HCB can still be found in human milk more than 40 years after their use was prohibited (Fång et al. 2015; Van den Berg et al. 2017).
2.2 Polychlorinated Biphenyls
Polychlorinated biphenyls (PCB) are complex technical mixtures with a chlorine content between around 30 and 60%. Although 209 different PCB with 1–10 chlorine atoms, termed congeners, are theoretically possible, only up to 130 congeners are likely to occur in the commercial products (WHO 1993). PCB were commercially introduced around 1928 and found broad use in open and closed application.
Determination of PCB in the environment and biota was quite challenging. Although in use since more than 30 years, it was only in 1966 that Jensen identified PCB for the first time in biota (Jensen 1966, 1972). At that time the analyses for organochlorine pesticides were performed by GC-ECD with packed GC columns. Jensen noticed a series of peaks behind DDE in the chromatograms which increased in samples through different levels of the food chain. When analysing a white-tailed eagle that was found dead in the archipelago of Stockholm and contained “enormous” amounts of the unknown compounds, Jensen was able to isolate these unknown compounds by thin-layer chromatography and identified them as PCB by GC-MS (Jensen 1972).
In their investigations of human milk on organochlorine pesticides, Acker and Schulte also noticed unknown peaks eluting behind DDT and assumed that these could be the same which Jensen found in biota. Performing GC on two columns of different polarity, they could identify PCB for the first time in human milk (Acker and Schulte 1970b). The initial analyses for PCB in human milk were hampered by the lack of separation power, as the PCB congeners could only be determined as “humps” in the chromatograms. By analysing different technical PCB mixtures, it was found that those products with a chlorine content of 60% best resembled the pattern found in human milk. Consequently, technical PCB mixtures with a chlorine content of 60%, such as Aroclor 1260 and Clophen A60 were initially used as reference standards for quantification of PCB concentrations in human milk. For quantification, two or three characteristic peaks of the technical PCB mixture were related to the corresponding peaks in the human milk samples. Applying this approach, Acker and Schulte reported a mean PCB concentration of 3.5 ppm determined in 43 human milk samples on a lipid base. This mean concentration was similar to the mean level of 3.8 ppm (on a lipid base) reported for the sum of DDT and DDE (Acker and Schulte 1970a, b).
The determination of total PCB using a technical mixture as reference standard for calibration and quantification of PCB concentrations in humans does not take the metabolization of individual congeners into account resulting in markedly different compositions of individual PCB in the technical mixture compared to human milk. Results obtained with this technique varied widely between laboratories and were considerably influenced by the method of quantification chosen and by the technical PCB mixture used as a reference standard. Thus, results calculated as total PCB are prone to overestimation of the actual PCB concentration in human milk which must be considered when comparing recent results with historical analytical data generated in the 1970s based on determinations using packed GC columns. Chemical conversion methods, especially perchlorination, have also been used to determine total PCB concentrations in environmental and biological matrices. These methods are quite sensitive, but do not allow for peak pattern identification. Another drawback of perchlorination is that conversion of less chlorinated biphenyls is not quantitative (WHO 1993).
A breakthrough in the analysis of PCB in environmental and biological samples is attributed to the availability of capillary columns for gas chromatographic determination, which substantially enhanced the separation of complex mixtures (Schulte and Acker 1974). In 1980, Ballschmiter and Zell investigated the composition of seven technical PCB mixtures by high-resolution thin-film glass capillary gas chromatography with electron-capture detection. Moreover, they developed a scheme of numbering the PCB congeners that follows the IUPAC rules of substituent characterization in biphenyls (Ballschmiter and Zell 1980). In 1983, Schulte and Malisch determined the contents of all individual PCB congeners in the two technical PCB mixtures Clophen A 30 and Clophen A 60 (Schulte and Malisch 1983).
The separation power provided by capillary columns allowed for the congener-specific analysis of the PCB pattern not only in technical PCB mixtures, but also in human milk. Moreover, it enabled an unequivocal determination of PCB congeners at trace concentrations due to separation from potentially overlapping lipophilic co-extracts. The PCB congeners with Ballschmiter and Zell numbers 138 (2,2′,3,4,4′,5-hexachlorobiphenyl), 153 (2,2′,4,4′,5,5′-hexachlorobiphenyl), and 180 (2,2′,3,4,4′,5,5′-heptachlorobiphenyl) were identified as major contributors to total PCB contamination of human milk. A common approach to calculate the total PCB concentration in human milk based on the identified individual congeners was to multiply the concentrations of the PCB congeners 138, 153, and 180 by 7.03, 6.64, and 11.86, respectively. Summarizing these three products and dividing the sum by 3 yielded the total PCB content in human milk.
Schulte and Malisch showed that this approach leads to an overestimation of the actual PCB content in human milk. They reported that the sum of the dominating PCB congeners 138, 153, and 180 amounts to 55–70% (mean: 61%) of the PCB pattern in human milk. Based on this investigation, the authors proposed to calculate the “real” PCB content by multiplying the sum of the three PCB congeners by a factor of 1.64 (Schulte and Malisch 1984).
In 1984, Mullins et al. reported on the synthesis of all 209 PCB congeners, their spectroscopic properties, molar response factors, and retention times on a 50 m narrow bore fused silica capillary column (Mullins et al. 1984). Based on this investigation, Safe et al. reported on the first congener-specific analysis of the technical PCB mixture Aroclor 1260 and the PCB composition of a human milk extract (Safe et al. 1985).
Concentrations of PCB in human milk analysed in the 1970s/1980s are compiled in the WHO publication “Environmental Health Criteria 140” (WHO 1993). The data demonstrate the global distribution of PCB. In summary, it is stated that “the average concentrations of total PCB in human milk fat are in the range of 0.5-1.5 mg/kg fat, depending on the donor’s residence, lifestyle, and the analytical methods used. Women who live in heavily industrialized, urban areas, or who consume a lot of fish, especially from heavily contaminated waters, may have higher PCB concentrations in their breast milk” (WHO 1993).
As mentioned above, the interpretation and comparison of these results, in particular for the evaluation of temporal trends is hampered by the different analytical approaches for the determination and quantification of the PCB concentrations in the human milk samples. A reliable temporal trend analysis can only be performed on a congener-specific basis. Reports on global surveys and/or spatial temporal trends of PCB comprising different time spans were repeatedly published (Schade and Heinzow 1998; Solomon and Weiss 2002; Fürst 2006; Zietz et al. 2008; Ryan and Rawn 2014; Fång et al. 2015; Van den Berg et al. 2017; Brajenović et al. 2018). All these reports indicate a substantial decrease of PCB in human milk in countries where PCB have been banned or otherwise regulated. These publications often also contain a section related to the global contamination and temporal trend data on other POPs. The most comprehensive global data are compiled in the publication by Fång et al. (2015).
A subgroup of PCB consists of congeners that are not or mono-chlorinated at the ortho-positions of the PCB molecule. These compounds can adopt a co-planar structure and show toxic effects similar to specific polychlorinated dibenzo-p-dioxins and dibenzofurans and are thus denoted dioxin-like PCB (dl-PCB). Generally, their concentrations in human milk are substantially lower than those of the predominant PCB congeners. Due to the low levels and limited analytical sensitivity, a reliable analysis of dl-PCB in human milk was only possible by gas chromatography/high-resolution mass spectrometry from the mid-1990s onwards (see also Sect. 2.4).
2.3 Brominated Flame Retardants
2.3.1 Polybrominated Biphenyls
Due to the relatively easy non-invasive accessibility and its high lipid content, human milk was occasionally used as a matrix to explore the extent of human exposure to lipophilic persistent organic pollutants due to contamination incidents. One prominent example is the poisoning in Michigan 1973 (Fries 1985). In autumn 1973, the flame retardant Firemaster FF-1® was accidentally used instead of magnesium oxide in livestock feed and was fed to thousands of food producing animals resulting in severe adverse effects in these animals. Firemaster FF-1® consisted of Firemaster BP-6® and 2% calcium silicate as an anticaking agent. The flame retardant is a technical mixture of polybrominated biphenyls (PBB), predominately consisting of hexabromobiphenyls. Its main application was the use in polymers. The poisoning by Firemaster continued until April 1974 when the mix-up of feed additive and flame retardant was discovered and PBB were identified as the contamination source for the poisoning of the animals. The long contamination period led to high concentrations of these lipophilic compounds, in particular in cattle, chickens, and sheep. More than 30,000 cattle, 4500 swine, 1500 sheep, and 1.5 million chicken were killed (Fries 1985). Consumption of food derived from these animals resulted into a considerable human exposure. In an initial investigation in 1976 of 53 body tissue and 12 human milk samples from two areas in Michigan, concentrations were reported as 0.01–1.2 ppm, with a median of 0.068 ppm on a fat basis (Brilliant et al. 1978). In a larger investigation of 2986 human milk samples collected between May 1976 and December 1978 from all over Michigan, PBB were detected in 88% of the samples. The maximum concentration in milk was reported as 2.0 ppm, and the median and mean values were 0.06 and 0.1 ppm, respectively (Miller et al. 1984). In human milk fat from 32 directly exposed farmer’s wives, the PBB concentrations were higher with a maximum of 92 ppm and a mean value of 3.6 ppm (Sonawane 1995).
Initially, the analysis of human milk samples was not performed as congener-specific but based on comparison and quantification based on technical mixtures on packed GC columns, similarly to the initial determination of PCB. In the middle of the 1980s application of capillary columns with mass spectrometric detection, in particular in negative chemical ionization mode (NCI) enabled the separation of technical PBB mixtures and revealed the congener-specific PBB pattern in human milk from various countries (WHO 1994a; Krüger 1988; Krüger and Groebel 1988). Most of the research was conducted with the Firemaster products as the PBB flame retardant with the highest production and application numbers. The predominant congeners in these products were found to be 2,2′4,4′,5,5′-hexabromobiphenyl (PBB-153) and 2,2′,3,4,4′,5,5′-heptabromobiphenyl (PBB-180) (WHO 1994a). Thus, the predominant PBB congeners carry the same substitution pattern as the major polychlorinated biphenyl congeners. This holds also true for the similar persistence of these compounds. Although banned since more than 40 years, especially PBB-153, the most bioaccumulative PBB congener can occasionally still be found in minor amounts in human milk samples collected in the 2000s (Fång et al. 2015).
2.3.2 Polybrominated Diphenylethers
Another class of brominated flame retardants (BFR) of particular importance are polybrominated diphenylethers (PBDE) which generally have replaced PBB after their phasing out. As additives to technical products, they can more easily leach out in contrast to reactive BFRs, which react with the protected product. In total, 209 individual PBDE congeners are possible. There are three technical mixtures PentaBDE, OctaBDE, and DecaBDE, termed according to their mean number of bromine atoms in the constituents of the commercial mixtures. Due to their high production and use since the late 1960s, PBDE have found global distribution (WHO 1994b). In the late 1990s, PBDE gained huge scientific and public interest when Meironyte et al. (1999) reported a time trend study concerning PBDE in Swedish human milk samples. The authors analysed archived pooled human milk samples which were collected at eight time periods between 1972 and 1997 for the PBDE congeners BDE-28, -47, -66, -85, -99, -100, -153, and -154. The sum of the concentrations of the eight PBDE congeners in human milk increased from 0.07 to 4.02 ng/g lipid during the 25-year period studied. While levels of other POPs in human milk decreased during this period, the PBDE showed a doubling of the concentration every 4–5 years. BDE-47, the predominant congener in the PentaBDE technical mixture, was found as the most abundant congener in all milk samples analysed. These results caused serious scientific and public concern and initiated worldwide comprehensive investigations on PBDE in human milk. The number of congeners studied varied widely. While almost all studies on human milk reported on the occurrence of BDE-47, -99, -100, and -153, the major constituents of the PentaBDE and OctaBDE technical mixtures, other studies covered a diverse range of further congeners. BDE-209, the predominant congener in the DecaBDE technical mixture was only occasionally analysed in the early human milk studies on PBDE. With increasing importance of DecaBDE as an alternative and replacement for PentaBDE and OctaBDE technical mixtures, information on contamination of human milk by BDE-209 increased.
The variable number of PBDE congeners analysed and the reporting of total PBDE concentrations based on different congeners hampered the interpretation and comparison of results, especially in case of the early analysed human milk samples. This showed the need for a harmonized analysis of PBDE not only in human milk. Thus, in 2011, the Panel on Contaminants in the Food Chain (CONTAM Panel) of the European Food Safety Authority (EFSA) considered the following eight PBDE congeners to be of primary interest: BDE-28, -47, -99, -100, -153, -154, -183, and -209. The selection was based on the composition of the technical PBDE mixtures, occurrence in the environment, and available data on toxicity (EFSA 2011a). In 2014, the European Commission recommended that, besides other brominated flame retardants, further data on levels of these eight PBDE congeners in food and in humans should be gathered. Moreover, the European Commission recommended to also include BDE-49 and -138 into the analytical methods (EU Commission 2014). Therefore, the 10 above-mentioned congeners became more and more the standard set of analytes for the determination of PBDE in human milk.
Data on PBDE concentrations in human milk predominantly collected in European countries were compiled by EFSA in their risk assessments on PBDE in food (EFSA 2011a, 2023). Systematic reviews on global distribution and temporal trends of PBDE in human milk were performed by Lignell et al. (2009), Fång et al. (2015), Tang and Zhai (2017), Shi et al. (2018), Meng et al. (2021), and Gyllenhammar et al. (2021). The reviews showed that PBDE are globally found in human milk samples, however, with some distinct differences concerning their concentration. The contamination seems to be dependent on the application, type and extent of use, date of sample collection, and time point of legal restrictions for the different PBDE technical mixtures. In general, the reported levels, e.g., of BDE-47 were much higher in human milk samples from the USA compared to samples collected in other parts of the world (Fång et al. 2015), indicating the excessive use of the technical product PentaBDE in the USA due to certain flammability standards (Shaw et al. 2010; Charbonnet et al. 2020). The monitoring programmes also identified some hotspots, in particular in the vicinity of informal e-waste recycling where the PBDE contamination of human milk was substantially higher than in other areas with background contamination (Li et al. 2017).
Due to their persistence, bioaccumulation, and toxicological properties, the production and application of PBDE was widely banned or strictly regulated in the past 20 years and they were listed in the Stockholm Convention in 2009 (PentaBDE and OctaBDE) and 2017 (DecaBDE).
Lignell et al. (2009) and Gyllenhammar et al. (2021), in a follow-up study, investigated human milk samples collected between 1996 and 2017 from first-time mothers living in Uppsala/Sweden. The results showed decreasing levels of BDE-47, -99, and -100 during the study period. No significant time trend was found for BDE-153, however, a change point was observed around the year 2004 with increasing concentration before and decreasing levels after that year. This shift in the PBDE profile is obviously caused by the subsequent substitution of the technical mixtures PentaBDE and OctaBDE.
While derivations of temporal trends are feasible on a national level provided that the human milk samples are collected from comparable cohorts, the deduction of global temporal trends is not meaningful due to the above-mentioned factors that have an impact on the analytical results. Concepts to derive reliable time trends from human milk studies of the WHO/UNEP-coordinated exposure studies are based on minimization of possible sources of variation from the sampling design and from chemical analysis (see Malisch et al. 2023, in Part I of this compendium, and the chapters on results and discussion in Part III, and specifically on time trends in Part IV of this compendium).
2.3.3 Hexabromocyclododecanes
Another group of brominated flame retardants that have been used widely as alternative to restricted PBDE are hexabromocyclododecanes (HBCDDFootnote 2). Their technical mixtures consist of predominantly three isomers, denoted alpha-, beta-, and gamma-HBCDD of which gamma-HBCDD contributes most. As HBCDD isomers are lipophilic and persistent they could also be detected in human milk soon after their commercial introduction. Comprehensive overviews on occurrence of HBCDD in human milk were compiled by EFSA (2011b), Fång et al. (2015), Shi et al. (2018) and EFSA (2021). In contrast to the technical mixtures, where gamma-HBCDD dominates, the predominant isomer in human milk and other biological matrices is alpha-HBCDD which is the chemically most stable isomer and has the highest bioaccumulation potency (EFSA 2011b).
The analysis for HBCDD in human milk is either performed by GC-HRMS or by HPLC-MS/MS. The determination by GC-MS is mostly applied if HBCDD is analysed together with PBDE. However, this approach has the disadvantage that an isomer-specific determination is not possible as a separation of the three isomers cannot be achieved. Thus, the results represent the total HBCDD content. An isomer-specific determination of HBCDD which may be of importance for toxicological considerations is only feasible by application of HPLC-MS/MS. A direct comparison of HBCDD levels obtained by these two analytical approaches is hampered by the fact that the total HBCDD concentration determined by GC-HRMS is not necessarily equal to the sum of the three HBCDD isomer levels obtained isomer-specifically by HPLC-MS/MS analysis due to different response factors. This fact should be taken into account when data obtained with the two different analytical approaches are to be compared. This is especially important for the evaluation of time trends.
The above-mentioned publications indicated that the determination of a global time trend by consideration of occurrence data from different countries is not meaningful. This is due to the fact that the results are not only influenced by the variable characteristics of the participating cohorts but also depend on the HBCDD application date in the various countries, the length of use as well as on differences of entry into force of legal measures requiring prohibiting their further use.
The benefit of archived human milk samples was demonstrated for the determination of HBCDD concentrations by Fängström et al. (2008) who analysed human milk pools from Sweden which were archived between 1980 and 2004. The human milk pools showed a seven-fold increase of HBCDD concentrations between 1980 and 2002 with somewhat decreasing levels between 2002 and the end of the study in 2003/2004. Gyllenhammar et al. (2017) analysed several Swedish milk pools collected between 1996 and 2016 and reported a significant downward trend for the whole study period with a decrease of 2.0% per year. A significant change point was observed around the years 2002–2003 with an increasing trend before that year and a decreasing trend thereafter (Gyllenhammar et al. 2017).
2.4 Polychlorinated Dibenzo-p-Dioxins, Dibenzofurans and dl-PCB
Polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) are two classes of environmental chemicals that have no use and are not produced intentionally but are formed as unintentional and often unavoidable by-products in a number of combustion and technical processes. Due to their numerous sources, they have meanwhile found global distribution. Depending on the number of chlorine atoms and their structural position in the molecules, a total of 75 PCDD and 135 PCDF individual congeners can be differentiated. Both groups together are often termed “dioxins”. The physico-chemical properties, sources, and basic information on toxic effects were compiled by the World Health Organization in 1989 (WHO 1989b).
While environmental specimens generally contain numerous PCDD/PCDF congeners, which often allow information on the sources, samples collected from mammals, including humans show only a reduced PCDD/PCDF profile predominantly consisting of congeners that are chlorine-substituted at the 2, 3, 7, and 8 positions. These congeners are of special importance as they are the most toxic ones and are only slowly metabolized. Due to their persistence and lipophilic properties, they are stored in fatty tissues and accumulate in the food chain. The most important route for human exposure to PCDD/PCDF is food consumption contributing over 90% of total exposure, with products of animal origin and fish making the greatest contribution to this exposure (Fürst et al. 1992; Liem and Rappe 2000).
As the PCDD/PCDF congeners do not exhibit the same toxic potency, toxicity equivalency factors (TEFs) were introduced that take into account the different toxicity in relation to the most toxic congener 2,3,7,8-TCDD. By multiplying the concentrations of the individual congeners in the analysed samples with the respective TEFs and summing up these values one gets the result expressed as toxic equivalent (TEQ) relative to 2,3,7,8-TCDD. Due to increasing knowledge on the toxicity of PCDD/PCDF, the TEFs were repeatedly re-evaluated and updated in the past 40 years resulting in some substantial changes. The most frequently applied factors are I-TEFs (CCMS 1988), WHO1998-TEFs (Van den Berg et al. 1998), and WHO2005-TEFsFootnote 3 (Van den Berg et al. 2006). Currently, the WHO2005-TEFs which are also the basis for legal provisions are almost exclusively used. The differences in the various TEF models have to be taken into account when comparing results, as the same analytical raw data after conversion to TEQ with different TEF models may substantially deviate. This hampers the interpretation and comparison of data reported as TEQ concentrations that were generated in the course of time by applying different TEF models.
Before the early 1980s, the analysis for PCDD/PCDF was generally limited to the determination of 2,3,7,8-TCDD following contamination incidents, such as in Seveso (Fanelli et al. 1982), or in Vietnam (Buckingham 1982). However, due to limited analytical sensitivity only relatively high concentrations could be measured.
The first measurement of 2,3,7,8-TCDD in human milk was reported by Baughman (1974), when he analysed 17 samples from the south of Vietnam collected in areas which were sprayed by the US army during the Vietnam war with Agent Orange, a mixture consisting of the phenoxy herbicides 2,4-D and 2,4,5-T which was highly contaminated with 2,3,7,8-TCDD. Concentrations of 2,3,7,8-TCDD in the human milk samples were reported to range between <1 (LOD) and 55 pptFootnote 4 based on wet weight (Baughman 1974). Assuming a mean fat content in human milk of 3%, the 2,3,7,8-TCDD concentration in the sample with the highest contamination would amount to around 1830 ppt or pg/g lipid (Schecter et al. 1995).
In 1984, Rappe et al. were the first to report on the congener-specific determination of PCDD/PCDF in humans when they analysed five human milk samples from Germany (cited in WHO 1989b). Besides a concentration of 1.9 pg/g lipid for 2,3,7,8-TCDD, they also reported results for 14 further congeners with 2,3,7,8-chlorine substitution. While the highest level was found for octachlorodibenzo-p-dioxin (OCDD) at 434 pg/g lipid, the concentration of the other quantified congeners ranged between <1 and 72.8 pg/g lipid.
Until then, it was assumed that PCDD/PCDF, and in particular 2,3,7,8-TCDD can only be found in samples collected in areas following contamination incidents. As Germany was not considered as dioxin contaminated, the results were questioned but due to the great resonance in the public, great efforts were also initiated to evaluate the human milk results reported by Rappe et al. At the Dioxin Symposium 1985 in Bayreuth, Fürst et al. presented the results of the analysis of 50 human milk samples for PCDD/PCDF (Fürst et al. 1985, 1986). These results showed great similarities concerning congener profile and analyte concentrations with the data reported by Rappe et al. and thus confirmed that PCDD/PCDF cannot only be found in areas following specific contamination incidents but also in the background population. This was also confirmed by Ende (1986), Rappe et al. (1986), and Van den Berg et al. (1986) who performed first comprehensive surveys on PCDD/PCDF in human milk samples collected in different European countries. All samples showed a comparable congener profile almost exclusively consisting of PCDD/PCDF congeners with 2,3,7,8-chlorine substitution, i.e., the toxic congeners. Although the pattern showed great similarities, the concentrations of the individual samples varied to some extent.
Since the mid-1990s, the analysis of human milk for PCDD/PCDF was increasingly extended to the parallel determination of dioxin-like PCB (dl-PCB). Research on the toxicity of PCB has indicated that several congeners can adopt a co-planar structure and show toxic properties similar as certain PCDD/PCDF. The respective dl-PCB are either non-ortho or mono-ortho chlorine substituted. As a consequence, an expert group evaluated the different toxicities and proposed TEFs for a number of dl-PCB similar as for PCDD/PCDF (Ahlborg et al. 1994). These TEFs were re-evaluated by WHO in 1998 (Van den Berg et al. 1998) and 2005 (Van den Berg et al. 2006) and several changes were introduced. Due to their occurrence and toxic properties, the WHO in 1998 evaluated dl-PCB together with PCDD/PCDF in the derivation of a tolerable daily intake for the sum of these three contaminant classes (Van Leeuwen et al. 2000).
The contribution of dl-PCB-TEQ to total PCDD/PCDF/dl-PCB-TEQ in human milk differs depending on the TEF model applied. Generally, the PCDD/PCDF-TEQ is at least doubled if the dl-PCB-TEQ is added. In any case, a reliable interpretation and comparison of occurrence data expressed as TEQ results is only feasible if the same TEF model is used. Conversion of analytical raw data for PCDD/PCDF and dl-PCB in human milk with different TEF models can result in TEQ values that deviate by a factor of 2 or more.
Analyses of human milk, human blood, and adipose tissue have shown that levels of PCDD/PCDF and dl-PCB in human milk very well reflect the body burden as the concentration in these matrices is quite similar when expressed on a lipid basis (Todaka et al. 2010; Needham et al. 2011). This indicated that human milk can be a valuable matrix to estimate the human body burden with PCDD/PCDF and dl-PCB, and thus numerous studies were performed to assess the contamination of human milk with these contaminants at a global level. Comprehensive reviews on the contamination of human milk with PCDD/PCDF were published by Lakind et al. (2001), Lakind (2007), Srogi (2008), Ulaszewska et al. (2011), Fång et al. (2015), and Van den Berg et al. (2017). These reviews confirmed the global occurrence of PCDD/PCDF and dl-PCB in human milk and indicated that samples collected in industrialized areas generally show higher levels than samples collected in developing countries.
2.5 Chlorinated Paraffins
Although chlorinated paraffins (CP) have been produced since the 1930s, they only have attracted increased attention concerning human exposure in the past few years. CP are produced by chlorination of alkanes and consist of n-alkanes with varying degrees of chlorination, usually between 40 and 70% by weight. According to their chain length, they can be divided into short-chain CP (SCCP) comprising 10–13 carbon atoms, medium-chain CP (MCCP) comprising 14–17 carbon atoms, and long-chain CP (LCCP) with 18 or more carbon atoms. CP with ≤9 carbon atoms are denoted very short CP (vSCCP). It is estimated that more than one million tons of CP are produced annually which China being the major producer. CP are used inter alia as high-temperature lubricants, plasticizers, and flame retardants in a wide variety of products. Technical CP are very complex mixtures which may consist of tens of thousands of congeners (Fiedler 2010; Van Mourik et al. 2016). CP may enter the environment during production, use, and improper disposal. As for other lipophilic persistent contaminants, food especially of animal origin is considered the main route of human exposure to CP.
The complexity of the technical CP mixtures impeded their analytical determination in environmental and biological samples in the past as no analytical method is able to separate the various constituents. A comprehensive overview on different analytical methods, their capabilities, limitations, and their area of application is given by EFSA (2020). A promising approach seems to be the combination of comprehensive two-dimensional gas chromatography (GCxGC) coupled with time-of-flight (TOF) mass spectrometry. Applying this technique, a separation into various homologue groups is feasible and also information on different chlorine substitution pattern can be received. The EU Reference Laboratory for halogenated POPs in Feed and Food developed a Guidance Document on the Analysis of Chlorinated Paraffins. This document compiles a set of analytical parameters that would lead to satisfactory method performance, provides an example of a method, and discusses different approaches of quantitative analysis of CP groups (chain length specific patterns) (EURL POPs 2021).
EFSA (2020) also compiled publications on CP concentrations in human milk from various countries. As these data were generated with different analytical approaches, the results should be compared with caution. In any case, the results show a broad range of contamination regarding CP in human milk, generally with higher levels of SCCP compared to MCCP. The results of Krätschmer et al. (2021) demonstrating that the CP levels analysed in human samples from various countries exceeded the PCB levels determined in the same samples should be considered as concern.
2.6 Per- and Polyfluoroalkyl Substances
Because of their high stability towards thermal, chemical, and biological degradation processes, as well as their inert and non-adhering surface properties, per- and polyfluoroalkyl substances (PFAS) have found a wide range of industrial applications. The group of PFAS comprises far more than 1000 known individual compounds. They are used in numerous commercial products, such as surfactants, lubricants, fire extinguishing foams, textile impregnation, electroplating, polishes, paintings, and many others (Glüge et al. 2020). PFAS consist of a hydrophobic alkyl chain of varying length (typicallyC4–C16) and a hydrophilic end group, and thus exhibit amphiphilic properties. They may be released into the environment during production, use, and disposal, and have meanwhile found global distribution (Abunada et al. 2020). PFAS have been shown to be extremely persistent and some of them biomagnify.
First results on PFAS in human milk were only reported in 2004–2007. Kuklenyik et al. (2004) developed a high-throughput method for measuring trace levels of 13 PFAS (2 perfluorosulfonates, 8 perfluorocarboxylates, and 3 perfluorosulfonamides) in human serum and milk using an automated solid phase extraction (SPE) clean-up followed by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). When analysing two human milk samples from the USA, they only found detectable concentrations for perfluoropentanoic acid (PFPeA 1.56 ng/mL) in one, and perfluorohexanoic acid (PFHxA 0.82 ng/mL) in the other milk sample, but no other PFAS in both samples.
So et al. (2006) analysed PFAS in human milk samples from 19 women from China. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) were the two dominant compounds detected in all milk samples. Concentrations of PFOS and PFOA ranged from 0.05–0.36 ng/mL and 0.05–0.21 ng/mL, respectively. The maximum concentrations of other PFAS were 0.10 ng/mL for perfluorohexanesulfonate (PFHxS), 0.06 ng/mL for perfluorononanoic acid (PFNA), 0.015 ng/mL for perfluorodecanoic acid (PFDA), and 0.056 ng/mL for perfluoroundecanoic acid (PFUnDA). Similar results were reported for PFOS and PFOA in human milk samples from Japan by Nakata et al. (2007), and in human samples from Germany by Völkel et al. (2008).
Kärrman et al. (2007) studied occurrence levels of PFASs in human milk in relation to maternal serum together with the temporal trend in milk levels between 1996 and 2004 in Sweden. In this study, matched human milk and serum samples from 12 primiparous women in Sweden were analysed together with composite milk samples (25–90 women/year) collected from 1996 to 2004. PFOS and PFHxS were detected in all milk samples at mean concentrations of 0.201 ng/mL and 0.085 ng/mL, respectively. Perfluorooctanesulfonamide (PFOSA) was detected in eight milk samples with a mean concentration of 0.013 ng/mL, and PFNA was detected in two milk samples (0.020 and 0.014 ng/mL). PFOS and PFHxS levels in composite milk samples were relatively unchanged between 1996 and 2004, with a total variation of 20 and 32% coefficient of variation, respectively.
A key finding of the study by Kärrman et al. (2007) is the observation that the PFOS and PFHxS ratios between human serum and human milk are around 100:1 and 50:1, respectively. This indicates that in contrast to other POPs, human milk is not the matrix of choice in the evaluation of internal human body burden with PFAS because of the substantial lower concentrations in human milk compared to human serum.
2.7 Further POPs of Interest
The number of POPs analysed in human milk has constantly increased over the past 70 years. Following the identification of POPs in the environment, human milk samples were often analysed to monitor the extent of internal human exposure. Examples besides the already mentioned contaminants above which triggered comprehensive monitoring programmes, are e.g. polychlorinated naphthalenes, pentachlorophenol, endosulfan, dicofol, and emerging brominated flame retardants. Moreover, there are a number of chemicals that are proposed for further listing under the Stockholm Convention and thus may become part of human milk surveys, i.e., among others the pesticides methoxychlor and chlorpyrifos, and the UV absorber UV-328.
An overview of the Convention, the Global Monitoring Plan and its implementation by regional and global monitoring reports is given in Part I of this compendium (Šebková 2023). The regional and global monitoring reports as source of information for a wide range of POPs found in various environmental samples and human matrices should be pointed out.
So far, the analytical determination of POPs in human milk was generally performed by target analysis. This means that the method is optimized for the analyte of interest. Since a couple of years, the advances in analytical instrumentation in terms of sensitivity, resolution power, and data evaluation allow non-target analytical approaches, such as use of time-of-flight mass spectrometry (TOF-MS). Applying this technique, not only known POPs can be detected in human milk with high sensitivity, but also emerging contaminants which are hitherto unknown as contaminants in human milk (see also Šebková et al. 2023, in Part V).
3 First and Second Round of WHO Field Studies on Human Milk in the 1980s and 1990s
This section covers the early establishment of global harmonized human milk surveys studying impacts of exposure on human health. Because of the high toxicity of PCDD/PCDF, their occurrence, and levels observed in human milk in 1984 caused a considerable expert and policy debate and public concern about the safety of breastfeeding. Therefore, the WHO Regional Office for Europe in 1987 decided to carry out analytical studies on human milk, including interlaboratory quality control studies. The first results of the interlaboratory quality control study were already discussed during a WHO consultation in 1987, and the first part of the analytical field study was presented in early 1988. Eleven laboratories participated in the quality control study on PCDD/PCDF and six laboratories on PCB in human milk. In general, the results were in good agreement between the laboratories. The WHO Regional Office for Europe noted that interlaboratory quality control studies between laboratories performing the analyses of PCDD/PCDF, especially in human milk are vital to ensure the reliability and comparability of results.
The first part of the analytical field study comprised human milk samples collected between 1987 and 1988 in 19 countries. One of the main conclusions was that in general the levels of PCDD/PCDF in human milk tend to be highest in the most polluted and industrial areas. However, the differences were not significant as they were in about the same range as the analytical deviations found in the quality control studies. The results for the individual congeners analysed in human milk samples from the participating countries were compiled by WHO in 1989 (WHO 1989c). It was recommended that field studies on the determination of PCDD/PCDF and PCB should be repeated at 5 years intervals. Therefore, a second round of exposure studies was completed in 1992–1993. PCDD/PCDF and PCB were analysed in human milk samples from 47 areas in 19 countries. The data of the second field study which are reported on a congener-specific basis in detail showed that the levels of PCDD/PCDF were not increasing but tend to decrease in some participating countries (WHO 1996). The former recommendation to repeat field studies on human milk every 5 years was reconfirmed.
In addition, further quality control studies were completed in 1988–1989, and 1991–1992. Based on the results of the 1991–1992 quality control study, eight laboratories were accepted by WHO for the determination of PCDD/PCDF in human milk (WHO 1995).
In accordance with the recommendations from the third round of interlaboratory quality assessment studies, the WHO European Centre for Environment and Health (ECEH), Bilthoven Division, organized a fourth round of quality assessment studies on levels of PCB, PCDD, and PCDF in human milk and blood plasma in 1996/1997. The CVUA Freiburg, Germany, was the only laboratory that met all performance criteria for analyses of marker and dioxin-like PCB, PCDD, and PCDF in human milk. Consequently, this laboratory was designated as the WHO Reference Laboratory for the Third Round of the WHO-coordinated exposure studies (WHO 2000).
4 Lessons Learned from the Early Human Milk Surveys
Over the past 70 years, analysis of human milk has been demonstrated to be a good indicator for estimating human body burden in regions and on a global level. Benefits are the non-invasive sample collection and the relatively high lipid content which in connection with the modern instrumental capabilities allow an analytical identification and quantification of numerous POPs down to trace concentrations. These data can be used for estimation of exposure to the POPs for the breastfed infant.
There is presumably no other contaminant found in human milk that is as intensively researched as PCDD/PCDF. A number of surveys did not only focus on the concentrations of these POPs but also looked for parameters that have an impact on their levels in human milk. For this, comprehensive questionnaires were designed and send to the respective mother. These questionnaires usually ask for personal characteristics of the mother, such as age, weight, number of breastfed children, length of nursing period, food consumption, area of living, use of cosmetics, smoking habits, and others. EFSA (2018) gives a comprehensive overview on these parameters and their impact on the concentrations in human milk. While the results were obtained for PCDD/PCDF, the outcome and conclusions can also be transferred to most of the other POPs found in human milk. In brief, the number of breastfed children and the length of the nursing period have a substantial impact. The higher the number of breastfed babies and the longer the breastfeeding period, the lower the concentration in the human milk. The special importance of food for POPs exposure was already demonstrated by Hayes et al. (1958) who concluded that there is strong evidence that fat of animal origin in the diet is the main source of exposure to DDT and DDE in subjects who are not occupationally exposed. This was subsequently also confirmed by other studies which showed that especially food of animal origin is the main route to human exposure with lipophilic persistent contaminants (Fürst et al. 1992; Liem and Rappe 2000). On the other hand, breastfeeding women who consume a vegetarian or vegan diet generally show somewhat lower POPs level in their breast milk. Due to the persistence of POPs, the levels in human milk are somewhat higher in mothers who breastfeed their first child at a higher age than respective younger first-time mothers (EFSA 2018). The area of domicile whether living in an urban or rural area has generally no impact on the POPs level in human milk provided that there is no hot spot in the vicinity. Finally, the impact of active and passive smoking of the mother on the contaminant levels is not clear as respective reports are inconclusive (EFSA 2018).
A comparison of results and an estimation of time trends based on data from different countries reported in the literature is severely limited due to the differences in sampling concepts (whether pooled or individual samples), and by the consideration or non-consideration of the above individual factors (diet, age, length of breastfeeding period, etc.) that may have a considerable impact on the contaminant concentration in human milk.
The information on potential impacts on the POP levels in human milk demonstrate the need for harmonized approaches for surveys that enable a reliable interpretation and comparison of results. Especially strict protocols for recruitment of participants with respect to age, number of breastfed babies, length of breastfeeding, and timepoint of sample collection during the breastfeeding period are required. Only if the participants of the surveys have a comparable core characteristic, the results of the analyses can reliably be compared. Further decisive survey criteria are distinct rules for sample collection, handling (volume, pooling), storage, and shipment of the samples to a laboratory as well as selection of a competent laboratory. Finally, validated analytical methods that are repeatedly and successfully tested in proficiency tests are a particular prerequisite for a sound performance of the human milk surveys.
Between 2000 and 2019, WHO and UNEP performed five rounds of global surveys on concentrations and trends of POPs in human milk, partly as joint studies (Malisch et al. 2023). After adoption of the Stockholm Convention, the number of POPs increased gradually not only to cover PCDD/PCDF, PCB, and organochlorine pesticides as “initial POPs” listed under the Stockholm Convention but also other POPs, such as PBDE, HBCDD, polychlorinated naphthalenes, PFOS, PFOA, PFHxS, chlorinated paraffins, and others.Footnote 5
The lessons from the previous WHO-coordinated exposure studies have also inspired the development and gradual updates of harmonized protocols for the WHO/UNEP-coordinated surveys focusing on identification of POPs.
The following chapters of this compendium describe the results of the global surveys on POPs in human milk from the Third Round of the WHO- and WHO/UNEP-coordinated exposure studies from 2000 onwards. As these surveys were based on strict protocols and requirements for performance of analysis by designated reference laboratories for chlorinated and brominated, respectively, fluorinated POPs, the data allow a reliable conclusion on global occurrence and potential time trends.
Notes
- 1.
In earlier publications, the concentrations are reported as “ppm” rather than “mg/kg” or “mg/L”.
- 2.
Sometimes in the literature also wrongly denoted as HBCD.
- 3.
As the re-evaluation of the WHO-TEFs took place in 2005, but the outcome was only published in 2006, the WHO2005-TEFs are sometimes also denoted as WHO2006-TEFs.
- 4.
Reported as ppt (parts per trillion), equal to pg/g or pg/mL.
- 5.
References
Abunada Z, Alazaiza MY, Bashir MJ (2020) An overview of per-and polyfluoroalkyl substances (PFAS) in the environment: source, fate, risk and regulations. Water 12(12):3590
Acker L, Schulte E (1970a) The occurrence of chlorinated biphenyls and hexachlorobenzene along with chlorinated insecticides in human milk and human adipose tissue. Naturwissenschaften 57(10):497
Acker L, Schulte E (1970b) Occurrence of chlorinated hydrocarbons in human fatty tissue and human milk. Dtsch Lebensmitt Rundsch 66(11):385–390
Ahlborg UG, Becking GC, Birnbaum LS, Brouwer A, Derks H, Feeley M, Golor G, Hanberg A, Larsen JC, Liem AKD, Safe SH, Schlatter C, Waern F, Younes M, Yrjanheikki E (1994) Toxic equivalency factors for dioxin-like PCBs. Report on a WHO-ECEH and IPCS consultation, December 1993. Chemosphere 28:1049–1067
Ballschmiter K, Zell M (1980) Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius' Z Anal Chem 302(1):20–31
Baughman RW (1974) Tetrachlorodibenzo-para-dioxins in the environment. High resolution mass spectrometry at the picogram level. PhD thesis, Harvard University
Brajenović N, Brčić Karačonji I, Jurič A (2018) Levels of polychlorinated biphenyls in human milk samples in European countries. Arh Hig Rada Toksikol 69:135–153
Brilliant LB, van Amburg G, Isbister J, Humphrey H, Wilcox K, Eyster J, Bloomer AW, Price H (1978) Breast-milk monitoring to measure Michigan's contamination with polybrominated biphenyls. Lancet 2:643–646
Buckingham WA (1982) Operation ranch hand: the air force and herbicides in Southeast Asia, 1961–1971. Office of Air Force History, United States Air Force
Carter R (1947) Estimation of DDT in milk by determination of organic chlorine. Anal Chem 19(1):54–54
CCMS (1988) Committee on the challenges of modern society. International toxicity equivalency factor (I-TEF) method of risk assessment for complex mixtures of dioxins and related compounds. Pilot study on international information exchange on dioxins and related compounds. North Atlantic Treaty Organization. Committee on the challenges of modern society (CCMS) report number 176
Charbonnet J, Weber R, Blum A (2020) Flammability standards for furniture, building insulation and electronics: benefit and risk. Emerg Contam 6:432–441. https://doi.org/10.1016/j.emcon.2020.05.002
Courtney KD (1979) Hexachlorobenzene (HCB): a review. Environ Res 20(2):225–266
EFSA (2011a) Scientific opinion on Polybrominated diphenyl ethers (PBDEs) in food. EFSA panel on contaminants in the food chain (CONTAM). EFSA J 9(5):2156
EFSA (2011b) Scientific opinion on Hexabromocyclododecanes (HBCDDs) in food. EFSA J 9(7):2296
EFSA (2018) Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J 16(11)
EFSA (2020) Risk assessment of chlorinated paraffins in feed and food. EFSA J 18(3):5991
EFSA (2021) Update of the risk assessment of hexabromocyclododecanes (HBCDDs) in food. EFSA J 19(3)
EFSA (2023) Update of the risk assessment of polybrominated diphenyl ethers (PBDEs) in food. In preparation
Egan H, Goulding R, Roburn J, Tatton JO (1965) ORGANO-chlorine pesticide residues in human fat and human milk. Br Med J 2(5453):66–69
Ende M (1986) Bericht über das Muttermilchuntersuchungsprogramm Niedersachsen 1986. Niedersächsisches Ärzteblatt 13:18–19
EU Commission (2014) Commission Recommendation of 3 March 2014 on the monitoring of traces of brominated flame retardants in food (2014/118/EU). Off J L 65:39–40
EURL POPs (2021) Guidance document on the analysis of chlorinated paraffins
Evans W (1962) The paper-chromatographic separation and determination of chlorinated insecticide residues. Analyst 87(1036):569–575
Fanelli R, Chiabrando C, Bonaccorsi A (1982) TCDD contamination in the Seveso incident. Drug Metab Rev 13(3):407–422
Fång J, Nyberg E et al (2015) Spatial and temporal trends of the Stockholm convention POPs in mothers’ milk—a global review. Environ Sci Pollut Res 22:8989–9041
Fängström B, Athanassiadis I, Ödsjö T, Norén K, Bergman Å (2008) Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in milk from Stockholm mothers, 1980-2004. Mol Nutr Food Res 52:187–193
Faraday M (1825) XX. On new compounds of carbon and hydrogen, and on certain other products obtained during the decomposition of oil by heat. Philos Trans R Soc Lond 115:440–466
Fiedler H (2010) Short-chain chlorinated paraffins: production, use and international regulations. In: The handbook of environmental chemistry, vol 10 “Chlorinated paraffins”, Springer, pp 1–40
Fries GF (1985) The PBB episode in Michigan: an overall appraisal. Crit Rev Toxicol 16(2):105–156
Fürst P (2006) Dioxins, polychlorinated biphenyls and other organohalogen compounds in human milk. Levels, correlations, trends and exposure through breastfeeding. Mol Nutr Food Res 50:922–933
Fürst P, Meemken H-A, Groebel W (1985) Determination of polychlorinated dibenzodioxins and dibenzofurans in human milk. Poster/Abstract 159, Dioxin 85, Bayreuth
Fürst P, Meemken H-A, Groebel W (1986) Determination of polychlorinated dibenzodioxins and dibenzofurans in human milk. Chemosphere 15(9–12):1977–1980
Fürst P, Beck H, Theelen R (1992) Assessment of human intake of PCDDs and PCDFs from different environmental sources. Toxic Subst J 12:133–150
Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D et al (2020) An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ Sci Processes Impacts 22(12):2345–2373
Goodwin ES, Reynolds JG, Goulden R (1961) Rapid identification and determination of residues of chlorinated pesticides in crops by gas-liquid chromatography. Analyst 86(102):697
Gyllenhammar I, Glynn A, Friden U, Cantillana T, Aune M, Lignell S, Bignert A (2017) Levels of persistent halogenated organic pollutants (POP) in mother’s milk from first-time mothers in Uppsala, Sweden: results from year 2015 and 2016, and temporal trends for the time period 1996-2016. In: Report to the Swedish EPA (the Health-related environmental monitoring program)
Gyllenhammar I, Aune M, Fridén U, Cantillana T, Bignert A, Lignell S, Glynn A (2021) Are temporal trends of some persistent organochlorine and organobromine compounds in Swedish breast milk slowing down? Environ Res 197:111117
Hayes Jr WJ, Quinby GE, Walker KC, Elliott J, Upholt WM (1958) Storage of DDT and DDE in people with different degrees of exposure to DDT. Arch Indust Health 18(5):398–406
Hayes WJ, Laws ER (1991) Handbook of pesticide toxicology
Howell D (1948) A case of DDT storage in human fat. Proc Oklahoma Acad Sci
Jensen S (1966) Report of a new chemical hazard. New Sci 32:612
Jensen S (1972) The PCB story. Ambio:123–131
Jensen AA (1983) Chemical contaminants in human milk. Residue Rev:1–128
Jensen A (1991) Levels and trends of environmental chemicals in human milk. Chem Contam Hum Milk:45–198
Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A et al (2007) Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 115(2):226–230
Knoll W, Jayaraman S (1973) Zur Kontamination von Humanmilch mit chlorierten Kohlenwasserstoffen. Food Nahrung 17(5):599–615
Krätschmer K, Malisch R, Vetter W (2021) Chlorinated paraffin levels in relation to other persistent organic pollutants found in pooled human milk samples from primiparous mothers in 53 countries. Environ Health Perspect 129(8) 087004-1-087004-12
Krüger C (1988) Polybrominated biphenyls and polybrominated biphenyl ethers-detection and quantitation in selected foods. University of Münster (thesis) (in German)
Krüger CPF, Groebel W (1988) Detection and determination of polybrominated biphenyls in human milk. Dtsch Lebensmitt Rundsch 84:273–276. in German
Kuklenyik Z, Reich JA, Tully JS, Needham LL, Calafat AM (2004) Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol 38(13):3698–3704
Lakind JS (2007) Recent global trends and physiologic origins of dioxins and furans in human milk. J Expo Sci Environ Epidemiol 17:510–524
LaKind JS, Berlin CM, Naiman DQ (2001) Infant exposure to chemicals in breast milk in the United States: what we need to learn from a breast milk monitoring program. Environ Health Perspect 109:75–88
Laug EP, Kunze FM, Prickett CS (1951) Occurrence of DDT in human fat and milk. AMA Arch Ind Hyg Occup Med 3(3):245–246
Li Y (1999) Global technical hexachlorocyclohexane usage and its contamination consequences in the environment: from 1948 to 1997. Sci Total Environ 232(3):121–158
Li X, Tian Y, Zhang Y, Ben Y, Lv Q (2017) Accumulation of polybrominated diphenyl ethers in breast milk of women from an e-waste recycling center in China. J Environ Sci 52:305–313
Liem DPF, Rappe C (2000) Exposure of populations to dioxins and related compounds. Food Addit Contam 17(4):241–259
Lignell S, Aune M, Darnerud PO, Cnattingius S, Glynn A (2009) Persistent organochlorine and organobromine compounds in mother's milk from Sweden 1996–2006: compound-specific temporal trends. Environ Res 109(6):760–767
Malisch R, Malisch K, van Leeuwen FXR, Moy G, Tritscher A, Witt A, Alvarez J (2023) Overview of WHO- and UNEP-coordinated human milk studies and their link to the Stockholm convention on persistent organic pollutants. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Meironyte D, Noren K, Bergman A (1999) Analysis of polybrominated diphenylethers in Swedish human milk. A time-related trend study 1972-1997. J Toxicol Environ Health A 58(6):329–341
Meng T, Cheng J, Tang Z, Yin H, Zhang M (2021) Global distribution and trends of polybrominated diphenyl ethers in human blood and breast milk: a quantitative meta-analysis of studies published in the period 2000–2019. J Environ Manage 280:111696
Miller FD, Brilliant LB, Copeland R (1984) Polybrominated biphenyls in lactating Michigan women: persistence in the population. Bull Environ Contam Toxicol 32:125–133
Mullins MD, Pochini CM, McCrindle S, Romkes M, Safe SH, Safe LM (1984) High-resolution PCB analysis: synthesis and chromatographic properties of all 209 PCB congeners. Environ Sci Technol 18(6):468–476
Nakata A, Katsumata T, Iwasaki Y, Ito R, Saito K, Izumi S, Makino T, Kishi R, Nakazawa H (2007) Measurement of perfluorinated compounds in human milk and house dust. Organohalogen Compd 69:2844–2846
Needham LL, Grandjean P, Heinzow B et al (2011) Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol 45(3):1121–1126
Prickett CS, Kunze FM, Laug EP (1950) Modification of the Schechter method for the determination of Methoxychlor or DDT in biological materials. J Assoc Off Agric Chem 33(3):880–886
Quinby GE, Armstrong JF, Durham WF (1965) DDT in human milk. Nature 207(998):726–728
Rappe C, Nygren M, Lindström G, Hansson M (1986) Dioxins and dibenzofurans in biological samples of European origin. Chemosphere 15:1635–1639
Ryan JJ, Rawn DFK (2014) Polychlorinated dioxins, furans (PCDD/Fs), and polychlorinated biphenyls (PCBs) and their trends in Canadian human milk from 1992 to 2005. Chemosphere 102:76–86
Safe S, Safe L, Mullin M (1985) Polychlorinated biphenyls: congener-specific analysis of a commercial mixture and a human milk extract. J Agric Food Chem 33(1):24–29
Savage EP, Keefe TJ, Tessari JD, Wheeler HW, Applehans FM, Goes EA, Ford SA (1981) National study of chlorinated hydrocarbon insecticide residues in human milk, USA. I. Geographic distribution of dieldrin, heptachlor, heptachlor epoxide, chlordane, oxychlordane, and mirex. Am J Epidemiol 113(4):413–422
Schade G, Heinzow B (1998) Organochlorine pesticides and polychlorinated biphenyls in human milk of mothers living in northern Germany: current extent of contamination, time trend from 1986 to 1997 and factors that influence the levels of contamination. Sci Total Environ 215(1–2):31–39
Schecter A et al (1995) Agent Orange and the Vietnamese: the persistence of elevated dioxin levels in human tissues. Am J Public Health 85(4):516–522
Schulte E, Acker L (1974) Gas-Chromatographie mit Glascapillaren bei Temperaturen bis zu 320° C und ihre Anwendung zur Trennung von Polychlorbiphenylen. Fresenius' Z Anal Chem 268(4):260–267
Schulte E, Malisch R (1983) Berechnung der wahren PCB-Gehalte in Umweltproben. I. Ermittlung der Zusammensetzung zweier technischer PCB-Gemische. Fresenius' Z Anal Chem 314(6):545–551
Schulte E, Malisch R (1984) Calculation of the real PCB content in environmental samples. II. Gas chromatographic determination of the PCB concentration in human milk and butter. Fresenius’ Z Anal Chem 319(6):54–59
Schwemmer B, Cochrane WP, Polen PB (1970) Oxychlordane, animal metabolite of chlordane: isolation and synthesis. Science 169(3950):1087–1087
Šebková K (2023) The Stockholm convention and its implementation by regional and global monitoring reports. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Šebková K, Fürst P, Malisch R (2023) Outlook (towards future studies on human milk). In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part V)
Shaw SD, Blum A, Weber R, Kannan K, Rich D, Lucas D, Koshland CP, Dobraca D, Hanson S, Birnbaum LS (2010) Halogenated flame retardants: do the fire safety benefits justify the risks? Rev Environ Health 25(4):261–305
Shi Z, Zhang L, Li J, Wu Y (2018) Legacy and emerging brominated flame retardants in China: a review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere 198:522–536
Smith D (1999) Worldwide trends in DDT levels in human breast milk. Int J Epidemiol 28(2):179–188
So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, Lam PKS (2006) Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ Sci Technol 40(9):2924–2929
Solomon GM, Weiss PM (2002) Chemical contaminants in breast milk: time trends and regional variability. Environ Health Perspect 110(69):A339–A347
Sonawane BR (1995) Chemical contaminants in human milk: an overview. Environ Health Perspect 103(6):197–205
Srogi K (2008) Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: a review. Environ Chem Lett 6:1–28
Tang J, Zhai JX (2017) Distribution of polybrominated diphenyl ethers in breast milk, cord blood and placentas: a systematic review. Environ Sci Pollut Res 24:21548–21573
Todaka T, Hirakawa H, Kajiwara J et al (2010) Relationship between the concentrations of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls in maternal blood and those in breast milk. Chemosphere 78(2):185–192
Ulaszewska MM, Zuccato E, Davoli E (2011) PCDD/Fs and dioxin-like PCBs in human milk and estimation of infants’ daily intake: a review. Chemosphere 83:774–782
Van den Berg M, van der Wielen FWM, Olie K, van Boxtel CJ (1986) The presence of PCDDs and PCDFs inhuman breast milk from The Netherlands. Chemosphere 15:693–706
Van den Berg M, Birnbaum L, Bosveld AT, Brunström B, Cook P, Feeley M et al (1998) Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106(12):775–792
Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M et al (2006) The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93(2):223–241
Van den Berg M, Kypke K, Kotz A, Tritscher A, Yong Lee S, Magulova K, Fiedler H, Malisch R (2017) WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit–risk evaluation of breastfeeding. Arch Toxicol 91:83–96
van der Linden T (1912) Über die benzol-hexachloride und ihren Zerfall in Trichlor-benzole. Ber Dtsch Chem Ges 45(1):231–247
Van Haver W, Vandezande A, Gordts L (1977) Organochlorine pesticides in human milk. Archives Belges de Medecine Sociale, Hygiene, Medecine du Travail et Medecine legale Belgisch Archief van Sociale Geneeskunde, Hygiene, Arbeidsgeneeskunde en Gerechtelijke Geneeskunde 35(5):312–324
Van Leeuwen FR, Feeley M, Schrenk D, Larsen JC, Farland W, Younes M (2000) Dioxins: WHO’s tolerable daily intake (TDI) revisited. Chemosphere 40(9–11):1095–1101
Van Mourik LM, Gaus C, Leonards PE, de Boer J (2016) Chlorinated paraffins in the environment: a review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 155:415–428
Völkel W, Genzel-Boroviczény O, Demmelmair H, Gebauer C, Koletzko B, Twardella D, Raab U, Fromme H (2008) Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: results of a pilot study. Int J Hyg Environ Health 211(3–4):440–446
WHO (1979) Environmental health criteria no 9 “DDT and its derivatives”. World Health Organization
WHO (1984a) Heptachlor-environmental health criteria 38. World Health Organization
WHO (1984b) Chlordane-environmental health criteria 34. World Health Organization
WHO (1989a) Aldrin and Dieldrin-environmental health criteria 91. World Health Organization
WHO (1989b) Polychlorinated dibenzo-p-dioxins and dibenzofurans. Environmental Health Criteria 88. World Health Organization
WHO (1989c) Levels of PCBs, PCDD, and PCDFs in breast milk- results of WHO-coordinated interlaboratory quality control studies and analytical field studies. Environ Health Ser 34
WHO (1991a) Alpha- and Beta-Hexachlorocyclohexanes-environmental health criteria 123. World Health Organization
WHO (1991b) Lindane-environmental health criteria 124. World Health Organization
WHO (1993) Polychlorinated biphenyls and terphenyls-Environmental health criteria 140. World Health Organization
WHO (1994a) Polybrominated biphenyls. Environmental health criteria 152. World Health Organization
WHO (1994b) Polybrominated diphenylethers. Environmental health criteria 162. World Health Organization
WHO (1995) Quality assessment of PCB, PCDD and PCDF analysis: third round of WHO-coordinated study. Environmental Health in Europe No. 2
WHO (1996) Levels of PCBs, PCDDs and PCDFs in human milk. Second round of WHO-coordinated exposure study. Environmental health in Europe series no. 3
WHO (1997) Hexachlorobenzene-environmental health criteria 195, World Health Organization
WHO (2000) Interlaboratory quality assessment of levels of PCBs, PCDDs and PCDFs in human milk and blood plasma—fourth round of WHO-coordinated study. WHO European Centre for Environment and Health, Bilthoven, The Netherlands. Processed by WHO regional Office for Europe, Copenhagen, Denmark, in Health Documentation Services. WHO Report EUR/00/5020352
Zietz BP, Hoopmann M, Funcke R, Huppmann R, Suchenwirth E (2008) Long-term biomonitoring of polychlorinated biphenyls and organochlorine pesticides in human milk from mothers living in northern Germany. Int J Hyg Environ Health 211(5–6):624–638
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Fürst, P. (2023). Human Milk Surveys on Persistent Organic Pollutants from a Historical Perspective. In: Malisch, R., Fürst, P., Šebková, K. (eds) Persistent Organic Pollutants in Human Milk. Springer, Cham. https://doi.org/10.1007/978-3-031-34087-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-34087-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34085-7
Online ISBN: 978-3-031-34087-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)