Abstract
Anti-PD-1 immune checkpoint inhibitors have recently revolutionized the treatment of recurrent and/or metastatic head and neck squamous cell carcinoma (HNSCC) patients, both in the first and second recurrent and metastatic settings. However, not all patients respond to PD-1 blockade, nor derive prolonged benefit from these immunotherapies, requiring further development of immune-oncology strategies beyond PD-1/PD-L1 inhibitors. There has been an important therapeutic development with the evaluation of many new immune checkpoints molecules and other type of immunomodulatory molecules, along with combinations of these new agents with PD-1/PD-L1 inhibitors, but very few of these strategies have shown significant anti-tumor activity as single agent in HNSCC patients, and further results are awaited from ongoing trials. All randomized trials assessing novel immune-oncology drugs in combination with an anti-PD1/PD-L1 agents have failed so far in HNSCC patients. Many other immune-oncology drugs are still in early clinical development and will hopefully improve HNSCC patient outcomes.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
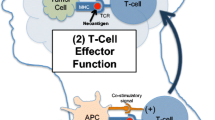
Tumor cells can induce T-cell immune tolerance via engagement of coinhibitory immune checkpoints molecules like cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or programmed cell death 1 (PD-1), leading to the escape from tumor-specific T-cell response and tumor progression [1, 2]. Therapeutic strategies to enhance cancer-specific T-cell immune response have been developed by inhibiting these specific coinhibitory immune checkpoints and re-activating T cells, such as anti-CTLA-4 or anti-PD-1/PD-L1 fully human monoclonal antibodies (MoAb) [1, 2].
Immune checkpoint inhibitors made a breakthrough in medical oncology in many different types of tumors. Regarding the treatment of recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) patients, two anti-PD-1 immune checkpoint inhibitors have been approved in the second-line setting for patients who have failed platinum-based therapy, i.e. pembrolizumab which received FDA approval in August 2016 in patients with PD-L1 positive tumors (defined by a tumor proportion score [TPS] ≥ 50%) and nivolumab in November 2016 regardless of the PD-L1 status [3, 4].
In the first-line R/M-HNSCC setting, it has been more than a decade that no new treatment option showed a survival benefit in comparison to the standard of care (SOC) EXTREME regimen (platinum/fluorouracil plus cetuximab) [5]. Recently, the results from the KEYNOTE-048 study changed the paradigm in that setting [6]. The KEYNOTE-048 study assessed the efficacy of pembrolizumab alone or in combination with platinum/fluorouracil-based chemotherapy versus the SOC EXTREME regimen in previously untreated patients with R/M-HNSCC and showed improvement in overall survival (OS) in both pembrolizumab arms compared to the EXTREME regimen in patients with PD-L1 positive tumors, defined by a combined positive score (CPS) ≥ 1 [6].
However, despite these encouraging results and impressive durable responses in a minority of patients, not all patients in the three above mentioned studies derived benefit from anti-PD-1 immune checkpoints inhibitors. In fact, observed overall response rates (ORR) ranged from 13 to 19% in the anti-PD-1 monotherapy arms in these three studies, and OS still remained poor [3, 4, 6, 7] (Table 5.1). Furthermore, although the majority of HNSCC patients have PD-L1 positive tumors (approximately 85%), around 15% of patients present PD-L1 negative tumors for whom SOC EXTREME is still indicated and for whom novel treatment options are urgently needed [6, 7]. This emphasizes the need to improve immunotherapy strategies for HNSCC patients beyond PD-1/PD-L1 inhibitors.
We will further discuss in this review the novel immunotherapy strategies in development beyond PD1/PD-L1 in head and neck cancer.
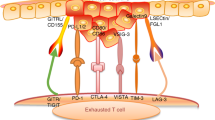
Targeting Other Immune Checkpoints
Several strategies have been assessed to further improve T-cell priming beyond the PD-1/PD-L1 blockade, either for patients who progressed under anti-PD-1/PD-L1 treatment, or to assess new immunotherapy strategies in immune checkpoint inhibitors naïve patients. Various novel antibodies targeting immune checkpoints have been investigated alone or in combination with anti-PD-1 agents, all showing disappointing results.
Several studies assessed the combination of an anti-CTLA4 MoAb with an anti-PD-1/PD-L1 agent in patients with R/M-HNSCC. In the first-line R/M disease setting, two phase III studies assessed whether the combined use of an anti-PD-1/PD-L1 MoAb with an anti-CTLA-4 MoAb (nivolumab [anti-PD-1] plus ipilimumab in the CheckMate-651 study and durvalumab [anti-PD-L1] plus tremelimumab in the KESTREL study) would be superior over the EXTREME regimen [8] (NCT02551159). However, both had a negative outcome in terms of OS benefit [8](AstraZeneca announcement 5.2.2021). In the second-line R/M-HNSCC setting in platinum-pretreated patients, the EAGLE study, a phase III assessing the combination of durvalumab plus tremelimumab versus treatment at the investigator’s choice (cetuximab, a taxane, methotrexate, or a fluoropyrimidine) showed no significant differences in OS [9].
Tiragolumab is a MoAb that targets the coinhibitory receptor T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif (ITIM) domain (TIGIT), which is expressed on lymphocytes and suppresses the immune response to cancer by limiting T cells and natural killer (NK) cells proliferation. Tiragolumab is currently assessed in combination with atezolizumab (an anti-PD-L1 MoAb) in a randomized phase II trial in the first line for patients with R/M previously untreated HNSCC, with PD-L1 positive tumors (TPS ≥ 5%) (Table 5.2) [10].
Another coinhibitory checkpoint molecule, lymphocyte-activation gene 3 (LAG-3) is currently targeted by different strategies. Relatlimab is a MoAb that is being investigated either in combination with nivolumab plus ipilimumab or with nivolumab plus an indoleamine 2,3-dioxygenase-1 (IDO1) inhibitor in a phase I/II study in the advanced setting (NCT03459222) (Table 5.2). Eftilagimod alpha is a soluble LAG-3 protein that binds to a subset of major histocompatibility complex (MHC) class II molecules to mediate antigen presenting cell activation and CD8 T-cell activation, that was studied in combination with pembrolizumab in a phase II trial for HNSCC patients who had progressed on or after a first-line platinum-based therapy (NCT03625323) [11]. First reported results showed an encouraging ORR of 31.4% (95% CI: 16.9–49.3%) in patients unselected for PD-L1 tumor expression and deserves further development [11] (Table 5.2).
Along with immune checkpoint inhibitors, another strategy has been to develop antibodies targeting costimulatory immune checkpoints on T cells, like the inducible T-cell co-stimulator (ICOS), to improve priming of T cells. The feladilimab, was a first-in-man ICOS agonist developed in heavily pre-treated HNSCC patients having an anti-PD-1/L1 treatment-naïve disease that showed encouraging efficacy results in early phase with an ORR of 26% (95% CI: 12.9–44.4) [12]. However, subsequent randomized phase II trials were stopped by a recommendation of the Independent Data Monitoring Committee after obtaining results from a pre-specified futility analysis.
Others agonist antibodies have been developed to target OX40, a potent costimulatory protein in the tumor necrosis factor receptor superfamily (CD134, TNFRSF4), allowing to stimulate effector T cells and inhibit regulatory T cells suppression [13, 14]. OX40 agonists are currently being assessed mainly in combination with other immunotherapies. ABBV-368, an immunoglobulin G1 agonistic anti-OX40 MoAb is evaluated in a phase Ib study in heavily pre-treated HNSCC patients when given together with intratumoral injection of tilsotolimod, a synthetic Toll-like receptor 9 (TLR9) agonist, in combination with nab-paclitaxel and/or budigalimab (ABBV-181), a MoAb targeting PD-1 modified to reduce Fc receptor interactions and limit effector function [13]. In this study, patients had to have failed an anti-PD-1/PD-L1 inhibitor and must have had at least one tumor lesion accessible for intratumoral injection. Other OX40 agonists are also in early-development, like ivuxolimab, a fully human immunoglobulin G2 agonistic MoAb, that unlike immunoglobulin G1-based approaches, does not induce antibody-dependent cellular cytotoxicity and does not deplete OX40-expressing cells (Table 5.2) [14].
NK cells play a critical role in immunosurveillance and control of tumor growth. NK cell activation is negatively regulated by inhibitory killer-cell immunoglobulin-like receptors (KIRs). Blocking KIR function may potentiate an anti-tumor immune response and complement other immuno-oncology strategies that enhance T-cell activity. Lirilumab, a fully human MoAb inhibiting KIRs on NK cells promoting NK cells activation, was studied in combination with nivolumab in a phase I/II trial including patients with advanced solid tumor who have failed at least one prior line of standard treatment (NCT01714739) [15]. In the subgroup of HNSCC patients, the combination of lirilumab plus nivolumab showed an ORR of 24.1% (7/29) and was therefore further investigated in a phase II randomized study versus placebo plus nivolumab. This study, however, showed negative results.
Another immune checkpoint inhibitor, monalizumab is currently being studied in R/M-HNSCC patients previously treated with platinum-based chemotherapy and PD-(L)1 inhibitors [16]. Monalizumab is a first-in-class antibody that inhibits the coinhibitory molecule CD94/NK group 2 member A (NKG2A) expressed by cytotoxic CD8 T cells and NK cells. Results from a phase II study investigating monalizumab in combination with cetuximab in this heavily pretreated population showed an ORR of 20% (95% CI: 11–35%) with a confirmed partial response in eight out of 40 patients included [16]. The phase III study is currently ongoing (NCT04590963) (Table 5.2).
Combination of Anti-immune Checkpoint MoAbs with Other Type of Molecules
In addition to immune checkpoint inhibitors, other molecules have been developed to stimulate the T-cell antitumoral immune response.
The IDO1, an enzyme that catalyzes the degradation of tryptophan, has been described to induce anergy and apoptosis of T cells and can be expressed by tumor cells, dendritic cells and macrophages. Epacadostat, a highly selective oral IDO1 inhibitor, has been assessed in combination with pembrolizumab in a phase III study in the first-line R/M-HNSCC setting [17], but the global development of the molecule was stopped after the negative results of a randomized phase III study published in patients with advanced melanoma [18].
Transforming growth factor β (TGF-β) is implicated in multiple tumorigenic processes and further has a pivotal function within the immune system by maintaining immunotolerance via the regulation of lymphocyte proliferation, differentiation, and survival [19]. Bintrafusp alfa, a first-in-class bifunctional fusion protein targeting TGF-β and PD-L1 was evaluated in a phase I dose-expansion cohort in patients with R/M-HNSCC [20]. The reported ORR was 13% with four partial responses among the 32 included patients, and four patients with disease stabilization. Clinical activity was shown irrespective of tumor PD-L1 expression. A particular adverse event to note related to the treatment was one case of grade 3 keratoacanthoma and one case of grade 3 squamous cell carcinoma of the skin, but these cases were managed with simple excision followed by clinical observation (Table 5.2) [20].
Another interesting strategy is the assessment of interleukin (IL)-2 recombinant cytokines. THOR-707 is a recombinant human IL-2 molecule that includes a polyethylene glycol (PEG) moiety irreversibly bound to a novel amino acid via click chemistry to block the alpha-binding domain (IL-2 receptor [IL-2R], CD25) while retaining near-native affinity for the beta/gamma subunits. This molecule reduced risk of immune toxicities, via blocking CD25 activation on regulatory T cells, and reduce vascular leak syndrome seen with standard human IL-2, along with a maintained selective activation of effector T cells via the beta/gamma subunits binding [21]. The dose escalation phase is ongoing (NCT04009681) in R/M-HNSCC patients, either in combination with cetuximab of pembrolizumab (Table 5.2).
Beside the combination of two immunotherapy agents, or combination with chemotherapy or targeted therapies already approved in the treatment of HNSCC, another strategy is to assess the effect of epidrugs (epigenetic enzyme inhibitors), like vorinostat, a Histone DesACetylases (HDAC) inhibitor in combination with immune checkpoint inhibitors, as preclinical evidence has suggested that modulating the epigenome might modulate antitumor response and improve the efficacy of current immunotherapies (NCT04357873) (Table 5.2) [22].
Immunotherapeutic Vaccines for Human Papillomavirus (HPV)-Positive HNSCC
MEDI0457, a therapeutic DNA vaccine containing plasmids for E6 and E7 oncogenes for HPV16/18 and IL-12 adjuvant, has been shown to be safe and to induce an immune response against the expressed antigens, along with interesting preliminary efficacy results (ORR of 22.2%) when given in combination with durvalumab to HPV16/18 positive R/M-HNSCC patients (Table 5.2) [23].
Tipapkinogene sovacivec (TG4001) is another therapeutic recombinant vaccine based on the non-propagative highly attenuated Modified Vaccinia Ankara (MVA) virus vector that contains inserted transgenes coding for three proteins, the HPV E6 and E7 oncoproteins and IL-2 as an adjuvant. TG4001 has shown promising activity in a phase Ib/II trial including HPV16-related malignancies with an ORR of 23.5%, but limited efficacy on liver metastases (NCT03260023) [24]. However, the further development of TG4001 in a phase II randomized study is now focusing on HPV16-positive anogenital cancer patients only, with limited hepatic involvement.
Immunotherapy in the Early Stage Setting
In parallel with the continued increase of the number of studies evaluating immuno-oncology molecules in the R/M setting, these agents are currently studied in primary disease and are being assessed in the neodjuvant/adjuvant setting of HNSCC, showing promising response rates, without any surgical delays [25,26,27].
One randomized phase II trial assessed 2 cycles of nivolumab or nivolumab plus ipilimumab in the neoadjuvant setting for the treatment of patients with resectable squamous cell carcinoma of the oral cavity (≥T2, or clinically node positive) [25]. No surgical delays were observed in this study and the pathologic response rate was 54% in the nivolumab arm and 73% in the nivolumab plus ipilimumab arm.
Pembrolizumab alone was investigated in a non-randomized phase II study in patients with resectable HPV-negative, locally advanced HNSCC [26]. In this study, the pathologic response rate after neoadjuvant pembrolizumab was 44% (16/36), without any delayed surgery, and a favorable one-year relapse rate of 16.7% (95%CI: 3.6–41.4%). The KEYNOTE-689 randomized phase III study further evaluates neoadjuvant and adjuvant pembrolizumab in combination with SOC adjuvant chemoradiotherapy in patients with previously untreated, resectable locally advanced HNSCC (NCT03765918).
Bintrafusp alfa has also been investigated in a window-of-opportunity phase II trial in patients with previously untreated, resectable HNSCC (NCT04428047). In this study the bintrafusp alfa was administered for 2 doses before surgery. The study was early terminated after sponsor decision following cases of hyperprogression and early toxicities reported in lung cancer trials.
The combination of lirilumab plus nivolumab was assessed in the neoadjuvant and adjuvant setting in patients with recurrent but resectable HNSCC [27]. In this open-label phase II trial, 28 patients received nivolumab plus lirilumab for one dose between 7 and 21 days prior the planned salvage surgery, then received 6 cycles in the adjuvant setting. Importantly there were no delays to surgery in this study, and pathological response to the combination was observed in 43% (12/28) of patients, with a favorable 1- and 2-year OS of 85.7% (95% CI: 66.3–94.4%) and 71.1% (95% CI: 48–85.3%), respectively.
However, it is not yet known if these preliminary results will translate into significant clinical benefit. Results from larger randomized studies and later survival endpoints are awaited. Furthermore, the definition of pathological responses needs to be standardized, and the establishment or pathological response as a surrogate for survival should be confirmed.
Conclusion
Despite a major craze for the development of immune-oncology drugs beyond PD1/PD-L1 inhibitors, for now, very few have shown significant antitumor activity as single agent in R/M-HNSCC patients, and further results are awaited from ongoing trials. All randomized trials assessing novel immune-oncology drugs in combination with an anti-PD-1/PD-L1 agent have failed in HNSCC patients so far. Many other immune-oncology drugs are in early clinical development and will hopefully improve patient outcomes.
References
Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–57.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–67.
Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–67.
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–27.
Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–28.
Burtness B, Rischin D, Greil R, Soulières D, Tahara M, de Castro Jr G, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. JCO. 2022;JCO.21.02198.
Argiris A, Harrington K, Tahara M, Ferris RL, Gillison M, Fayette J, et al. LBA36 Nivolumab (N) + ipilimumab (I) vs EXTREME as first-line (1L) treatment (tx) for recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): final results of CheckMate 651. Ann Oncol. 2021;32:S1310–1.
Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31(7):942–50.
Cohen E, Fayette J, Harrington KJ, Johnson ML, Kao HF, Lee SH, et al. 927TiP SKYSCRAPER-09: a phase II, randomised, double-blinded study of atezolizumab (Atezo) + tiragolumab (Tira) and atezo + placebo as first-line (1L) therapy for recurrent/metastatic (R/M) PD-L1+ squamous cell carcinoma of the head and neck (SCCHN). Ann Oncol. 2021;32:S814–5.
Brana I, Forster M, Lopez-Pousa A, Doger B, Roxburgh P, Bajaj P, et al. Results from a phase II study of eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab in patients with PD-L1 unselected metastatic second-line squamous head and neck carcinoma. JCO. 2021;39(15_suppl):6028.
Angevin E, Groenland SL, Lim AML, Martin-Liberal J, Moreno V, Trigo JM, et al. Updated analysis of the inducible T-cell co-stimulatory receptor (ICOS) agonist, GSK3359609 (GSK609), combination with pembrolizumab (PE) in patients (pts) with anti-PD-1/L1 treatment-naïve head and neck squamous cell carcinoma (HNSCC). JCO. 2020;38(15_suppl):6517.
Le X, Gluck I, Maurice-Dror C, Panwar A, Gold K, Berlin J, et al. 975TiP Phase Ib trial of ABBV-368 + tilsotolimod in combination with nab-paclitaxel and/or budigalimab (ABBV-181) in patients with recurrent/metastatic head and neck squamous cell carcinoma. Ann Oncol. 2020;31:S685.
Diab A, Hamid O, Thompson JA, Ros W, Eskens FALM, Doi T, et al. A Phase I, open-label, dose-escalation study of the OX40 agonist ivuxolimab in patients with locally advanced or metastatic cancers. Clin Cancer Res. 2022;28(1):71–83.
Leidner R, Kang H, Haddad R, Segal NH, Wirth LJ, Ferris RL, et al. Preliminary efficacy from a phase I/II study of the natural killer cell–targeted antibody lirilumab in combination with nivolumab in squamous cell carcinoma of the head and neck. Poster 2016;456, STIC.
Cohen RB, Bauman JR, Salas S, Colevas AD, Even C, Cupissol D, et al. Combination of monalizumab and cetuximab in recurrent or metastatic head and neck cancer patients previously treated with platinum-based chemotherapy and PD-(L)1 inhibitors. JCO. 2020;38(15_suppl):6516.
Cohen EEW, Rischin D, Pfister DG, Vermorken JB, Zhao Y, Gowda H, et al. A phase 3, randomized, open-label study of epacadostat plus pembrolizumab, pembrolizumab monotherapy, and the EXTREME regimen as first-line treatment for recurrent/metastatic head and neck squamous cell carcinoma (R/M SCCHN): ECHO-304/KEYNOTE-669. JCO. 2018;36(15_suppl):TPS6090.
Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20(8):1083–97.
Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-β and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13(18):5262–70.
Cho BC, Daste A, Ravaud A, Salas S, Isambert N, McClay E, et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in advanced squamous cell carcinoma of the head and neck: results from a phase I cohort. J Immunother Cancer. 2020;8(2): e000664.
Janku F, Abdul-Karim R, Azad A, Bendell J, Falchook G, Gan HK, et al. Abstract LB041: THOR-707 (SAR444245), a novel not-alpha IL-2 as monotherapy and in combination with pembrolizumab in advanced/metastatic solid tumors: interim results from HAMMER, an open-label, multicenter phase 1/2 Study. Cancer Res. 2021;81(13_Supplement):LB041.
De Guillebon E, Jimenez M, Mazzarella L, Betsou F, Stadler P, Petak I. Combining immunotherapy with an epidrug in squamous cell carcinomas of different locations: rationale and design of the PEVO basket trial. ESMO Open. 2021;6(3): 100106.
Aggarwal C, Saba N, Algazi AP, Sukari A, Seiwert TY, Haigentz M. Safety and efficacy of MEDI0457 plus durvalumab in patients (pts) with human papillomavirus-associated recurrent/metastatic head and neck squamous cell carcinoma (HPV+ R/M HNSCC). Ann Oncol. 2020; 31 (suppl_4): S599–S628 101016/annonc/annonc277.
Le Tourneau C, Cassier P, Rolland F, Salas S, Limacher JM, Capitain O, et al. 793 TG4001 (Tipapkinogene sovacivec) and avelumab for recurrent/metastatic (R/M) human papilloma virus (HPV)-16+ cancers: clinical efficacy and immunogenicity. J Immunother Cancer. 2020;8(Suppl 3).
Schoenfeld JD, Hanna GJ, Jo VY, Rawal B, Chen YH, Catalano PS, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in untreated oral cavity squamous cell carcinoma: a phase 2 open-label randomized clinical trial. JAMA Oncol. 2020;6(10):1563–70.
Uppaluri R, Campbell KM, Egloff AM, Zolkind P, Skidmore ZL, Nussenbaum B, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: a multicenter. Phase II Trial Clin Cancer Res. 2020;26(19):5140–52.
Hanna GJ, O’Neill A, Shin KY, Wong K, Jo VY, Quinn CT, et al. Neoadjuvant and adjuvant nivolumab and lirilumab in patients with recurrent, resectable squamous cell carcinoma of the head and neck. Clin Cancer Res. 2022;28(3):468–78.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest Disclosures
EB received honoraria and nonfinancial support from Eisai, MSD, Sandoz, and Daiichi Sankyo.
CLT participated in advisory boards from MSD, BMS, Merck Serono, Astra Zeneca, Celgene, Seattle Genetics, Roche, Novartis, Rakuten, Nanobiotix, and GSK.
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this paper
Cite this paper
Borcoman, E., Le Tourneau, C. (2023). Novel Immune Oncology Targets Beyond PD-1/PD-L1 in Head and Neck Cancer. In: Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, JP., Nicolai, P., O'Sullivan, B. (eds) Critical Issues in Head and Neck Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-23175-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-23175-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23174-2
Online ISBN: 978-3-031-23175-9
eBook Packages: MedicineMedicine (R0)