Abstract
Over 200,000 new leprosy cases are reported globally every year. A vaccine for leprosy can eliminate the debilitating, biblical, and stigmatised disease in the twenty-first century. Since the 1940s, many clinical studies have consistently shown that the BCG vaccine offers some level of protection but ranging between 18% and 90%. Throughout this time, different versions of BCG and new developments have resulted in new leprosy vaccine candidates and prevention strategies. Examples are the vaccine and drug combinatory therapy that has shown promise in decreasing transmission and the subunit vaccine candidate, LepVax, which has been shown to reduce bacterial count and delay nerve function impairment in animal models and safe in healthy adults in early studies. The WHO officially recommended the BCG vaccine as a leprosy vaccine in 2018, a century later after it was first used as a tuberculosis vaccine in 1921. However, a better leprosy vaccine and prevention strategy is still needed because we do not exactly know how Mycobacterium leprae spreads and causes neurological damage in leprosy patients. The history and latest developments in leprosy vaccines are explored in this chapter.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
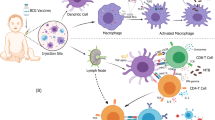
Leprosy is an age-old infectious disease that continues to be endemic in some regions of the Americas, Africa, and South-east Asia [1]. It is caused by the bacterium called Mycobacterium leprae, discovered by Gerhard Armauer Hansen in 1874 [2]. Hence, leprosy is also called Hansen’s disease. Leprosy primarily affects the skin and peripheral nerves. Every year, over 200,000 new leprosy cases are reported globally [1]. In 2019, India, Brazil, and Indonesia accounted for 79% of the 202,166 newly registered leprosy cases [1]. A third of the diagnosed patients experience disabilities because of nerve damage (Fig. 4.1). Consequently, leprosy is the leading infectious cause of disability worldwide [3, 4], and an estimated three to four million people are living with disabilities caused by leprosy [5].
Multi-drug therapy (MDT)Footnote 1 introduced by the World Health Organization (WHO) in 1981 remains highly effective to cure leprosy, but early diagnosis and treatment are paramount to preventing permanent nerve damage that can progressively lead to deformity and disability. Alarmingly, cases of drug resistance and disease relapses have been reported [6,7,8]. There have been many leprosy vaccine candidates and a leprosy vaccine does exist: in 2018, the WHO recommended one dose of the Bacille Calmette-Guérin (BCG) vaccine for healthy neonates at the earliest opportunity to reduce the risk of leprosy in countries or settings where it is common [9] (Fig. 4.2). However, meta-data analyses of clinical trials found that the BCG vaccine has variable protection ranging from 18% to 90% against leprosy [10,11,12].
Photographs of vaccines. Left: a leprosy vaccine of unknown composition produced by the Wellcome Physiological Research Laboratories in London, United Kingdom, circa 1978. (Image source: Wellcome Collection. Attribution 4.0 International (CC BY 4.0)). Right: a BCG vaccine to prevent tuberculosis, manufactured by Aventis Pasteur Canada in 2002. (Image source: Sanofi Pasteur Canada Archives)
In the next decade, the WHO Global Leprosy Strategy, 2021–2030, boldly aims ‘towards zero leprosy’ [5], focusing on interrupting transmission and achieving zero autochthonous cases. To ultimately bring leprosy to zero, an effective leprosy vaccine is essential and pivotal as part of the global strategic effort to eradicate the debilitating disease in the twenty-first century. In this chapter, we highlight the leprosy vaccine successes and investigate current leprosy vaccine developments and strategies.
2 The BCG Vaccine Has Variable Protection Against Leprosy
A misconception is that there is no leprosy vaccine. Studies show that the BCG vaccine used to prevent tuberculosis caused by M. tuberculosis, a bacterium closely related to M. leprae, offers more protection against leprosy than against tuberculosis [12, 13]!
The BCG vaccine is live attenuated M. bovis BCG strain. It was originally developed by Jean-Marie Camille Guérin and Léon Charles Albert Calmette in the early 1900s using attenuated M. bovis, a bacterium more closely related to M. tuberculosis, as an experimental vaccine to protect cattle from bovine tuberculosis [14]. In 1921, BCG was administered for the first time to a newborn baby in Paris to prevent human tuberculosis [15]. Now, BCG is one of the most widely used vaccines worldwide. In 1987, the Brazilian Ministry of Health recommended BCG vaccination or repeat vaccination of contacts to reduce the incidence of leprosy [16]. However, it was only in 2018 that leprosy was included in the WHO BCG vaccine program. Why did it take so long?
BCG vaccination against leprosy was first suggested by J. M. M. Fernandez in 1939 [17], who reported leprominFootnote 2 conversation among children following BCG administration. It was postulated that BCG may confer some protection against leprosy due to possible common antigens between M. bovis BCG and M. leprae. The finding initiated five early small-scale trials in the 1950s in Brazil [18], India [19], Argentina [20], Venezuela [21], and Japan [22]. The trials showed that BCG vaccine has partial or wide protection (26–96%) against leprosy, but they had inadequate controls to draw any definitive conclusion. Furthermore, because leprosy has a long incubation period, on average of 5 or more years before the disease manifests in a clinically diagnosable form [5], long follow-ups and large-scale trials are needed to provide the necessary robust data. A plethora of clinical trials and community surveys then followed from the 1960s to the 2000s in Uganda [23,24,25], New Guinea [26, 27], India [11, 28,29,30,31,32,33], Myanmar (Burma) [34,35,36,37,38], Malawi [39,40,41], Kenya [42], Venezuela [43], Vietnam [44, 45], Brazil [46,47,48,49,50,51,52,53], and Indonesia [54]. Interestingly, the trial data in BCG protection were heterogeneous but showed protection wherever they were studied. To make sense of the heterogeneity, Setia et al. [10], Zodpey [11], and Merle et al. [12] carried out meta-data analyses and found that BCG protection against leprosy remained variable, between 18% and 90%. While the extrema are wide and with no definitive reasons for the heterogeneity, the authors agreed the trials consistently showed that BCG protects against leprosy. The authors commented that the variability between studies was due to several factors: study population (genetics, household contact, geography), environmental bacteria (cross-reaction), BCG dose number, M. bovis BCG diversity of sub-strains (genotype, phenotype, and vaccine manufacturer), nutrition, economic background, study bias, publication bias, and data collection/methodology.Footnote 3 These are ongoing factors to consider and to address for future studies.
In 2013, the WHO published new recommendations for manufacturing and evaluating BCG vaccine (for tuberculosis) [55]. In 2018, the WHO officially included leprosy in the single-dose BCG vaccination recommendation [9]. The inclusion of leprosy for BCG vaccination has huge implications for public health and research moving forward. It recognises that the BCG vaccine is important to prevent both tuberculosis and leprosy.
Generally, a single dose of BCG showed higher protection against leprosy in young individuals. The BCG protection wanes over time but can last for 10–30 years [12, 13].
3 The Recombinant BCG Vaccines to Improve Efficacy Against Leprosy
Strategies to increase BCG vaccine immunogenicity include mixed vaccine with the addition of killed M. leprae or killed M. vaccae (an environmental mycobacterium) and recombinant BCG (rBCG) that expresses foreign molecules.
In three large clinical trial studies in Venezuela [56], Malawi [57], and India [58] comparing the efficacy between BCG and BCG + killed M. leprae, no significant difference in protection was found in Venezuela (56% vs. 54%) after 5-year follow-up and in Malawi (49% vs. 49%) after 6- to 9-year follow-up. However, an improvement was found in the Indian study (34% vs. 64%), after 4- to 7-year follow-up.
There are contradictory conjectures and a lack of studies on the premise that pre-sensitisation to environmental mycobacteria may improve, diminish, or mask BCG immunogenicity [59,60,61,62,63,64,65]. In a small population vaccination trial of children in close contact with leprosy in Vietnam [66], BCG + killed M. vaccae was found to have a modest improvement in protection at 66%, compared to BCG (58%) and M. vaccae alone (55%). Further studies are needed but killed M. leprae is a scare material. M. leprae cannot be cultured with an artificial growth medium and is therefore difficult to isolate in large quantities for experimental studies. Currently, M. leprae cultivation requires animals such as mice [67,68,69] or armadillos [70,71,72], which is costly, with months of maintenance and growth time required to isolate sufficient bacterial samples.
The rBCG was first introduced by Stover et al. in 1991 [73] and enabled the expression of foreign antigens in BCG. In essence, BCG is immunogenic and is used as a vector to elicit specific immune responses guided by the foreign antigen. Since then, a repertoire of antigenic rBCG candidates have shown promise, in improving immunogenicity not only against tuberculosis [74] but also against viruses (respiratory syncytial virus [75, 76], human metapneumovirus [77], measles [78], human immunodeficiency virus type 1 [79, 80]); bladder cancer [81, 82]; the protozoa parasites Leishmania [83], Plasmodium spp. [84, 85], and Toxoplasma gondii [86]; and the bacteria Streptococcus pneumoniae [87], Borrelia burgdorferi [88], and Bordetella pertussis [89,90,91].
Several rBCG candidates have been developed for leprosy. Ohara et al. [92, 93] first constructed the rBCG/85A vaccine with M. leprae antigen Ag85A and then the rBCG/BA51 vaccine with M. leprae antigen Ag85 and M. tuberculosis major protein MBP51. They found that a repeat immunisation in C57BL/6 mice with rBCG/85A vaccine drastically inhibited the multiplication of M. leprae in the mouse footpads compared to control and BCG. This was improved with the rBCG/BA51 vaccine with one-dose immunisation inhibiting multiplication of M. leprae in the mouse footpads, compared to control and BCG in C57BL/6 and BALB/c mice. Furthermore, M. leprae lysate stimulated a higher level of interferon-γ (IFN-γ) production in spleen cells from rBCG/BA51 immunised C57BL/6 mice than BCG and rBCG/85A, an indication of improved host immune defence against M. leprae.
Makino et al. [94,95,96] constructed the rBCG-SM vaccine secreting M. leprae major membrane protein II (MMP-II). MMP-II is an antigen that can stimulate dendritic cells (DC) to produce interleukin (IL)-12 p70 and activate T cells to produce IFN-γ during the pro-inflammatory response important for adaptive and innate immunity. In the initial in vitro and ex vivo studies, the rBCG-SM-infected DC stimulated BCG-vaccinated donor naïve and memory type CD4+ and CD8+ T cells, to produce significantly higher levels of IFN-γ than the rBCG-vector and killed rBCG-SM. A similar outcome was found for IFN-γ production by splenic T cells of C57BL/6 mice infected with rBCG-SM. This was also later confirmed by Maeda et al. [97]. Furthermore, Makino et al. [95] found that rBCG-SM-infected DC increased intracellular production of perforin in CD8+ T cells. Perforin is a pore-forming cytolytic protein produced by cytotoxic T cells that allows passive diffusion of pro-apoptotic proteases to enter target cells to control infection [98]. In a subsequent study, rBCG-SM-stimulated macrophages induced granulocyte-macrophage colony-stimulating factor (GM-CSF) cytokine production and inhibited the production of IL-10 [96]. The T cell activation was found to be dependent on GM-CSF production. IL-10 can block the reactivation of memory T cells. Therefore, the inhibition can potentially benefit anti-mycobacterial immune responses. This has been found in IL-10-deficient mice with a decreased bacterial burden [99].
Tabouret et al. [100] designed the rBCG::PGL-1 vaccine to study the role of PGL-1 in the pathogenesis of leprosy. PGL-1 is a species-specific phenolic glycolipid 1 from M. leprae with virulence, protective, and immunomodulatory properties. They found that rBCG::PGL-1 enhanced invasion via the complement receptor 3 (CR3) of human monocyte-derived macrophages, increased uptake by DCs, and impaired inflammatory responses. Recently, Doz-Deblauwe et al. [101] found that rBCG::PGL-1 enhanced CR3-mediated non-opsonic phagocytosis in polymorphonuclear neutrophils and DCs and activated Syk-calcineurin/nuclear factor of activated T cells signalling to rewire host cytokine responses to M. leprae. Although no M. leprae infection challenge was carried out, the insights on the PGL-1 could help rBCG vaccine development, by considering immune responses during leprosy pathogenesis and the mechanisms of nerve damage causation.
Horwitz et al. [102] designed the rBCG30 vaccine to overexpress M. tuberculosis 30 kDa major secretory protein antigen 85B, which they found to offer better protection than BCG against M. tuberculosis and M. bovis challenge in animal models. Gillis et al. [103] further evaluated rBCG30 and found that it could stimulate CD4+ and CD8+ in cytokine responses from BCG-immunised BALB/c mice and needed boosting with purified M. tuberculosis 30 kDa antigen 85B to reduce M. leprae burden in mouse footpads.
Now, there is only one rBCG vaccine in clinical trials, the VPM1002 vaccine. The clinical trial evaluations are in phases II and III for tuberculosis (ClinicalTrials.gov Identifier: NCT03152903, NCT04351685), in phases I and II for recurrent non-muscle invasive bladder cancer (ClinicalTrials.gov Identifier: NCT02371447), and in phase III for SARS-CoV-2 infectionFootnote 4 (ClinicalTrials.gov Identifier: NCT04439045, NCT04387409). The VPM1002 vaccine has not been evaluated as a vaccine candidate for leprosy. The VPM1002 is a genetically modified BCG that has the urease C encoding gene replaced by the listeriolysin O encoding gene from Listeria monocytogenes [115,116,117]. Urease C neutralises phagosomes that contribute to mycobacteria survival, whereas listeriolysin O forms transmembrane β-barrel pores in the phagolysosome membrane. Therefore, VPM1002 can effectively release mycobacterial antigens into the cytosol to trigger immunogenic responses. The VPM1002 system can potentially be used and further modified as a leprosy vaccine. Now that BCG is more widely recognised as a vaccine for leprosy, this offers promise for rBCGs such as VPM1002, rBCG/85A, rBCG-SM, rBCG::PGL-b, and rBCG::PGL-1 and the tuberculosis rBCGs as leprosy vaccine candidates in clinical studies.
4 The Cross-Reactivity and Subunit Leprosy Vaccines
Other leprosy vaccine candidates besides the M. bovis BCG and rBCGs include (1) non-pathogenic or closely related M. leprae mycobacterium species to induce cross-reactivity such as the ICRC (Indian Cancer Research Centre bacilli), M. vaccae, M. duvalii, M. welchii (M. w) or M. indicus pranii (MIP) [118],Footnote 5 and M. habana and (2) recombinant protein subunits, such as the LEP-F1 + GLA-SE (LepVax), to induce target-specific immune responses. M. vaccae, as previously discussed, is like BCG in leprosy protection. M. duvalii is an early vaccine candidate proposed in 1974 [119] that showed some cross-reactivity. However, Shepard et al. in 1976 [120] later found that M. duvalii and M. duvalii + BCG offered less protection and no change in protection, respectively, when compared to BCG in mice footpad immunisation studies.
The M. habana vaccine was reported by Singh et al. [121, 122] to reduce M. leprae counts better than BCG in mice footpad immunisation studies. Furthermore, Singh et al. [123] found that M. habana induced a positive Mitsuda reaction in monkeys. Additionally, Chaturvedi et al. [124] identified M. habana proteins in the cell wall and cell membrane fractions that were recognised by leprosy antisera, and the 65 kDa protein [125] and 23 kDa proteins [126] were found to induce cell-mediated immune responses. The latest study identified two additional M. habana proteins, an enoyl-coenzyme A hydratase and antigen 85B, both recognised by leprosy antisera [127]. These proteins can be used in vaccine studies and as serodiagnosis tools. However, the M. habana efficacy as a leprosy vaccine remains uncertain. A small vaccination study of 31 lepromatous leprosy patients and 36 household contacts found positive lepromin reaction only after 15 weeks, but also had systemic side effects [128]. It is a short time frame to draw a conclusion considering that leprosy has a long incubation period. Therefore, further studies are required to understand the efficacy and the safety profile.
The ICRC vaccine is a gamma-radiation inactivated group of leprosy-derived cultivable slow-growing mycobacteria belonging to the M. avium complex isolated in 1958 from a leprosy patient [129,130,131]. Early immunological studies from 1974 to 1978 all demonstrated reactivity [132,133,134]. Bhide et al. [135] reported in 1978 that ICRC offered protection against M. leprae infection in the mouse footpad model. This led to small trials by Deo et al. [136] and Bhatki et al. in the early 1980s [137] that continued to show promising outcomes. ICRC resulted in negative to positive lepromin conversion in 58% of lepromatous leprosy patients and 91% of borderline lepromatous patients. Chaturvedi et al. [138] reported that ICRC has a dose-dependent lepromin conversion at eighth week (high dose and 1/30th dose resulted in 79% and 46% lepromin conversion, respectively) and resulted in >90% lepromin conversion in healthy subjects from household contacts of leprosy patients and non-contacts in a general population in Bombay at the end of 1 year; patients remained stable up to 3 years; and no nerve toxicity was reported, as hypersensitivity to M. leprae antigens can lead to nerve damage. In a large-scale comparative study in India, Gupte et al. [58] reported 66% protection by ICRC versus 34% protection by BCG after 4–7-year follow-up. Interestingly in the same comparative study, BCG combined with killed M. leprae offered 64% protection, similar to ICRC. A recent ICRC formula evaluation found that ICRC candidate strain C-44 is coated with human immunoglobulin G that may play a role in the immune responses [139].
The MIP vaccine was developed in the National Institute of Immunology, India, and showed promising early initial outcomes. Chaudhuri et al. [140] and Talwar et al. [141] reported that 20 of the 32 patients had negative to positive lepromin reaction conversion after 4–6 weeks from a single administration and remained stable after 6–11 months. However, in the large-scale comparative study in India reported by Gupte et al. [58], MIP only offered 26% protection compared to 66% protection by ICRC, 34% protection by BCG, and 64% protection by BCG + killed M. leprae, after 4–7-year follow-up. In a double-blind immunoprophylactic trial conducted in an endemic area of Kanpur Dehat, Uttar Pradesh, Sharma et al. [142] showed that the low MIP protection was attributable to a decrease in protection over time and offered greater protection for contacts. They found that MIP had protective efficacy of 69%, 59%, and 39% at, 3-, 6-, and 9-year follow-up, respectively, for household contacts after the initial vaccination. Similarly, the protective efficacy was 68%, 60%, and 28% at 3-, 6-, and 9-year follow-up, respectively, for both patients and contacts after the initial vaccination. The MIP vaccine was less effective for patients: the protective efficacy was 43%, 31%, and 3% at 3-, 6-, and 9-year follow-up, respectively. However, smaller studies have found that MDT and MIP as immunotherapy for multibacillary leprosy patients could shorten recovery time, reduce bacterial load, clear granuloma, and reduce neuritis [143,144,145,146,147]. The MIP vaccine has received approval by the Drugs Controller General of India and the US Food and Drug Administration [148]. In 2017, the Indian Council for Medical Research launched a vaccine programme to eradicate leprosy in leprosy endemic districts [149,150,151]. The patients, family members, and contacts will receive two doses of autoclaved MIP at 6 months intervals. Studies are ongoing to evaluate the efficacy of MDT and MIP immunotherapy.
LepVax is the latest vaccine candidate moving in the clinical trial pipeline [152] (Fig. 4.3). LepVax is a defined subunit vaccine containing a chimeric recombinant protein (LEP-F1) consisting of a tandem linkage of M. leprae antigens ML2531, ML2380, ML2055, and ML2028 and a synthetic Toll-like receptor 4 (TLR4) agonist glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE). In the M. leprae mouse challenge studies, LepVax raised an immune response not affected by prior BCG immunisation. Additionally, immunised mice infected with M. leprae had significantly fewer bacteria recovered in the mouse footpad experiments, compared to unimmunised control mice. After 12 months, the bacterial burden in immunised mice was approximately 85% lower than mice immunised with GLA-SE adjuvant formulation alone. Importantly, LepVax immunisation delayed motor nerve function impairment in M. leprae-infected nine-band armadillos, demonstrated as post-exposure immunoprophylaxis. LepVax dosage, safety, and immunogenicity parameters were evaluated in the phase 1a clinical trial on 24 healthy adult volunteers in the United States [153]. The study outcome published in 2020 concluded that LepVax was safe and immunogenic and LepVax will start phase 1b/2a clinical trial in 2022 to carry out the same evaluation in leprosy endemic regions (ClinicalTrials.gov Identifier: NCT03947437).
5 Vaccine and Drug Combinatory Therapy
The combination of immunotherapy and chemotherapy can shorten leprosy treatment time and potentially improve the treatment outcome. When the WHO recommended MDT for leprosy in 1981, patients were required to be on the regimen for at least 2 years.Footnote 6 An early evaluation of the MIP vaccine candidate by Talwar et al. [154] found there was more rapid bacterial clearance in vaccinated patients who were also receiving MDT. Zaheer et al. [155] investigated if chemotherapy in combination with immunotherapy, i.e. MDT + MIP, could reduce the treatment time by inducing cell-mediated immune responses. They reported that MIP helped overall in the treatment; 13 of 31 BL and LL patients or multibacillary leprosy patients who received MDT and MIP were bacteriologically negative within 2 years, compared to 5 of 25 controls. The vaccinated patients had either upgraded in disease spectrum or were completely cleared of granuloma. Furthermore, 80% of vaccinated BL and LL patients had lepromin conversions, compared to 14% of the controls.
Sharma et al. [156] also reported a faster bacterial clearance for patients receiving both MDT and MIP within 2 or 3 years. They found that 90% of the vaccinated patients had negative to positive lepromin conversions compared to 38% of the placebo group, and the patients released from treatment had no incidence of relapses in a 5-year follow-up. They concluded that the addition of MIP to MDT could reduce treatment time from 4–5 years to 2–3 years. Kaur et al. [145] and Kamal et al. [144] have similarly reported that MDT + MIP improves treatment outcomes. Due to the long incubation period of leprosy, long-term follow-up is needed for the safety and efficacy of shortening the treatment time.
Katoch et al. [157] reported a comparative study between MIP and BCG with MDT. They found that the patient groups receiving MDT and MIP, or BCG, had no detectable viable bacilli in the local and distal sites by mouse footpad analysis, whereas viable bacilli were detected in the patients on MDT alone within 2 years. Additionally, patients receiving MDT and MIP, or BCG, also had accelerated granuloma clearance. As with the previous studies, they also concluded that the addition of immunotherapy to achieve negative bacteriology could reduce treatment time by about 40% and found no relapses in the 10–12 years post-treatment follow-up. Interestingly, MIP did perform slightly better than BCG in bacterial and granuloma clearance. In contrast, Narang et al. [147] found that although MIP or BCG improved clinical outcomes, BCG performed better than MIP. However, immunisation by BCG on its own of close contacts of leprosy patients has been reported to precipitate PB leprosy on potentially asymptomatic infected or previously exposed individuals [158,159,160].
The addition of immunotherapy to patients under MDT generally shows positive clinical outcomes. What about close contacts of leprosy patients and transmission? It has been shown that a single dose of rifampicin (one of the drugs in the leprosy MDT) to close contacts of patients is 57% effective at preventing leprosy within 2 years, but with no effect after 2 years [161, 162]. Richardus et al. [163, 164] investigated whether chemoprophylaxis with rifampicin and immunoprophylaxis with BCG on contacts of leprosy patients could reduce transmission. Although they found a 42% reduction in PB leprosy cases of close contacts of leprosy patients in the first year, they noted that it was not statistically significant, due to low patient cases. Thus, more studies are needed to understand the clinical benefits of the combination of MIP or BCG with MDT on reducing transmission.
The Leprosy Post-Exposure Prophylaxis (LPEP) programme (Fig. 4.4), funded and coordinated by Novartis Foundation, launched in 2015, and ended in 2018, was established to explore contact tracing and to evaluate single-dose rifampicin post-exposure prophylaxis (SDR-PEP) to reduce and curb transmission in Brazil, Cambodia, India, Indonesia, Myanmar, Nepal, Sri Lanka, and Tanzania [165,166,167]. The programme outcome varied in countries that showed an increase in the number of detected cases in the first year but followed by a reduction in cases, indicating a reduction in leprosy incidence. Furthermore, a 2040 projection model indicates that LPEP could have a huge impact in interrupting M. leprae transmission. Future programmes to include immunotherapy may demonstrate greater impact.
Overall, the studies indicate that a combination of chemotherapy and immunotherapy is a powerful therapeutic intervention to treat leprosy patients and potentially as a control strategy to reduce transmission.
6 Conclusion and Vaccine Outlook
The current BCG vaccine for leprosy offers only partial protection. Leprosy is not eliminated, despite early ‘elimination’ declaration by WHO defined as ‘the reduction of prevalence to a level below one case per 10,000 population’ [168]. This has drawn major criticism, because it changed public perception and shifted away the resources and financial support needed to carry out fundamental and long-term epidemiological studies [169,170,171,172]. M. leprae remains a bacterium that requires animals for cultivation. We still do not exactly know how M. leprae transmission occurs, how it induces immune responses, and what is the mechanism underlying the nerve damage.
The recognition that BCG is a leprosy vaccine by the WHO is a critical admission that can help push current vaccine research forwards and support social changes. Historically, leprosy sufferers are stigmatised and discriminated against by their community [5, 173]. Unfortunately, stigma and discrimination are still happening today. According to the WHO, there are 127 discriminatory laws in 22 countries based on leprosy [5]. A widely recognised leprosy vaccine that is already in use can change the dialogues within communities and perceptions about the disease. Table 4.1 summarises the leprosy vaccines and strategies to reduce treatment time and transmission discussed in this chapter. The development of rBCGs, killed related mycobacteria, and subunit recombinant vaccine candidates is showing promise in clinical trials for the future, with an improved and effective leprosy vaccine as immunoprophylaxis, a supplement to chemotherapy, and post-exposure immunoprophylaxis.
Notes
- 1.
Rifampicin, clofazimine, and dapsone.
- 2.
Lepromin is a skin test to classify the type of leprosy. It is carried out by an intradermal injection of inactivated M. leprae extract to check if the body responds to the bacterial antigens. An early reaction within 48 h (Fernández reaction) of erythema and induration indicates tuberculoid leprosy. A late reaction at 3 weeks (Mitsuda reaction) of nodule and indurated lesion indicates borderline tuberculoid leprosy. A lepromatous leprosy patient will not have a positive reaction.
- 3.
Numerous classifications have been used over the years to recognise leprosy as a disease that can be characterised on a spectrum due to the different immune responses. There are two classification systems that are commonly used, the Ridley and Jopling classification and the WHO classification. The Ridley and Jopling classification of leprosy was proposed in 1966 and is based on clinical and histopathologic observations: polar tuberculoid (TT), borderline tuberculoid (BT), borderline (BB), borderline lepromatous (BL), subpolar lepromatous (LLs), and polar lepromatous (LLp). In 1982, the WHO simplified the classification with paucibacillary (PB) leprosy that correlates with TT and BT and multibacillary (MB) leprosy that correlates with BB, BL, and LL.
- 4.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the ‘coronavirus disease 2019’ (COVID-19) outbreak. Before an effective COVID-19 vaccine was made available for the public, existing approved vaccines were assessed for COVID-19 mitigation. The BCG vaccine has been found to train the immune system to fight off infections caused by viruses and therefore an attractive candidate as a COVID-19 vaccine [104,105,106,107,108,109]. However, there is no direct evidence that BCG provides protection against COVID-19 in humans [110,111,112,113]. In mice and hamster studies, BCG vaccination provided no protection against SARS-CoV-2 [114]. The phase III clinical trial is a small population group evaluation to determine if BCG can mitigate COVID-19, before moving to phase IV clinical trial with a large population group to assess safety and efficacy. Clinical trials to assess the efficacy of BCG vaccination against COVID-19 are being performed around the world (ClinicalTrials.gov).
- 5.
Previously M. welchii (M. w). It was renamed to MIP in 2009 after its lineage and to avoid confusion with M. tuberculosis-W Beijing.
- 6.
The latest WHO 2018 ‘Guidelines for the Diagnosis, Treatment and Prevention of Leprosy’ recommends ‘a 3-drug regimen of rifampicin, dapsone and clofazimine for all leprosy patients, with a duration of treatment of 6 months for PB leprosy and 12 months for MB leprosy’.
References
WHO. THE GLOBAL HEALTH OBSERVATORY: leprosy (Hansen’s disease). Geneva: World Health Organization; 2020.
Hansen GA. Undersøgelser Angående Spedalskhedens Årsager (Investigations concerning the etiology of leprosy). Norsk Mag Laegervidenskaben. 1874;4:1–88.
Chaptini C, Marshman G. Leprosy: a review on elimination, reducing the disease burden, and future research. Lepr Rev. 2015;86(4):307–15.
Nanjan Chandran SL, Tiwari A, Lustosa AA, Demir B, Bowers B, Albuquerque RGR, et al. Revised estimates of leprosy disability weights for assessing the global burden of disease: a systematic review and individual patient data meta-analysis. PLoS Negl Trop Dis. 2021;15(3):e0009209. https://doi.org/10.1371/journal.pntd.0009209.
WHO. Towards zero leprosy. Global leprosy (Hansen’s disease) strategy 2021–2030. Geneva: World Health Organization; 2021.
Cambau E, Saunderson P, Matsuoka M, Cole ST, Kai M, Suffys P, et al. Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009-15. Clin Microbiol Infect. 2018;24(12):1305–10. https://doi.org/10.1016/j.cmi.2018.02.022.
Williams DL, Araujo S, Stryjewska BM, Scollard D. Dapsone resistance in leprosy patients originally from American Samoa, United States, 2010-2012. Emerg Infect Dis. 2018;24(8):1584–5. https://doi.org/10.3201/eid2408.180033.
Rosa PS, D’Espindula HRS, Melo ACL, Fontes ANB, Finardi AJ, Belone AFF, et al. Emergence and transmission of drug-/multidrug-resistant mycobacterium leprae in a former leprosy colony in the Brazilian Amazon. Clin Infect Dis. 2020;70(10):2054–61. https://doi.org/10.1093/cid/ciz570.
WHO. BCG vaccine: WHO position paper, February 2018 - recommendations. Vaccine. 2018;36(24):3408–10. https://doi.org/10.1016/j.vaccine.2018.03.009.
Setia MS, Steinmaus C, Ho CS, Rutherford GW. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect Dis. 2006;6(3):162–70. https://doi.org/10.1016/S1473-3099(06)70412-1.
Zodpey SP. Protective effect of bacillus Calmette Guerin (BCG) vaccine in the prevention of leprosy: a meta-analysis. Indian J Dermatol Venereol Leprol. 2007;73(2):86–93. https://doi.org/10.4103/0378-6323.31891.
Merle CS, Cunha SS, Rodrigues LC. BCG vaccination and leprosy protection: review of current evidence and status of BCG in leprosy control. Expert Rev Vaccines. 2010;9(2):209–22. https://doi.org/10.1586/erv.09.161.
Glynn JR, Fielding K, Mzembe T, Sichali L, Banda L, McLean E, et al. BCG re-vaccination in Malawi: 30-year follow-up of a large, randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2021;9(10):e1451–9. https://doi.org/10.1016/S2214-109X(21)00309-0.
Calmette A. L’infection bacillaire et la tuberculose chez l’homme et chez animaux : processus d’infection et de defense; étude biologique et expérimentale. Paris: Masson; 1920.
Calmette A, Guérin C, Bouquet A, Nègre L. La vaccination préventive contre la tuberculose par le “BCG”. Paris: Masson; 1927.
Gillini L, Cooreman E, Wood T, Pemmaraju VR, Saunderson P. Global practices in regard to implementation of preventive measures for leprosy. PLoS Negl Trop Dis. 2017;11(5):e0005399. https://doi.org/10.1371/journal.pntd.0005399.
Fernandez JMM. [Comparative study of the Mitsuda reaction with the tuberculin reaction]. Revista Argentina dermatosifilis. 1939;23:425–53.
Campos DS. [Primary results of BCG in leprosy prophylaxis]. In: Memoria del VI Congreso Internacional de Leprologia, Madrid; 1953. p. 518–20.
De Souza CN. BCG in the prophylaxis of leprosy; a preliminary report. Int J Lepr. 1953;21(3):307–12.
Fernandez JM. Influence of the tuberculosis factor on the clinical and immunological evolution of child contacts with leprosy patients. Int J Lepr. 1955;23(3):243–58.
Convit J. Studies of leprosy in the German ethnic group of Colonia Tovar, Venezuela. V. The morbidity rates in BCG-vaccinated and unvaccinated groups during five years. Int J Lepr. 1956;24(3):269–74.
Yanagisawa K. On the immunological relationship between tuberculosis and leprosy with special reference to the effect of BCG administration upon the prophylaxis of leprosy. La Lepro. 1960;28:37–47.
Brown JA, Stone MM. B.C.G. vaccination of children against leprosy: first results of a trial in Uganda. Br Med J. 1966;1(5478):7–14. https://doi.org/10.1136/bmj.1.5478.7.
Brown JA, Stone MM, Sutherland I. B.C.G. vaccination of children against leprosy in Uganda: results at end of second follow-up. Br Med J. 1968;1(5583):24–7. https://doi.org/10.1136/bmj.1.5583.24.
Stanley SJ, Howland C, Stone MM, Sutherland I. BCG vaccination of children against leprosy in Uganda: final results. J Hyg (Lond). 1981;87(2):233–48. https://doi.org/10.1017/s002217240006945x.
Russell DA, Scott GC, Wigley SC. BCG vaccination in leprosy. A preliminary report of a “blind” controlled trial. Int J Lepr. 1964;32:235–45.
Bagshawe A, Scott GC, Russell DA, Wigley SC, Merianos A, Berry G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea, 1963-79. Bull World Health Organ. 1989;67(4):389–99.
Tripathy SP. The case for B.C.G. Ann Natl Acad Med Sci. 1983;19(1):11–21.
Gupte MD. Field trials of antileprosy vaccines. Indian J Lepr. 1998;70(4):363–7.
Zodpey SP, Shrikhande SN, Salodkar AD, Maldhure BR, Kulkarni SW. Effectiveness of bacillus Calmette-Guerin (BCG) vaccination in the prevention of leprosy; a case-finding control study in Nagpur, India. Int J Lepr Other Mycobact Dis. 1998;66(3):309–15.
Zodpey SP, Ambadekar NN, Thakur A. Effectiveness of Bacillus Calmette Guerin (BCG) vaccination in the prevention of leprosy: a population-based case-control study in Yavatmal District, India. Public Health. 2005;119(3):209–16. https://doi.org/10.1016/j.puhe.2004.04.007.
Zodpey SP, Shrikhande SN, Kulkarni SW, Maldhure BR. Scar size and effectiveness of Bacillus Calmette Guerin (BCG) vaccination in the prevention of tuberculosis and leprosy: a case-control study. Indian J Public Health. 2007;51(3):184–9.
Rahete NP, Zodpey SP, Kamble KM. Effectiveness of Bacillus Calmette Guerin (BCG) vaccination in the prevention of leprosy: a population-based case-control study in Raipur, India. Indian J Public Health. 2007;51(2):86–90.
Bechelli LM, Garbajosa G, Uemura K, Engler V, Martinez Dominguez V, Paredes L, et al. BCG vaccination of children against leprosy. Preliminary findings of the WHO-controlled trial in Burma. Bull World Health Organ. 1970;42(2):235–81.
Bechelli LM, Garbajosa PG, Gyi MM, Uemura K, Engler V, Dominguez VM, et al. BCG vaccination of children against leprosy. Preliminary findings of the WHO-controlled trial in Burma up to January 1970. Int J Lepr Other Mycobact Dis. 1971;39(2):609.
Bechelli LM, Garbajosa PG, Gyi MM, Uemura K, Sundaresan T, Martinez Dominguez V, et al. BCG vaccination of children against leprosy: seven-year findings of the controlled WHO trial in Burma. Bull World Health Organ. 1973;48(3):323–34.
Bechelli LM, Lwin K, Gallego Garbajosa P, Mg Mg G, Uemura K, Sundaresan T, et al. BCG vaccination of children against leprosy: nine-year findings of the controlled WHO trial in Burma. Bull World Health Organ. 1974;51(1):93–9.
Bertolli J, Pangi C, Frerichs R, Halloran ME. A case-control study of the effectiveness of BCG vaccine for preventing leprosy in Yangon, Myanmar. Int J Epidemiol. 1997;26(4):888–96. https://doi.org/10.1093/ije/26.4.888.
Fine PE, Ponnighaus JM, Maine N, Clarkson JA, Bliss L. Protective efficacy of BCG against leprosy in Northern Malawi. Lancet. 1986;2(8505):499–502. https://doi.org/10.1016/s0140-6736(86)90367-3.
Ponnighaus JM, Fine PE, Sterne JA, Wilson RJ, Msosa E, Gruer PJ, et al. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339(8794):636–9. https://doi.org/10.1016/0140-6736(92)90794-4.
Baker DM, Nguyen-Van-Tam JS, Smith SJ. Protective efficacy of BCG vaccine against leprosy in southern Malawi. Epidemiol Infect. 1993;111(1):21–5. https://doi.org/10.1017/s0950268800056636.
Orege PA, Fine PE, Lucas SB, Obura M, Okelo C, Okuku P. Case-control study of BCG vaccination as a risk factor for leprosy and tuberculosis in western Kenya. Int J Lepr Other Mycobact Dis. 1993;61(4):542–9.
Convit J, Smith PG, Zuniga M, Sampson C, Ulrich M, Plata JA, et al. BCG vaccination protects against leprosy in Venezuela: a case-control study. Int J Lepr Other Mycobact Dis. 1993;61(2):185–91.
Abel L, Cua VV, Oberti J, Lap VD, Due LK, Grosset J, et al. Leprosy and BCG in southern Vietnam. Lancet. 1990;335(8704):1536. https://doi.org/10.1016/0140-6736(90)93086-5.
Thuc NV, Abel L, Lap VD, Oberti J, Lagrange PH. Protective effect of BCG against leprosy and its subtypes: a case-control study in southern Vietnam. Int J Lepr Other Mycobact Dis. 1994;62(4):532–8.
de Matos HJ, Duppre N, Alvim MF, MachadoVieira LM, Sarno EN, Struchiner CJ. [Leprosy epidemiology in a cohort of household contacts in Rio de Janeiro (1987-1991)]. Cad Saude Publica. 1999;15(3):533–42. https://doi.org/10.1590/s0102-311x1999000300010.
Cunha SS, Rodrigues LC, Pedrosa V, Dourado IM, Barreto ML, Pereira SM. Neonatal BCG protection against leprosy: a study in Manaus, Brazilian Amazon. Lepr Rev. 2004;75(4):357–66.
Düppre NC, Camacho LA, da Cunha SS, Struchiner CJ, Sales AM, Nery JA, et al. Effectiveness of BCG vaccination among leprosy contacts: a cohort study. Trans R Soc Trop Med Hyg. 2008;102(7):631–8. https://doi.org/10.1016/j.trstmh.2008.04.015.
Goulart IM, Bernardes Souza DO, Marques CR, Pimenta VL, Goncalves MA, Goulart LR. Risk and protective factors for leprosy development determined by epidemiological surveillance of household contacts. Clin Vaccine Immunol. 2008;15(1):101–5. https://doi.org/10.1128/CVI.00372-07.
Rodrigues ML, Silva SA, Neto JC, de Andrade AL, Martelli CM, Zicker F. Protective effect of intradermal BCG against leprosy; a case-control study in central Brazil. Int J Lepr Other Mycobact Dis. 1992;60(3):335–9.
Lombardi C, Pedrazzani ES, Pedrazzani JC, Ferreira Filho P, Zicker F. [The protective efficacy of BCG against leprosy in Sao Paulo, Brazil]. Bol Oficina Sanit Panam. 1995;119(5):415–21.
Lombardi C, Pedrazzani ES, Pedrazzani JC, Filho PF, Zicker F. Protective efficacy of BCG against leprosy in Sao Paulo. Bull Pan Am Health Organ. 1996;30(1):24–30.
Kerr-Pontes LR, Barreto ML, Evangelista CM, Rodrigues LC, Heukelbach J, Feldmeier H. Socioeconomic, environmental, and behavioural risk factors for leprosy in north-east Brazil: results of a case-control study. Int J Epidemiol. 2006;35(4):994–1000. https://doi.org/10.1093/ije/dyl072.
Boelens JJ, Kroes R, van Beers S, Lever P. Protective effect of BCG against leprosy in South Sulawesi, Indonesia. Int J Lepr Other Mycobact Dis. 1995;63(3):456–7.
World Health Organization. WHO Expert Committee on biological standardization. World Health Organ Tech Rep Ser. 2013;(979):1–366, back cover.
Convit J, Sampson C, Zuniga M, Smith PG, Plata J, Silva J, et al. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet. 1992;339(8791):446–50. https://doi.org/10.1016/0140-6736(92)91056-e.
Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi, Karonga Prevention Trial Group. Lancet. 1996;348(9019):17–24.
Gupte MD, Vallishayee RS, Anantharaman DS, Nagaraju B, Sreevatsa, Balasubramanyam S, et al. Comparative leprosy vaccine trial in south India. Indian J Lepr. 1998;70(4):369–88.
Stanford JL, Shield MJ, Rook GA. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle. 1981;62(1):55–62. https://doi.org/10.1016/0041-3879(81)90037-4.
Price DN, Kusewitt DF, Lino CA, McBride AA, Muttil P. Oral tolerance to environmental Mycobacteria interferes with intradermal, but not pulmonary, immunization against tuberculosis. PLoS Pathog. 2016;12(5):e1005614. https://doi.org/10.1371/journal.ppat.1005614.
Demangel C, Garnier T, Rosenkrands I, Cole ST. Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect Immun. 2005;73(4):2190–6. https://doi.org/10.1128/IAI.73.4.2190-2196.2005.
Mehta KP, Merchant SM, Korde U. Environmental influence on immunity due to B.C.G. vaccination. Indian Pediatr. 1976;13(7):525–32.
Beverley P, Ronan E, Lee L, Arnold I, Bolinger B, Powrie F, et al. Environmental effects on protection against Mycobacterium tuberculosis after immunization with Ad85A. Vaccine. 2013;31(7):1086–93. https://doi.org/10.1016/j.vaccine.2012.12.024.
Andersen P, Doherty TM. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3(8):656–62. https://doi.org/10.1038/nrmicro1211.
von Reyn CF. BCG, latitude, and environmental mycobacteria. Clin Infect Dis. 2014;59(4):608. https://doi.org/10.1093/cid/ciu331.
Van Truoc L, Ly HM, Thuy NK, Trach DD, Stanford CA, Stanford JL. Vaccination against leprosy at Ben San Leprosy Centre, Ho Chi Minh City, Vietnam. Vaccine. 2001;19(25–26):3451–8. https://doi.org/10.1016/S0264-410x(01)00052-4.
Colston MJ, Hilson GR. Growth of Mycobacterium leprae and M. marinum in congenitally athymic (nude) mice. Nature. 1976;262(5567):399–401. https://doi.org/10.1038/262399a0.
Levy L, Ji B. The mouse foot-pad technique for cultivation of Mycobacterium leprae. Lepr Rev. 2006;77(1):5–24.
Shepard CC. The experimental disease that follows the injection of human leprosy bacilli into foot-pads of mice. J Exp Med. 1960;112(3):445–54. https://doi.org/10.1084/jem.112.3.445.
Kirchheimer WF, Storrs EE. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int J Lepr Other Mycobact Dis. 1971;39(3):693–702.
Truman RW, Ebenezer GJ, Pena MT, Sharma R, Balamayooran G, Gillingwater TH, et al. The Armadillo as a model for peripheral neuropathy in leprosy. ILAR J. 2014;54(3):304–14. https://doi.org/10.1093/ilar/ilt050.
Storrs EE, Walsh GP, Burchfield HP, Binford CH. Leprosy in the Armadillo: new model for biomedical research. Science. 1974;183(4127):851–2. https://doi.org/10.1126/science.183.4127.851.
Stover CK, Delacruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, et al. New use of BCG for recombinant vaccines. Nature. 1991;351(6326):456–60. https://doi.org/10.1038/351456a0.
Nieuwenhuizen NE, Kaufmann SHE. Next-generation vaccines based on Bacille Calmette-Guerin. Front Immunol. 2018;9:121. https://doi.org/10.3389/fimmu.2018.00121.
Abarca K, Rey-Jurado E, Munoz-Durango N, Vazquez Y, Soto JA, Galvez NMS, et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine. 2020;27:100517. https://doi.org/10.1016/j.eclinm.2020.100517.
Bueno SM, Gonzalez PA, Cautivo KM, Mora JE, Leiva ED, Tobar HE, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci U S A. 2008;105(52):20822–7. https://doi.org/10.1073/pnas.0806244105.
Palavecino CE, Cespedes PF, Gomez RS, Kalergis AM, Bueno SM. Immunization with a recombinant Bacillus Calmette-Guerin strain confers protective Th1 immunity against the human metapneumovirus. J Immunol. 2014;192(1):214–23. https://doi.org/10.4049/jimmunol.1300118.
Fennelly GJ, Flynn JL, Termeulen V, Liebert UG, Bloom BR. Recombinant Bacille Calmette-Guerin priming against measles. J Infect Dis. 1995;172(3):698–705. https://doi.org/10.1093/infdis/172.3.698.
Honda M, Matsuo K, Nakasone T, Okamoto Y, Yoshizaki H, Kitamura K, et al. Protective immune-responses induced by secretion of a chimeric soluble-protein from a recombinant Mycobacterium-Bovis Bacillus-Calmette-Guerin vector candidate vaccine for human-immunodeficiency-virus Type-1 in small animals. Proc Natl Acad Sci U S A. 1995;92(23):10693–7. https://doi.org/10.1073/pnas.92.23.10693.
Chapman R, Chege G, Shephard E, Stutz H, Williamson AL. Recombinant Mycobacterium bovis BCG as an HIV vaccine vector. Curr HIV Res. 2010;8(4):282–98. https://doi.org/10.2174/157016210791208686.
Chade DC, Borra RC, Nascimento IP, Villanova FE, Leite LCC, Andrade E, et al. Immunomodulatory effects of recombinant BCG expressing pertussis toxin on TNF-alpha and IL-10 in a bladder cancer model. J Exp Clin Cancer Res. 2008;27:78. https://doi.org/10.1186/1756-9966-27-78.
Singh AK, Srikrishna G, Bivalacqua TJ, Bishai WR. Recombinant BCGs for tuberculosis and bladder cancer. Vaccine. 2021;39:7321. https://doi.org/10.1016/j.vaccine.2021.09.040.
Connell ND, Medinaacosta E, Mcmaster WR, Bloom BR, Russell DG. Effective immunization against cutaneous leishmaniasis with recombinant Bacille Calmette-Guerin expressing the leishmania surface proteinase Gp63. Proc Natl Acad Sci U S A. 1993;90(24):11473–7. https://doi.org/10.1073/pnas.90.24.11473.
Teo WH, Nurul AA, Norazmi MN. Immunogenicity of recombinant BCG-based vaccine expressing the 22 kDa of serine repeat antigen (SE22) of Plasmodium falciparum. Trop Biomed. 2012;29(2):239–53.
Matsumoto S, Yukitake H, Kanbara H, Yamada T. Recombinant Mycobacterium bovis bacillus Calmette-Guerin secreting merozoite surface protein 1 (MSP1) induces protection against rodent malaria parasite infection depending on MSP1-stimulated interferon gamma and parasite-specific antibodies. J Exp Med. 1998;188(5):845–54. https://doi.org/10.1084/jem.188.5.845.
Yu QL, Huang XS, Gong PT, Zhang Q, Li JH, Zhang GC, et al. Protective immunity induced by a recombinant BCG vaccine encoding the cyclophilin gene of toxoplasma gondii. Vaccine. 2013;31(51):6065–71. https://doi.org/10.1016/j.vaccine.2013.10.015.
Langermann S, Palaszynski SR, Burlein JE, Koenig S, Hanson MS, Briles DE, et al. Protective humoral response against pneumococcal infection in mice elicited by recombinant Bacille Calmette-Guerin vaccines expressing pneumococcal surface protein-A. J Exp Med. 1994;180(6):2277–86. https://doi.org/10.1084/jem.180.6.2277.
Stover CK, Bansal GP, Hanson MS, Burlein JE, Palaszynski SR, Young JF, et al. Protective immunity elicited by recombinant Bacille Calmette-Guerin (Bcg) expressing outer surface protein-A (OspA) lipoprotein - a candidate Lyme-disease vaccine. J Exp Med. 1993;178(1):197–209. https://doi.org/10.1084/jem.178.1.197.
Nascimento IP, Dias WO, Mazzantini RP, Miyaji EN, Gamberini M, Quintilio W, et al. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect Immun. 2000;68(9):4877–83. https://doi.org/10.1128/Iai.68.9.4877-4883.2000.
Medeiros MA, Armoa GRG, Dellagostin OA, McIntosh D. Induction of humoral immunity in response to immunization with recombinant Mycobacterium bovis BCG expressing the S1 subunit of Bordetella pertussis toxin. Can J Microbiol. 2005;51(12):1015–20. https://doi.org/10.1139/W05-095.
Kanno AI, Goulart C, Leite LCC, Pagliarone AC, Nascimento IP. A bivalent recombinant Mycobacterium bovis BCG expressing the S1 subunit of the pertussis toxin induces a polyfunctional CD4(+) T cell immune response. Biomed Res Int. 2019;2019:9630793. https://doi.org/10.1155/2019/9630793.
Ohara N, Matsuoka M, Nomaguchi H, Naito M, Yamada T. Inhibition of multiplication of Mycobacterium leprae in mouse foot pads by recombinant Bacillus Catmette-Guerin (BCG). Vaccine. 2000;18(14):1294–7. https://doi.org/10.1016/S0264-410x(99)00420-X.
Ohara N, Matsuoka M, Nomaguchi H, Naito M, Yamada T. Protective responses against experimental Mycobacterium leprae infection in mice induced by recombinant Bacillus Calmette-Guerin over-producing three putative protective antigen candidates. Vaccine. 2001;19(15–16):1906–10. https://doi.org/10.1016/S0264-410x(00)00439-4.
Makino M, Maeda Y, Ishii N. Immunostimulatory activity of major membrane protein-II from Mycobacterium leprae. Cell Immunol. 2005;233(1):53–60. https://doi.org/10.1016/j.cellimm.2005.04.001.
Makino M, Maeda Y, Inagaki K. Immunostimulatory activity of recombinant Mycobacterium bovis BCG that secretes major membrane protein II of Mycobacterium leprae. Infect Immun. 2006;74(11):6264–71. https://doi.org/10.1128/Iai.00878-06.
Makino M, Maeda Y, Kai M, Tamura T, Mukai T. GM-CSF-mediated T-cell activation by macrophages infected with recombinant BCG that secretes major membrane protein-II of Mycobacterium leprae. FEMS Immunol Med Microbiol. 2009;55(1):39–46. https://doi.org/10.1111/j.1574-695X.2008.00495.x.
Maeda Y, Tamura T, Matsuoka M, Makino M. Inhibition of the multiplication of Mycobacterium leprae by vaccination with a recombinant M. bovis BCG strain that secretes major membrane protein II in mice. Clin Vaccine Immunol. 2009;16(10):1399–404. https://doi.org/10.1128/Cvi.00203-09.
Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15(6):388–400. https://doi.org/10.1038/nri3839.
Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67(6):3087–95. https://doi.org/10.1128/Iai.67.6.3087-3095.1999.
Tabouret G, Astarie-Dequeker C, Demangel C, Malaga W, Constant P, Ray A, et al. Mycobacterium leprae phenolglycolipid-1 expressed by engineered M. bovis BCG modulates early interaction with human phagocytes. PLoS Pathog. 2010;6(10):e1001159. https://doi.org/10.1371/journal.ppat.1001159.
Doz-Deblauwe E, Carreras F, Arbues A, Remot A, Epardaud M, Malaga W, et al. CR3 engaged by PGL-I triggers Syk-calcineurin-NFATc to rewire the innate immune response in leprosy. Front Immunol. 2019;10:2913. https://doi.org/10.3389/fimmu.2019.02913.
Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A. 2000;97(25):13853–8. https://doi.org/10.1073/pnas.250480397.
Gillis TP, Tullius MV, Horwitz MA. rBCG30-induced immunity and cross-protection against Mycobacterium leprae challenge are enhanced by boosting with the Mycobacterium tuberculosis 30-kilodalton antigen 85B. Infect Immun. 2014;82(9):3900–9. https://doi.org/10.1128/Iai.01499-13.
Spencer JC, Ganguly R, Waldman RH. Nonspecific protection of mice against influenza-virus infection by local or systemic immunization with Bacille Calmette-Guerin. J Infect Dis. 1977;136(2):171–5. https://doi.org/10.1093/infdis/136.2.171.
Stensballe LG, Nante E, Jensen IP, Kofoed PE, Poulsen A, Jensen H, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23(10):1251–7. https://doi.org/10.1016/j.vaccine.2004.09.006.
Wardhana, Datau EA, Sultana A, Mandang VV, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;43(3):185–90.
Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. https://doi.org/10.1126/science.aaf1098.
Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang SY, Oosting M, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100.e5. https://doi.org/10.1016/j.chom.2017.12.010.
Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–8. https://doi.org/10.1016/j.cmi.2019.04.020.
O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20(6):335–7. https://doi.org/10.1038/s41577-020-0337-y.
WHO. Bacille Calmette-Guérin (BCG) vaccination and COVID-19. WHO Headquarters (HQ), WHO Worldwide; 2020.
Li JL, Zhan LJ, Qin C. The double-sided effects of Mycobacterium Bovis bacillus Calmette-Guerin vaccine. NPJ Vaccines. 2021;6(1):14. https://doi.org/10.1038/s41541-020-00278-0.
Lobo N, Brooks NA, Zlotta AR, Cirillo JD, Boorjian S, Black PC, et al. 100 years of Bacillus Calmette-Guerin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021;18(10):611–22. https://doi.org/10.1038/s41585-021-00481-1.
Kaufmann E, Khan N, Tran KA, Ulndreaj A, Pernet E, Fontes G, et al. BCG vaccination provides protection against IAV but not SARS-CoV-2. Cell Rep. 2022;38:110502. https://doi.org/10.1016/j.celrep.2022.110502.
Geoffroy C, Gaillard JL, Alouf JE, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin-O from Listeria-monocytogenes. Infect Immun. 1987;55(7):1641–6. https://doi.org/10.1128/Iai.55.7.1641-1646.1987.
Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Eddine AN, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis Bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115(9):2472–9. https://doi.org/10.1172/Jci24617.
Farinacci M, Weber S, Kaufmann SHE. The recombinant tuberculosis vaccine rBCG Delta ureC::hly(+) induces apoptotic vesicles for improved priming of CD4(+) and CD8(+) T cells. Vaccine. 2012;30(52):7608–14. https://doi.org/10.1016/j.vaccine.2012.10.031.
Talwar GP, Ahmed N, Saini V. The use of the name Mycobacterium w for the leprosy immunotherapeutic bacillus creates confusion with M-tuberculosis-W (Beijing strain): a suggestion. Infect Genet Evol. 2008;8(1):100–1. https://doi.org/10.1016/j.meegid.2007.07.009.
Godal T, Myrvang B, Stanford JL, Samuel DR. Recent advances in the immunology of leprosy with special reference to new approaches in immunoprophylaxis. Bull Inst Pasteur. 1974;72(3):273–310.
Shepard CC, Vanlandingham R, Walker LL. Immunity to Mycobacterium-leprae infections in mice stimulated by M-Leprae, Bcg, and graft versus host reactions. Infect Immun. 1976;14(4):919–28. https://doi.org/10.1128/Iai.14.4.919-928.1976.
Singh NB, Srivastava, Gupta HP, Sreevatsa, Desikan KV. Immunological potential of a cultivable mycobacterial strain M. habana against leprosy bacillus in mouse foot pad. Indian J Lepr. 1985;57(2):278–81.
Singh NB, Lowe AC, Rees RJ, Colston MJ. Vaccination of mice against Mycobacterium leprae infection. Infect Immun. 1989;57(2):653–5. https://doi.org/10.1128/iai.57.2.653-655.1989.
Singh NB, Srivastava A, Gupta HP, Kumar A, Srivastava S. Induction of lepromin positivity in monkeys by a candidate antileprosy vaccine: Mycobacterium habana. Int J Lepr Other Mycobact Dis. 1991;59(2):317–20.
Chaturvedi V, Singh NB, Sinha S, Immunoreactive antigens of a candidate leprosy vaccine: Mycobacterium habana. Lepr Rev. 1995;66(1):31–8. https://doi.org/10.5935/0305-7518.19950005.
Singh NB, Srivastava K, Malaviya B, Kandpal H, Srivastava A, Gupta HP. The 65 kDa protein of Mycobacterium habana and its putative role in immunity against experimental tuberculosis. Immunol Cell Biol. 1995;73(4):372–6. https://doi.org/10.1038/icb.1995.57.
Bisht D, Mehrotra J, Dhindsa MS, Singh NB, Sinha S. A major T-cell-inducing cytosolic 23 kDa protein antigen of the vaccine candidate Mycobacterium habana is superoxide dismutase. Microbiology (Reading). 1996;142(Pt 6):1375–83. https://doi.org/10.1099/13500872-142-6-1375.
Serafin-Lopez J, Talavera-Paulin M, Amador-Molina JC, Alvarado-Riveron M, Vilchis-Landeros MM, Mendez-Ortega P, et al. Enoyl-coenzyme a hydratase and antigen 85B of Mycobacterium habana are specifically recognized by antibodies in sera from leprosy patients. Clin Vaccine Immunol. 2011;18(7):1097–103. https://doi.org/10.1128/Cvi.00519-10.
Wakhlu A, Gaur SPS, Kaushal GP, Misra A, Asthana OP, Sircar AR. Response of Mycobacterium habana vaccine in patients with lepromatous leprosy and their household contacts. A pilot clinical study. Lepr Rev. 2001;72(2):179–91.
Bapat CV, Ranadive KJ, Khanolkar VR. Growth characteristics of an acid-fast Mycobacterium isolated from human lepromatous leprosy. Int J Lepr. 1961;29:329–42.
Ranadive KJ, Nerurkar RV, Khanolkar VR. In vitro studies on human leprosy. I. Indian J Med Sci. 1958;12(10):791–6.
Bapat CV. Immunological properties of M. leprae culture isolates ICRC bacilli: hypothesis on relationship between M. leprae and ML-culture isolates. Acta Leprol. 1984;2(2–4):175–94.
Gangal SG, Khanolkar SR. Delayed hypersensitivity in vitro to an acid fast mycobacterium cultivated from human lepromatous leprosy. Indian J Med Res. 1974;62(2):290–6.
Girdhar BK, Desikan KV. Results of skin tests with five different mycobacteria. Lepr India. 1978;50(4):555–9.
Mustafa AS, Talwar GP. Five cultivable mycobacterial strains giving blast transformation and leukocyte migration inhibition of leukocytes analogous to mycobacterium leprae. Lepr India. 1978;50(4):498–508.
Bhide MB, Pradhan KS, Bapat CV. A vaccine from ICRC bacilli against M. leprae infection in mouse foot-pad. Lepr India. 1978;50(3):334–44.
Deo MG, Bapat CV, Bhalerao V, Chaturvedi RM, Bhatki WS, Chulawala RG. Antileprosy potentials of ICRC vaccine. A study in patients and healthy volunteers. Int J Lepr Other Mycobact Dis. 1983;51(4):540–9.
Bhatki WS, Chulawala RG, Bapat CV, Deo MG. Reversal reaction in lepromatous patients induced by a vaccine containing killed ICRC bacilli—a report of five cases. Int J Lepr Other Mycobact Dis. 1983;51(4):466–72.
Chaturvedi RM, Chirmule NB, Yellapurkar MV, Shaikh SU, Deo MG. Effects of Icrc antileprosy vaccine in healthy-subjects. Int J Leprosy. 1987;55(4):657–66.
Yadav AR, Mohanty K, Sengupta U. ICRC bacillus a vaccine candidate strain (C-44) is coated with human IgG. Open J Immunol. 2017;7:45–50.
Chaudhuri S, Fotedar A, Talwar GP. Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium w. Int J Lepr Other Mycobact Dis. 1983;51(2):159–68.
Talwar GP, Fotedar A. Two candidate antileprosy vaccines—current status of their development. Int J Lepr Other Mycobact Dis. 1983;51(4):550–2.
Sharma P, Mukherjee R, Talwar GP, Sarathchandra KG, Walia R, Parida SK, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76(2):127–43.
Kamal R, Natrajan M, Katoch K, Arora M. Clinical and histopathological evaluation of the effect of addition of immunotherapy with Mw vaccine to standard chemotherapy in borderline leprosy. Indian J Lepr. 2012;84(4):287–306.
Kamal R, Pathak V, Kumari A, Natrajan M, Katoch K, Kar HK. Addition of Mycobacterium indicus pranii vaccine as an immunotherapeutic to standard chemotherapy in borderline leprosy: a double-blind study to assess clinical improvement (preliminary report). Br J Dermatol. 2017;176(5):1388–9. https://doi.org/10.1111/bjd.14971.
Kaur I, Dogra S, Kumar B, Radotra BD. Combined 12-month WHO/MDT MB regimen and Mycobacterium w. vaccine in multibacillary leprosy: a follow-up of 136 patients. Int J Lepr Other Mycobact Dis. 2002;70(3):174–81.
De Sarkar A, Kaur I, Radotra BD, Kumar B. Impact of combined Mycobacterium w vaccine and 1 year of MDT on multibacillary leprosy patients. Int J Lepr Other Mycobact Dis. 2001;69(3):187–94.
Narang T, Kaur I, Kumar B, Radotra BD, Dogra S. Comparative evaluation of immunotherapeutic efficacy of BCG and mw vaccines in patients of borderline lepromatous and lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2005;73(2):105–14.
Talwar GPSP, Atrey N, Gupta JC. Making of a highly useful multipurpose vaccine. J Transl Med. 2016;1(3):69–73.
Talwar GP, Gupta JC, Mustafa AS, Kar HK, Katoch K, Parida SK, et al. Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics. 2017;11:55–63. https://doi.org/10.2147/BTT.S128308.
Duthie MS, Casper C, Reed SG. Second coming: the re-emergence and modernization of immunotherapy by vaccines as a component of leprosy control. Future Microbiol. 2018;13:1449–51. https://doi.org/10.2217/fmb-2018-0186.
Talwar GPGJ. Launching of immunization with the vaccine Mycobacterium indicus pranii for eradication of leprosy in India. Int J Vaccine Res. 2017;2(3):1–5.
Duthie MS, Pena MT, Ebenezer GJ, Gillis TP, Sharma R, Cunningham K, et al. LepVax, a defined subunit vaccine that provides effective pre-exposure and post-exposure prophylaxis of M. leprae infection. NPJ Vaccines. 2018;3:12. https://doi.org/10.1038/s41541-018-0050-z.
Duthie MS, Frevol A, Day T, Coler RN, Vergara J, Rolf T, et al. A phase 1 antigen dose escalation trial to evaluate safety, tolerability and immunogenicity of the leprosy vaccine candidate LepVax (LEP-F1 + GLA-SE) in healthy adults. Vaccine. 2020;38(7):1700–7. https://doi.org/10.1016/j.vaccine.2019.12.050.
Talwar GP, Zaheer SA, Mukherjee R, Walia R, Misra RS, Sharma AK, et al. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine. 1990;8(2):121–9. https://doi.org/10.1016/0264-410x(90)90134-8.
Zaheer SA, Mukherjee R, Ramkumar B, Misra RS, Sharma AK, Kar HK, et al. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J Infect Dis. 1993;167(2):401–10. https://doi.org/10.1093/infdis/167.2.401.
Sharma P, Misra RS, Kar HK, Mukherjee A, Poricha D, Kaur H, et al. Mycobacterium w vaccine, a useful adjuvant to multidrug therapy in multibacillary leprosy: a report on hospital based immunotherapeutic clinical trials with a follow-up of 1–7 years after treatment. Lepr Rev. 2000;71(2):179–92. https://doi.org/10.5935/0305-7518.20000020.
Katoch K, Katoch VM, Natrajan M, Sreevatsa, Gupta UD, Sharma VD, et al. 10-12 years follow-up of highly bacillated BL/LL leprosy patients on combined chemotherapy and immunotherapy. Vaccine. 2004;22(27–28):3649–57. https://doi.org/10.1016/j.vaccine.2004.03.037.
Duppre NC, Camacho LA, Sales AM, Illarramendi X, Nery JA, Sampaio EP, et al. Impact of PGL-I seropositivity on the protective effect of BCG vaccination among leprosy contacts: a cohort study. PLoS Negl Trop Dis. 2012;6(6):e1711. https://doi.org/10.1371/journal.pntd.0001711.
Richardus RA, Butlin CR, Alam K, Kundu K, Geluk A, Richardus JH. Clinical manifestations of leprosy after BCG vaccination: an observational study in Bangladesh. Vaccine. 2015;33(13):1562–7. https://doi.org/10.1016/j.vaccine.2015.02.017.
van Hooij A, van den Eeden SJF, Khatun M, Soren S, Franken KLMC, Chandra Roy J, et al. BCG-induced immunity profiles in household contacts of leprosy patients differentiate between protection and disease. Vaccine. 2021;39:7230.
Moet FJ, Pahan D, Oskam L, Richardus JH, Group CS. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: cluster randomised controlled trial. BMJ. 2008;336(7647):761–4. https://doi.org/10.1136/bmj.39500.885752.BE.
Feenstra SG, Pahan D, Moet FJ, Oskam L, Richardus JH. Patient-related factors predicting the effectiveness of rifampicin chemoprophylaxis in contacts: 6 year follow up of the COLEP cohort in Bangladesh. Lepr Rev. 2012;83(3):292–304.
Richardus RA, Alam K, Pahan D, Feenstra SG, Geluk A, Richardus JH. The combined effect of chemoprophylaxis with single dose rifampicin and immunoprophylaxis with BCG to prevent leprosy in contacts of newly diagnosed leprosy cases: a cluster randomized controlled trial (MALTALEP study). BMC Infect Dis. 2013;13:456. https://doi.org/10.1186/1471-2334-13-456.
Richardus R, Alam K, Kundu K, Chandra Roy J, Zafar T, Chowdhury AS, et al. Effectiveness of single-dose rifampicin after BCG vaccination to prevent leprosy in close contacts of patients with newly diagnosed leprosy: a cluster randomized controlled trial. Int J Infect Dis. 2019;88:65–72. https://doi.org/10.1016/j.ijid.2019.08.035.
Barth-Jaeggi T, Steinmann P, Mieras L, van Brakel W, Richardus JH, Tiwari A, et al. Leprosy post-exposure prophylaxis (LPEP) programme: study protocol for evaluating the feasibility and impact on case detection rates of contact tracing and single dose rifampicin. BMJ Open. 2016;6(11):e013633. https://doi.org/10.1136/bmjopen-2016-013633.
Richardus JH, Tiwari A, Barth-Jaeggi T, Arif MA, Banstola NL, Baskota R, et al. Leprosy post-exposure prophylaxis with single-dose rifampicin (LPEP): an international feasibility programme. Lancet Glob Health. 2021;9(1):e81–90. https://doi.org/10.1016/S2214-109X(20)30396-X.
Blok DJ, Steinmann P, Tiwari A, Barth-Jaeggi T, Arif MA, Banstola NL, et al. The long-term impact of the leprosy post-exposure prophylaxis (LPEP) program on leprosy incidence: a modelling study. PLoS Negl Trop Dis. 2021;15(3):e0009279. https://doi.org/10.1371/journal.pntd.0009279.
WHO. In: Diseases CoNT, editor. The final push strategy to eliminate leprosy as a public health problem. Geneva: World Health Organization; 2003. p. 31.
Lockwood DN, Shetty V, Penna GO. Hazards of setting targets to eliminate disease: lessons from the leprosy elimination campaign. BMJ. 2014;348:g1136. https://doi.org/10.1136/bmj.g1136.
Smith WC, van Brakel W, Gillis T, Saunderson P, Richardus JH. The missing millions: a threat to the elimination of leprosy. PLoS Negl Trop Dis. 2015;9(4):e0003658. https://doi.org/10.1371/journal.pntd.0003658.
Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. https://doi.org/10.1186/s13071-015-1143-4.
The Lancet Infectious Disease. Leprosy elimination not yet in sight. Lancet Infect Dis. 2005;5(6):321. https://doi.org/10.1016/S1473-3099(05)70117-1.
van Brakel WH, Sihombing B, Djarir H, Beise K, Kusumawardhani L, Yulihane R, et al. Disability in people affected by leprosy: the role of impairment, activity, social participation, stigma and discrimination. Glob Health Action. 2012;5:18394. https://doi.org/10.3402/gha.v5i0.18394.
Acknowledgements
H.W. gratefully acknowledges the support from the University of Glasgow pump-prime funds to develop an independent research career and the research funding support from The Royal Society (Research Grant: RGS\R2\192167) and The Academy of Medical Sciences (Springboard: SBF007\100061). The Springboard scheme is supported by the Wellcome Trust; the British Heart Foundation; the Government Department of Business, Energy, and Industrial Strategy; and Diabetes UK.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Wang, H. (2023). Leprosy Vaccines: Developments for Prevention and Treatment. In: Christodoulides, M. (eds) Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges . Springer, Cham. https://doi.org/10.1007/978-3-031-24355-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-24355-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24354-7
Online ISBN: 978-3-031-24355-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)