Abstract
Rectal prolapse or procidentia is a debilitating condition that typically presents in parous older women but can occur in men and women of all ages. Surgery is the mainstay for the treatment of rectal prolapse and can be performed through a transabdominal or a perineal approach. Ventral mesh rectopexy was first described by D’Hoore in 2004 and involves pure anterior rectal mobilization to reduce the risk of autonomic nerve injury and postoperative constipation. The need for dissecting along the rectovaginal (or rectovesical) septum as well as suturing within the confined space of the deep pelvis makes ventral mesh rectopexy a procedure ideally suited for robotic surgery. Data from recent meta-analyses suggest that the robotic platform may reduce intraoperative blood loss, length of hospital stay, and postoperative complication rates when compared with conventional laparoscopy. Prospective data on long-term functional outcomes and recurrence are needed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Ventral mesh rectopexy
- Robotic rectopexy
- Rectal prolapse
- Rectal procidentia
- Pelvic organ prolapse
- Robotic surgery
1 Introduction
Rectal prolapse or procidentia is a pelvic floor disorder that typically presents in parous older women but can occur in men and women of all ages. It is a debilitating condition that results in local symptoms (seepage of mucus, bleeding, pain, rectal and pelvic pressure), bowel dysfunction (irregularity, incomplete evacuation, fecal urgency, fecal incontinence, outlet dysfunction constipation), and an impaired quality of life.
Surgery is the mainstay for the treatment of rectal prolapse and can be performed through a transabdominal or a perineal approach [1]. Abdominal repairs may offer lower recurrence rates than perineal surgery, allow for correction of a concomitant pelvic organ prolapse, and should be offered to physically fit patients. Abdominal surgery involves either posterior or anterior rectopexy by using sutures or a mesh. Posterior rectopexy can produce or worsen constipation maybe due to autonomic denervation from posterior mobilization of the rectum or to angulation of a redundant sigmoid colon. Adding a sigmoid resection to posterior suture rectopexy (also known as the Frykman-Goldberg procedure: see Video 18.1) decreases the risk of postoperative constipation and is a good option for patients who present with this complaint preoperatively and often have a redundant sigmoid colon, although anastomotic leak may occur [2].
Ventral mesh rectopexy was first described by D’Hoore in 2004 for the treatment of rectal procidentia. It involves a pure anterior rectal mobilization and mesh suspension of the anterior rectal wall to the sacral promontory. Ventral mesh rectopexy avoids injury to the parasympathetic and sympathetic innervation that can occur with posterior rectal mobilization and division of the lateral stalks, thus reducing the risk of postoperative constipation and the need for a sigmoid resection [3]. This approach gives the opportunity to correct symptomatic internal rectal prolapse as well as concomitant rectocele and enterocele, and can be combined with vaginal prolapse procedures, such as colpopexy, in patients with multicompartment pelvic floor defects. Due to the good functional results and low recurrence rates, ventral mesh rectopexy has rapidly gained acceptance as a favored surgical therapy for rectal prolapse [4].
A laparoscopic approach is usually selected for ventral mesh rectopexy due to improved morbidity and faster recovery compared to open surgery. However, the need for dissecting along the rectovaginal (or rectovesical) septum as well as suturing within the confined space of the deep pelvis makes ventral mesh rectopexy a procedure ideally suited for robotic surgery. Indeed, improved visualization, fine motions, and a stable exposure of the surgical field may optimize anatomical dissection, preservation of critical structures (autonomic nerves, presacral venous plexus, and right ureter) as well as mesh fixation. To date, robotic ventral mesh rectopexy has been reported as a feasible and safe procedure [5]. Few studies and with relatively small sample sizes have compared outcomes after robotic and laparoscopic ventral mesh rectopexy. It is important to note that most studies are performed by surgeons who are experts in laparoscopy but relatively new to robotic surgery [6]. With this limitation, perioperative as well as functional outcomes and recurrence rates have been shown to be similar regardless of the approach used. However, data from recent meta-analyses suggest that the robotic platform may reduce intraoperative blood loss, length of hospital stay, and postoperative complication rates when compared with conventional laparoscopy [7, 8]. This may offset the additional theatre costs associated with robotic surgery. Also, it has been shown that operative time – which is one of the main criticisms of robotic rectopexy – decreases with increasing experience and that the trend toward a longer duration of the robotic procedure may not be statistically significant. Furthermore, a shorter learning curve has been demonstrated, with nearly twenty cases needed to gain proficiency with the robotic approach compared to almost one hundred cases for the laparoscopic approach [9].
The type of mesh material, whether synthetic or biologic, continues to be a matter of debate regarding mesh-related complications and recurrence rates. Synthetic mesh is usually made of lightweight or heavyweight polypropylene, with polyester not being recommended due to a much higher risk of erosion. To date, it is difficult to draw definitive conclusions on this topic. However, current data do not support the idea that biologic mesh entails a higher risk of recurrence compared to synthetic mesh. There might be a small advantage of a lower risk on mesh-related complaints in favor of biologic mesh, which should be considered against the higher costs. This may suggest the use of a biologic mesh in high-risk patients such as smokers, diabetics, patients with inflammatory bowel disease, previous pelvic irradiation, and intraoperative leak from the rectum or vagina [10].
Preoperative diagnostic evaluation includes a careful history and full physical exam, colonoscopy as per screening guidelines, defecography, anorectal physiology studies, and colonic transit study in patients with a severe or lifelong history of constipation. A multidisciplinary evaluation can improve outcomes. Perioperative care is provided according to an enhanced recovery pathway.
2 Operating Room Setup, Patient Position and Port Placement
A full robotic procedure is performed by using a da Vinci Xi surgical system (Intuitive Surgical, Inc., Sunnyvale, CA, USA). The patient cart is docked from the patient’s left side, while the assistant surgeon and scrub nurse stand on the patient’s right side.
Surgery is performed under general anesthesia. An orogastric tube and a Foley catheter are inserted. The patient is placed supine with both arms alongside the body and legs apart on Allen stirrups. A viscoelastic mat (CarePad) is placed on the operating table to prevent the patient sliding throughout the surgical procedure and to reduce the risk of pressure injuries. A lateral support with adequate padding is also placed at the level of the right shoulder.
A 12-mmHg pneumoperitoneum is achieved by using a Veress needle through a small incision at Palmer’s point in the left hypochondrium.
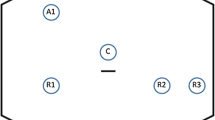
Four 8-mm robotic ports and one 12-mm assistant port (AirSeal Access Port) are used. Three robotic ports are placed in the right abdomen at least 8 cm from each other along a straight line that is parallel and approximately 4 cm lateral to the costofemoral line. An additional robotic port is placed in the left flank, while the assistant port is placed in the right subcostal region, 5–10 cm away from the robotic ports.
The robotic port for the endoscope is placed first after a saline drop test, while the remaining working ports are placed under direct vision. Limited laparoscopic lysis is performed to allow positioning of ports when adhesions are encountered. Then adhesiolysis is completed under robotic assistance.
The patient is positioned in a steep Trendelenburg with right tilt (20–25°), allowing the small bowel to be displaced out of the pelvis under gravity, thus obtaining a good surgical field exposure. The patient cart is deployed and, after a 30° endoscope has been installed on robotic arm 3 (R3), targeting is done towards the pelvis. Next, the rest of the arms are docked and positioned, and the instruments are inserted. A tip-up fenestrated grasper, a force bipolar and a permanent cautery hook are mounted on arm 1 (R1), arm 2 (R2), and arm 4 (R4), respectively. A medium-large clip applier, or a large SutureCut needle driver are used in R4 during the procedure. Curved scissors may be employed instead of the cautery hook according to surgeon’s preference.
3 Rectal Mobilization
If present, the uterus is retracted by placing a straight needle 2–0 polypropylene suture which passes through the fundus and the anterior abdominal wall and is tied extracorporeally over the pubis to better expose the rectovaginal plane. The rectosigmoid junction is retracted cranially, anteriorly, and to the left by the tip-up grasper in R1, exposing the right pararectal fossa.
The right lateral peritoneum of the rectosigmoid mesentery is divided starting over the sacral promontory and advancing distally toward the rectovaginal septum (Fig. 18.1). The plane of the peritoneal incision is made medial to the right common iliac artery. Care is taken to avoid damage to the right hypogastric nerve and ureter, which may be visible through the lining of pelvic peritoneum. Dissection along the right pararectal fossa should remain superficial and limited to about 3 cm in width – just enough to admit a strip of mesh and without performing posterior mobilization of the rectum.
At the level of the pouch of Douglas, the peritoneal incision curves from right to left over the ventral aspect of the rectum in the shape of a smooth inverted letter “J” (Fig. 18.2). Then dissection is performed in an anterior plane between the vagina and rectum. A uterine and vaginal manipulator may be used to lift the posterior vaginal wall and helps identifying the rectovaginal plane. Once identified, the tip-up grasper is used as a retractor deep in the pelvis, while the assistant grasper retracts the rectum cranially. Dissection along the anterior rectal wall is carried out inferiorly down to the level of the pelvic floor and laterally to the cardinal ligaments. Rectal examination may help in assessing the distance from the anal verge, which should not be more than 3–4 cm from the pectinate line. The posterior and lateral attachments of the rectum are left intact to avoid injury to the autonomic nerves and reduce the risk of postoperative constipation and pelvic floor dysfunction.
4 Mesh Placement
A strip of lightweight macroporous polypropylene mesh, 3 cm wide and 15 to18 cm long, is inserted into the abdomen through the assistant port. The mesh is secured to anterior aspect of the distal rectum by using four 2–0 Ethibond interrupted stitches (Fig. 18.3). Care is taken to pierce the seromuscular layer of the rectal wall without penetrating the rectal lumen.
The mesh is passed on the right side of the rectum and its proximal end is fixed to the sacral promontory with two 2–0 Ethibond sutures, while taking care to avoid injury to the presacral veins, hypogastric nerves, right ureter, and iliac vessels (Fig. 18.4). The mesh should lie without tension or redundancy. The peritoneum is then re-approximated over the mesh with a 3–0 PDS (polydioxanone) barbed running suture. This provides elevation of the pelvic floor and leaves the mesh extraperitoneal to prevent mesh-related complications. No drain is routinely left in place. If placed, the suture for uterus retraction is removed. The trocars are removed under direct vision, and the fascial defect of the 12-mm assistant port is closed with absorbable sutures.
5 Conclusions
Robotic ventral mesh rectopexy is an effective approach for the surgical treatment of rectal prolapse. The robotic approach helps to overcome the limitations of conventional laparoscopy in confined spaces like the pelvis and may potentially become the gold standard for ventral mesh rectopexy. Prospective high-quality data are needed to validate the preliminary results and to draw conclusions on the long-term functional outcomes and recurrence.
References
Wexner SD, Fleshman JW. Colon and rectal surgery: abdominal operations. 2nd ed. Lippincott Williams & Wilkins; 2018.
Sciuto A, Pirozzi REM, Pede A, et al. Robotic Frykman-Goldberg procedure for complete rectal prolapse – a video vignette. Color Dis. 2021;23(11):3046–7.
Murphy M, Vogler SA. Rectal prolapse. In: Steele SR, Hull TR, Hyman N, et al., editors. The ASCRS textbook of colon and rectal surgery. 4th ed. Springer; 2022.
Loh KC, Umanskiy K. Ventral rectopexy. Clin Colon Rectal Surg. 2021;34(1):62–8.
van Iersel JJ, Formijne Jonkers HA, Paulides TJC, et al. Robot-assisted ventral mesh rectopexy for rectal prolapse: a 5-year experience at a tertiary referral center. Dis Colon Rectum. 2017;60(11):1215–23.
Faucheron JL, Trilling B, Girard E. Robotic ventral mesh rectopexy for rectal prolapse: a few years until this becomes the gold standard. Tech Coloproctol. 2019;23(5):407–9.
Bao X, Wang H, Song W, et al. Meta-analysis on current status, efficacy, and safety of laparoscopic and robotic ventral mesh rectopexy for rectal prolapse treatment: can robotic surgery become the gold standard? Int J Color Dis. 2021;36(8):1685–94.
Flynn J, Larach JT, Kong JCH, et al. Robotic versus laparoscopic ventral mesh rectopexy: a systematic review and meta-analysis. Int J Color Dis. 2021;36(8):1621–31.
Formisano G, Ferraro L, Salaj A, et al. Update on robotic rectal prolapse treatment. J Pers Med. 2021;11(8):706.
van der Schans EM, Boom MA, El Moumni M, et al. Mesh-related complications and recurrence after ventral mesh rectopexy with synthetic versus biologic mesh: a systematic review and meta-analysis. Tech Coloproctol. 2022;26(2):85–98.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Robotic Frykman-Goldberg procedure (MP4 1315456 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Sciuto, A., Montesarchio, L., Pede, A., Pirozzi, F. (2024). Robotic Ventral Rectopexy for Rectal Prolapse. In: Ceccarelli, G., Coratti, A. (eds) Robotic Surgery of Colon and Rectum. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-33020-9_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-33020-9_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33019-3
Online ISBN: 978-3-031-33020-9
eBook Packages: MedicineMedicine (R0)