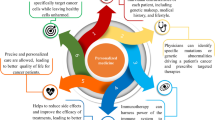
Abstract
Targeted therapy and personalized medicine are novel emerging disciplines of cancer research intended for treatment and prevention. One of the most significant advancements in modern oncology is the shift from an organ-centric strategy to a personalized strategy guided by deep molecular analysis. This shift in view, which focuses on the tumour’s precise molecular changes, has paved the way for individualized treatment. Researchers and clinicians are using targeted therapies to select the best treatment available based on the molecular characterization of malignant cancer. In the treatment of a cancer, personalized medicine entails the use of genetic, immunological, and proteomic profiling to provide therapeutic alternatives as well as prognostic information about cancer. In this book, targeted therapies and personalized medicine have been covered for specific malignancies, including latest FDA-approved targeted therapies and it also sheds light on effective anti-cancer regimens and drug resistance. This will help to enhance our ability to conduct individualized health planning, make early diagnoses, and choose optimal medications for each cancer patient with predictable side effects and outcomes in a quickly evolving era. Various applications and tools’ capacity have been improved for early diagnosis of cancer and the growing number of clinical trials that choose specific molecular targets reflects this predicament. Nevertheless, there are several limitations that must need to be addressed. Hence, in this chapter, we will discuss recent advancements, challenges, and opportunities in personalized medicine for various cancers, with a specific emphasis on target therapies in diagnostics and therapeutics.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- Acute lymphocytic leukaemia:
-
ALL
- Acute myeloid leukaemia:
-
AML
- Adenosine triphosphate:
-
ATP
- Anaplastic lymphoma kinase:
-
ALK
- Chronic lymphocytic leukaemia:
-
CLL
- Chronic myeloid (or myelogenous) leukaemia:
-
CML
- Colorectal cancer:
-
CRC
- Computed tomography:
-
CT
- Cyclin dependent kinase 4 and 6:
-
CDK 4/6
- Cytotoxic T-lymphocyte antigen 4:
-
CTLA-4
- Epidermal growth factor receptor:
-
EGFR
- Fms-related receptor tyrosine kinase 3:
-
FLT3
- Folate-receptor:
-
FR
- Hepatocellular carcinoma:
-
HCC
- Human epidermal growth factor receptor-2:
-
HER
- Internal tandem duplications:
-
FLT3-ITD
- Magnetic resonance imaging:
-
MRI
- Metastatic CRC:
-
mCRC
- Multidrug resistance:
-
MDR
- National cancer institute:
-
NCI
- Non-small cell lung cancer:
-
NSCLC
- Oestrogen receptor:
-
ER
- Overall response rate:
-
ORR
- Platelet derived growth factor receptor:
-
PDGFR
- Poly-ADP ribose polymerase:
-
PARP
- Positron emission tomography:
-
PET
- Precision and personalized medicine:
-
PPM
- Progesterone receptor:
-
PR
- Programmed death-ligand 1:
-
PD-L1
- Progression-free survival:
-
PFS
- Prostate-specific antigen:
-
PSA
- Receptor tyrosine kinase:
-
RTK
- Relapsed/refractory:
-
R/R
- RNA interference:
-
RNAi
- Small interfering RNA:
-
SiRNA
- Tyrosine kinase domain:
-
FLT3-TKD
- Tyrosine kinase inhibitors:
-
TKIs
- Vascular epidermal growth factor receptor:
-
VEGFR
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Lim Z-F, Ma PC (2019) Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol 12(1):134
Goldblatt EM, Lee W-H (2010) From bench to bedside: the growing use of translational research in cancer medicine. Am J Transl Res 2(1):1–18
Liu D (2019) Cancer biomarkers for targeted therapy. Biomark Res 7(1):25
Majithia N, Rajkumar SV, Lacy MQ, Buadi FK, Dispenzieri A, Gertz MA et al (2016) Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia 30(11):2208–2213
Saijo N, Nishio K, Tamura T (2003) Translational and clinical studies of target-based cancer therapy. Int J Clin Oncol 8(4):187–192
Pucci C, Martinelli C, Ciofani G (2019) Innovative approaches for cancer treatment: current perspectives and new challenges. Ecancermedicalscience 13:961
Carr KM, Rosenblatt K, Petricoin EF, Liotta LA (2004) Genomic and proteomic approaches for studying human cancer: prospects for true patient-tailored therapy. Hum Genomics 1(2):134
Rodriguez H, Zenklusen JC, Staudt LM, Doroshow JH, Lowy DR (2021) The next horizon in precision oncology: proteogenomics to inform cancer diagnosis and treatment. Cell 184(7):1661–1670
Khan T, Ali M, Khan A, Nisar P, Jan SA, Afridi S et al (2019) Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 10(1):47
Anand David AV, Arulmoli R, Parasuraman S (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10(20):84–89
Greenwell M, Rahman PKSM (2015) Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res 6(10):4103–4112
Tauchen J, Huml L, Rimpelova S, Jurášek M (2020) Flavonoids and related members of the aromatic polyketide group in human health and disease: do they really work? Molecules 25(17):3846
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y et al (2020) Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 7(193)
Goldberg MS (2019) Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer 19(10):587–602
Jia L-T, Chen S-Y, Yang A-G (2012) Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat Rev 38(7):868–876
Agrawal N, Dasaradhi PVN, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK (2003) RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev 67(4):657–685
Dana H, Chalbatani GM, Mahmoodzadeh H, Karimloo R, Rezaiean O, Moradzadeh A et al (2017) Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci 13(2):48–57
Meng Y, Sun J, Qu N, Zhang G, Yu T, Piao H (2019) Application of radiomics for personalized treatment of cancer patients. Cancer Manag Res 11:10851–10858
Baudino TA (2015) Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol 12(1):3–20
Gerber DE (2008) Targeted therapies: a new generation of cancer treatments. Am Fam Physician 77(3):311–319
Sorokin P (2000) Mylotarg approved for patients with CD33+ acute myeloid leukemia. Clin J Oncol Nurs 4(6):279–280
Lode HN, Xiang R, Becker JC, Gillies SD, Reisfeld RA (1998) Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther 80(3):277–292
Kawakami K, Nakajima O, Morishita R, Nagai R (2006) Targeted anticancer immunotoxins and cytotoxic agents with direct killing moieties. TheScientificWorldJOURNAL 6:781–790
Yap TA, Workman P (2012) Exploiting the cancer genome: strategies for the discovery and clinical development of targeted molecular therapeutics. Annu Rev Pharmacol Toxicol 52:549–573
Frigerio B, Bizzoni C, Jansen G, Leamon CP, Peters GJ, Low PS et al (2019) Folate receptors and transporters: biological role and diagnostic/therapeutic targets in cancer and other diseases. J Exp Clin Cancer Res CR 38(1):125
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21(9)
Munroe DJ, Harris TJ (2010) Third-generation sequencing fireworks at Marco Island. Nat Biotechnol 28(5):426–428
Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M et al (2018) The growing role of precision and personalized medicine for cancer treatment. Technology 6(3–4):79–100
Karczewski KJ, Snyder MP (2018) Integrative omics for health and disease. Nat Rev Genet 19(5):299–310
Schwaederle M, Zhao M, Lee JJ, Lazar V, Leyland-Jones B, Schilsky RL et al (2016) Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: a meta-analysis. JAMA Oncol 2(11):1452–1459
Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Sci (N Y) 304(5676):1497–1500
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350(21):2129–2139
Di Martino S, Rainone A, Troise A, Di Paolo M, Pugliese S, Zappavigna S et al (2015) Overview of FDA-approved anti cancer drugs used for targeted therapy. WCRJ 2:e553
Alberg AJ, Nonemaker J (2008) Who is at high risk for lung cancer? Population-level and individual-level perspectives. Semin Respir CritAl Care Med 29(3):223–232
Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J 48(3):889–902
Matakidou A, Eisen T, Houlston RS (2005) Systematic review of the relationship between family history and lung cancer risk. Br J Cancer 93(7):825–833
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL et al (2017) Lung cancer: current therapies and new targeted treatments. Lancet (Lond, Engl) 389(10066):299–311
Martine NE, Hahn SM, McKenna WG (2005) Molecular biology and genetics of lung cancer. In: Jeremić B (ed) Advances in radiation oncology in lung cancer. Springer-Verlag, Berlin, p 6
Wu K, House L, Liu W, Cho WCS (2012) Personalized targeted therapy for lung cancer. Int J Mol Sci 13(9):11471–11496
Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N et al (2009) Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957
Yang JC, Sequist LV, Geater SL, Tsai C-M, Mok TSK, Schuler M et al (2015) Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 16(7):830–838
Jänne PA, Yang JC-H, Kim D-W, Planchard D, Ohe Y, Ramalingam SS et al (2015) AZD9291 in EGFR inhibitor–resistant non–small-cell lung cancer. N Engl J Med 372(18):1689–1699
Shaw AT, Ou S-HI, Bang Y-J, Camidge DR, Solomon BJ, Salgia R et al (2014) Crizotinib in ROS1-rearranged non–small-cell lung cancer. N Engl J Med 371(21):1963–1971
Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T et al (2010) EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 363(18):1734–1739
Duruisseaux M, Mc Leer-Florin A, Moro-Sibilot D, Cadranel J (2017) Are ALK rearrangement variants promising predictive biomarker of ALK tyrosine kinase inhibitors efficacy? Ann Oncol 28(6):1401
Mazières J, Zalcman G, Crinò L, Biondani P, Barlesi F, Filleron T et al (2015) Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol Off J Am Soc Clin Oncol 33(9):992–999
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W et al (2013) Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res Off J Am Assoc Cancer Res 19(8):2240–2247
Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274(2):113–126
Li J, Wang Z, Shao Z (2019) Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: a review. Cancer Med 8(5):1943–1957
Hu R, Hilakivi-Clarke L, Clarke R (2015) Molecular mechanisms of tamoxifen-associated endometrial cancer. Oncol Lett 9(4):1495–1501
Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR et al (2002) Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 20(16):3396–3403
Osborne C, Pippen J, Jones S, Parker L, Ellis M, Come S et al (2002) Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 20(16):3386–3395
Robertson JF, Osborne CK, Howell A, Jones SE, Mauriac L, Ellis M et al (2003) Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer 98(2):229–238
von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE et al (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol Off J Am Soc Clin Oncology 27(12):1999–2006
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A et al (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol Off J Am Soc Clin Oncology 27(33):5529–5537
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2(2):127–137
Von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G et al (2017) Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377(2):122–131
Scaltriti M, Baselga J (2006) The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 12(18):5268–5272
Fang L, Barekati Z, Zhang B, Liu Z, Zhong X (2011) Targeted therapy in breast cancer: what’s new? Swiss Med Wkly 141(2526)
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M et al (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 28(7):1124–1130
Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G et al (2012) Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol Off J Am Soc Clin Oncol 30(21):2585–2592
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E et al (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly (ADP-ribose) polymerase. Nature 434(7035):913–917
Helleday T (2011) The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol 5(4):387–393
Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N et al (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377(6):523–533
Karginova O, Siegel MB, Van Swearingen AE, Deal AM, Adamo B, Sambade MJ et al (2015) Efficacy of carboplatin alone and in combination with ABT888 in intracranial murine models of BRCA-mutated and BRCA–wild-type triple-negative breast cancer. Mol Cancer Ther 14(4):920–930
Yu KD, Ye FG, He M, Fan L, Ma D, Mo M et al (2020) Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: a phase 3 randomized clinical trial. JAMA Oncol 6(9):1390–1396
Meisel JL, Venur VA, Gnant M, Carey L (2018) Evolution of targeted therapy in breast cancer: where precision medicine began. Am Soc Clin Oncol Educ Book 38:78–86
Baselga J, Campone M, Piccart M, Burris HA III, Rugo HS, Sahmoud T et al (2012) Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med 366(6):520–529
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Thomas E, Holmes FA, Smith TL, Buzdar AU, Frye DK, Fraschini G et al (2004) The use of alternate, non–cross-resistant adjuvant chemotherapy on the basis of pathologic response to a neoadjuvant doxorubicin-based regimen in women with operable breast cancer: long-term results from a prospective randomized Trial. J Clin Oncol 22(12):2294–2302
Strumberg D, Nitiss JL, Rose A, Nicklaus MC, Pommier Y (1999) Mutation of a conserved serine residue in a quinolone-resistant type II topoisomerase alters the enzyme-DNA and drug interactions. J Biol Chem 274(11):7292–7301
Noguchi K, Katayama K, Sugimoto Y (2014) Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmacogenomics Pers Med 7:53
Redmond KM, Wilson TR, Johnston PG, Longley DB (2008) Resistance mechanisms to cancer chemotherapy. Front Biosci 13:5138–5154
Raguz S, Adams C, Masrour N, Rasul S, Papoutsoglou P, Hu Y et al (2013) Loss of O6-methylguanine-DNA methyltransferase confers collateral sensitivity to carmustine in topoisomerase II-mediated doxorubicin resistant triple negative breast cancer cells. Biochem Pharmacol 85(2):186–196
Li W-J, Zhong S-L, Wu Y-J, Xu W-D, Xu J-J, Tang J-H et al (2013) Systematic expression analysis of genes related to multidrug-resistance in isogenic docetaxel-and adriamycin-resistant breast cancer cell lines. Mol Biol Rep 40(11):6143–6150
Christowitz C, Davis T, Isaacs A, van Niekerk G, Hattingh S, Engelbrecht A-M (2019) Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 19(1):757
Reis-Filho JS, Pusztai L (2011) Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet (Lond, Engl) 378(9805):1812–1823
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691
Rawla P, Sunkara T, Barsouk A (2019) Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 14(2):89–103
Graham DM, Coyle VM, Kennedy RD, Wilson RH (2016) Molecular subtypes and personalized therapy in metastatic colorectal cancer. Curr Color Cancer Rep 12:141–150
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M et al (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol Off J Am Soc Clin Oncol 28(31):4697–4705
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 26(14):2311–2319
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol Off J Am Soc Clin Oncol 30(28):3499–3506
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312.
Sforza V, Martinelli E, Ciardiello F, Gambardella V, Napolitano S, Martini G et al (2016) Mechanisms of resistance to anti-epidermal growth factor receptor inhibitors in metastatic colorectal cancer. World J Gastroenterol 22(28):6345
Moorcraft SY, Smyth EC, Cunningham D (2013) The role of personalized medicine in metastatic colorectal cancer: an evolving landscape. Therap Adv Gastroenterol 6(5):381–395
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M et al (2016) Hepatocellular carcinoma. Nat Rev Dis Prim 2:16018
Kew MC (2014) Hepatocellular carcinoma: epidemiology and risk factors. J Hepatocell Carcinoma 1:115
Galun D, Basaric D, Zuvela M, Bulajic P, Bogdanovic A, Bidzic N et al (2015) Hepatocellular carcinoma: from clinical practice to evidence-based treatment protocols. World J Hepatol 7(20):2274
Deng G-L, Zeng S, Shen H (2015) Chemotherapy and target therapy for hepatocellular carcinoma: new advances and challenges. World J Hepatol 7(5):787
Tai W-T, Cheng A-L, Shiau C-W, Huang H-P, Huang J-W, Chen P-J et al (2011) Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol 55(5):1041–1048
Stotz M, Gerger A, Haybaeck J, Kiesslich T, Bullock MD, Pichler M (2015) Molecular targeted therapies in hepatocellular carcinoma: past, present and future. Anticancer Res 35(11):5737–5744
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34
Benson AB 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM et al (2009) NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Cancer Netw JNCCN 7(4):350–391
Capozzi M, De Divitiis C, Ottaiano A, von Arx C, Scala S, Tatangelo F et al (2019) Lenvatinib, a molecule with versatile application: from preclinical evidence to future development in anti-cancer treatment. Cancer Manag Res 11:3847
Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173
Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA et al (2017) NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Cancer Netw 15(5):563–73
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C et al (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383(9911):31–39
Perol M, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Göksel T et al (2014) REVEL: a randomized, double-blind, phase III study of docetaxel (DOC) and ramucirumab (RAM; IMC-1121B) versus DOC and placebo (PL) in the second-line treatment of stage IV non-small cell lung cancer (NSCLC) following disease progression after one prior platinum-based therapy. American Society of Clinical Oncology
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA et al (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203(4):883–895
Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E et al (2003) Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 125(1):89–97
Wen L, Liang C, Chen E, Chen W, Liang F, Zhi X et al (2016) Regulation of multi-drug resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci Rep 6:23269
Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, Otsuka M et al (2012) Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 12:56
Gao L, Wang X, Tang Y, Huang S, Hu CA, Teng Y (2017) FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J Exp Clin Cancer Res CR 36(1):8
Miki D, Ochi H, Hayes CN, Aikata H, Chayama K (2012) Hepatocellular carcinoma: towards personalized medicine. Cancer Sci 103(5):846–850
Forner A, Da Fonseca LG, Díaz-González Á, Sanduzzi-Zamparelli M, Reig M, Bruix J (2019) Controversies in the management of hepatocellular carcinoma. JHEP Reports. 1(1):17–29
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M et al (2018) Cancer today (powered by GLOBOCAN 2018). Lyon (FR)
Daver N, Schlenk RF, Russell NH, Levis MJ (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33(2):299–312
Stone RM, Manley PW, Larson RA, Capdeville R (2018) Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv 2(4):444–453
Antar AI, Otrock ZK, Jabbour E, Mohty M, Bazarbachi A (2020) FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions. Leukemia 34(3):682–696
Zhao J, Song Y, Liu D (2019) Gilteritinib: a novel FLT3 inhibitor for acute myeloid leukemia. Biomark Res 7(1):1–6
Sidaway P (202) Gilteritinib improves outcomes in AML. Nat Rev Clin Oncol 17(2):69
Zhang H, Savage S, Schultz AR, Bottomly D, White L, Segerdell E et al (2019) Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat Commun 10(1):1–13
Sandmaier BM, Khaled S, Oran B, Gammon G, Trone D, Frankfurt O (2018) Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol 93(2):222–231
Altman JK, Foran JM, Pratz KW, Trone D, Cortes JE, Tallman MS (2018) Phase 1 study of quizartinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed acute myeloid leukemia. Am J Hematol 93(2):213–221
O’Farrell A-M, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA et al (2003) An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res 9(15):5465–5476
Man CH, Fung TK, Ho C, Han HH, Chow HC, Ma AC et al (2012) Sorafenib treatment of FLT3-ITD+ acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood, J Am Soc Hematol 119(22):5133–5143
O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F et al (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348(11):994–1004
Kong Y, Wu Y-L, Song Y, Shi M-M, Cao X-N, Zhao H-Y et al (2017) Ruxolitinib/nilotinib cotreatment inhibits leukemia-propagating cells in Philadelphia chromosome-positive ALL. J Transl Med 15(1):1–13
Verstovsek S, Odenike O, Singer JW, Granston T, Al-Fayoumi S, Deeg HJ (2016) Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies. J Hematol Oncol 9(1):1–12
Zhang J, Gu Y, Chen B (2019) Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther 12:1937–1945
Williams SC (2015) News feature: capturing cancer’s complexity. Proc Natl Acad Sci USA 112(15):4509–4511
Duarte TT, Spencer CT (2016) Personalized proteomics: the future of precision medicine. Proteomes 4(4)
Verma M (2012) Personalized medicine and cancer. J Pers Med 2(1):1–14
FDA (2016) FDA advances precision medicine initiative by issuing draft guidances on next generation sequencing-based tests. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm509814.htm. Last Accessed 9 Oct 2021
Maciejko L, Smalley M, Goldman A (2017) Cancer immunotherapy and personalized medicine: emerging technologies and biomarker-based approaches. J Mol Biomark Diagn 8(5)
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S et al (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448(7153):561–566
Chae YK, Pan AP, Davis AA, Patel SP, Carneiro BA, Kurzrock R et al (2017) Path toward precision oncology: review of targeted therapy studies and tools to aid in defining “Actionability” of a molecular lesion and patient management support. Mol Cancer Ther 16(12):2645–2655
Meric-Bernstam F, Johnson A, Holla V, Bailey AM, Brusco L, Chen K et al (2015) A decision support framework for genomically informed investigational cancer therapy. J Natl Cancer Inst 107(7)
Conley BA (2015) Genomically guided cancer treatments: from “promising” to “clinically useful”. Oxford University Press, US
McShane LM, Polley MY (2013) Development of omics-based clinical tests for prognosis and therapy selection: the challenge of achieving statistical robustness and clinical utility. Clin Trials (Lond, Engl) 10(5):653–665
McShane LM, Cavenagh MM, Lively TG, Eberhard DA, Bigbee WL, Williams PM et al (2013) Criteria for the use of omics-based predictors in clinical trials. Nature 502(7471):317–320
Butcher EC, Berg EL, Kunkel EJ (2004) Systems biology in drug discovery. Nat Biotechnol 22(10):1253–1259
Schram AM, Chang MT, Jonsson P, Drilon A (2017) Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 14(12):735–748
Metro G, Finocchiaro G, Toschi L, Bartolini S, Magrini E, Cancellieri A et al (2006) Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC). Rev Recent Clin Trials 1(1):1–13
Keum N, Giovannucci E (2019) Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 16(12):713–732
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD et al (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377(5):454–464
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Saeed, R.F., Awan, U.A., Saeed, S., Mumtaz, S., Akhtar, N., Aslam, S. (2023). Targeted Therapy and Personalized Medicine. In: Qazi, A.S., Tariq, K. (eds) Therapeutic Approaches in Cancer Treatment. Cancer Treatment and Research, vol 185. Springer, Cham. https://doi.org/10.1007/978-3-031-27156-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-27156-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-27155-7
Online ISBN: 978-3-031-27156-4
eBook Packages: MedicineMedicine (R0)