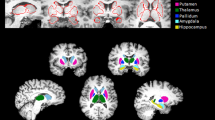
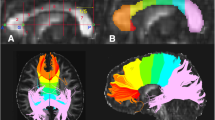
Abstract
Resveratrol can initiate anti-alcoholic functions in the human body. Magnetic resonance diffusion kurtosis imaging (DKI) can comprehensively evaluate ethanol associated brain changes at different time points. This study aimed at utilizing the DKI technique to evaluate the protective effects of resveratrol on brain. Ten volunteers were recruited into this study. Participants in Group A were orally administered with a high alcoholic dose. Those in Group R only took resveratrol, and each participant in Group AR received resveratrol before taking alcohol with the same dose in Group A. Each group included the same 10 volunteers. Each volunteer was subjected to 3 batches of DKI tests. DKI data and images were obtained before drinking, 1.5 h after drinking and at 2 h after drinking. The data obtained before drinking were set as the control group, and the rest data were compared with it. Both Group A and Group AR showed the decrease of mean diffusion (MD) values in multiple brain regions (Group A, n = 12; Group AR, n = 3), Group A showed increased fractional anisotropy (FA) values (n = 16) and Group AR showed increased mean kurtosis (MK) values (n = 7) in multiple brain regions. The MK values of Group A and FA values of Group AR showed biphasic changes in different brain regions. Comparisons between Group A and Group AR, Group AR showed lower FA values in 9 brain regions. The results implied that alcohol caused less damage to the brains of the AR group. DKI can be used to quantify changes in brain tissue microstructure during acute alcohol exposure. The ability of resveratrol consumption to mitigate fluctuations in DKI parameters associated with acute alcohol intake suggests that resveratrol has a protective effect on the brain in a state of alcohol exposure.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Topiwala, A., Allan, C.L., Valkanova, V., et al.: Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ (Clin. Res. Ed.) 357, j2353 (2017). https://doi.org/10.1136/bmj.j2353
Geneva: World Health Organization: Global status report on alcohol and health 2018: executive summary. (WHO/ MSD/MSB/18.2). Licence: CC BY-NC-SA 3.0 IGO (2018)
Fritz, M., Klawonn, A.M., Zahr, N.M.: Neuroimaging in alcohol use disorder: from mouse to man. J. Neurosci. Res. 100(5), 1140–1158 (2022). https://doi.org/10.1002/jnr.24423
Takahashi, N., Senda, M., Lin, S., et al.: Auraptene regulates gene expression involved in lipid metabolism through PPARα activation in diabetic obese mice. Mol. Nutr. Food Res. 55(12), 1791–1797 (2011). https://doi.org/10.1002/mnfr.201100401
Kidani, Y., Bensinger, S.J.: Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol. Rev. 249(1), 72–83 (2012). https://doi.org/10.1111/j.1600-065X.2012.01153.x
Berger, J., Moller, D.E.: The mechanisms of action of PPARs. Annu. Rev. Med. 53, 409–435 (2002). https://doi.org/10.1146/annurev.med.53.082901.104018
Jonker, J.W., Suh, J.M., Atkins, A.R., et al.: A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 485(7398), 391–394 (2012). https://doi.org/10.1038/nature10998
Huang, J., Jia, Y., Fu, T., et al.: Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. Official Publ. Fed. Am. Soc. Exp. Biol. 26(2), 628–638 (2012). https://doi.org/10.1096/fj.11-194019
Kong, L.M., Zheng, W.B., Lian, G.P., et al.: Acute effects of alcohol on the human brain: diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 33(5), 928–934 (2012). https://doi.org/10.3174/ajnr.A2873
Chen, X.R., Zeng, J.Y., Shen, Z.W., et al.: Diffusion kurtosis imaging detects microstructural changes in the brain after acute alcohol intoxication in rats. Biomed. Res. Int. 2017, 4757025 (2017). https://doi.org/10.1155/2017/4757025
Liu, H.M.: A study of acute EtOH intoxication on the brain of rat using 7.0T DWI and 1H-MRS. Medical College of Shantou University (2013)
Kong, L., Lian, G., Zheng, W., et al.: Effect of alcohol on diffuse axonal injury in rat brainstem: diffusion tensor imaging and aquaporin-4 expression study. Biomed. Res. Int. 2013, 798261 (2013). https://doi.org/10.1155/2013/798261
Yamakami, I., Vink, R., Faden, A.I., et al.: Effects of acute ethanol intoxication on experimental brain injury in the rat: neurobehavioral and phosphorus-31 nuclear magnetic resonance spectroscopy studies. J. Neurosurg. 82(5), 813–821 (1995). https://doi.org/10.3171/jns.1995.82.5.0813
Lu, Y., Gao, G.S., Yu, X.J., et al.: Effects of ethanol on Ngb, Hif-1α and Na+, K+ -ATPase activity of brain and mechanism of death. 23(2), 96–99 (2009)
McDonough, K.H., Giaimo, M.E., Miller, H.I., et al.: Low-dose ethanol alters the cardiovascular, metabolic, and respiratory compensation for severe blood loss. J. Trauma 53(3), 541–548 (2002). https://doi.org/10.1097/00005373-200209000-00024
Matés, J.M., Pérez-Gómez, C., Núñez de Castro, I.: Antioxidant enzymes and human diseases. Clin. Biochem. 32(8), 595–603 (1999). https://doi.org/10.1016/s0009-9120(99)00075-2
Yoo, H.Y., Chang, M.S., Rho, H.M.: Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene 234(1), 87–91 (1999). https://doi.org/10.1016/s0378-1119(99)00176-6
Chen, C.C., Yin, S.J.: Alcohol abuse and related factors in Asia. Int. Rev. Psychiatry (Abingdon, England) 20(5), 425–433 (2008). https://doi.org/10.1080/09540260802344075
Karadayian, A.G., Bustamante, J., Czerniczyniec, A., et al.: Alcohol hangover induces mitochondrial dysfunction and free radical production in mouse cerebellum. Neuroscience 304, 47–59 (2015). https://doi.org/10.1016/j.neuroscience.2015.07.012
Koch, O.R., Pani, G., Borrello, S., et al.: Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol. Aspects Med. 25(1–2), 191–198 (2004). https://doi.org/10.1016/j.mam.2004.02.019
Snopek, L., Mlcek, J., Sochorova, L., et al.: Contribution of red wine consumption to human health protection. Molecules (Basel, Switzerland) 23(7) (2018). https://doi.org/10.3390/molecules23071684
Petrella, C., Carito, V., Carere, C., et al.: Oxidative stress inhibition by resveratrol in alcohol-dependent mice. Nutrition (Burbank, Los Angeles County, Calif) 79–80, 110783 (2020). https://doi.org/10.1016/j.nut.2020.110783
Van Cauter, S., Veraart, J., Sijbers, J., et al.: Gliomas: diffusion kurtosis MR imaging in grading. Radiology 263(2), 492–501 (2012). https://doi.org/10.1148/radiol.12110927
Cernak, I., Vink, R., Zapple, D.N., et al.: The pathobiology of moderate diffuse traumatic brain injury as identified using a new experimental model of injury in rats. Neurobiol. Dis. 17(1), 29–43 (2004). https://doi.org/10.1016/j.nbd.2004.05.011
Acknowledgments
This study was funded by Special Funds of Department of Science and Technology of Guangdong Province (Grant Number: 202055, 202063) and was supported by Joint Research Fund for Enterprise and basic and applied basic research Programs of Guangdong Province of China (Grant No. 2021A1515 220112).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Zhang, G., Lu, Y., Zheng, H., Kong, L., Zheng, W. (2023). Protective Effects of Resveratrol on Brain Edema and Microstructural Changes in Human Brain After Acute Alcohol Intake: Assessment by Diffusion Weighted Kurtosis Imaging. In: Wen, S., Yang, C. (eds) Biomedical and Computational Biology. BECB 2022. Lecture Notes in Computer Science(), vol 13637. Springer, Cham. https://doi.org/10.1007/978-3-031-25191-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-25191-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25190-0
Online ISBN: 978-3-031-25191-7
eBook Packages: Computer ScienceComputer Science (R0)